准备扩大的奇廷泡沫及其用于去除水中的铜
Summary
这项研究描述了一种通过不需要专门设备的化学技术将奇丁扩展成泡沫的方法。
Abstract
奇廷是一种开发不足、自然丰富、机械坚固、耐化学的生物聚合物。这些品质在吸附剂中是可取的,但奇丁缺乏必要的特定表面积,其修改涉及专门的技术和设备。这里描述了一种新的化学程序,将来自虾壳废物的辣椒片膨胀成表面积较高的泡沫。这个过程依赖于H2 气体的进化,从水的反应与NaH被困在一个奇丁凝胶。制备方法不需要专用设备。粉末X射线衍射和N2-物理吸收表明晶体大小从6.6纳米减少到4.4纳米,特定表面积从12.6±2.1米2/g增加到73.9±0.2米2/克。然而,红外光谱学和热重力学分析表明,这个过程不会改变奇廷的化学特性。扩大的奇丁的特定Cu吸附能力与特定表面积成正比,从13.8±2.9毫克/克增加到73.1±2.0毫克/克。然而,Cu吸附能力作为表面密度保持相对恒定,平均为10.1±0.8原子/nm2,这再次表明甲酰丁的化学特性没有变化。这种方法提供了在不牺牲其理想特性的情况下将奇丁转化为更高表面积材料的方法。虽然这里的奇丁泡沫被描述为吸附剂,但它可以设想为催化剂支撑、热绝缘体和结构材料。
Introduction
奇廷是一种机械坚固和化学惰性生物聚合物,仅次于天然丰度1中的纤维素。它是节肢动物外骨骼和真菌和酵母2细胞壁的主要成分。奇丁与纤维素相似,但每个单体的羟基组被乙酰胺组(图1A,B)所取代。这种差异增加了相邻聚合物链之间的氢粘结强度,并赋予奇丁其特有的结构弹性和化学惰性2,3。由于其特性和丰富性,奇廷吸引了巨大的工业和学术兴趣。它已被研究为组织生长的支架4,5,6,作为复合材料的成分7,8,9,10,11,并作为支持吸附剂和催化剂11,12,13,14。其化学稳定性,特别是使奇丁吸引吸附应用,涉及条件不适宜常见的吸附剂14。此外,丰富的胺组使奇丁对金属离子15的有效吸附。然而,酸性条件下胺组的质子会降低奇丁16的金属吸附能力。一个成功的策略是引入更耐质子17,18的吸附位点。相反,这里描述了一个简单的方法来增加特定的表面积,因此,在奇廷吸附点的数量。
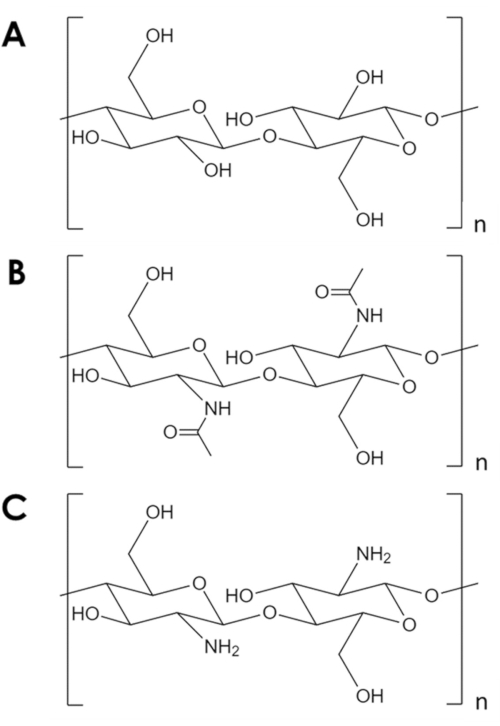
图1。化学结构。(A)纤维素, (B)奇廷, (C)奇托桑。请点击这里查看这个数字的更大版本。
尽管它有许多潜在的用途,但奇丁的利用不足。奇廷加工具有挑战性,因为它在大多数溶剂中的溶解性较低。其在催化和吸附中的使用的主要限制是其低特异性表面积。而典型的碳和金属氧化物支架有特定的表面积在顺序102-103 m2/g,商业辣椒片有表面积在10米2/克19,20,21的顺序。将辣椒素扩展到泡沫的方法是存在的,但它们总是依赖于高温高压、强酸和碱基,或代表重大进入屏障的专用设备,这些设备代表着重要的进入屏障5、21、22、23、24、25。此外,这些方法倾向于去乙酰丁,形成甲壳素(图1C)– 一种更可溶性和反应性生物聚合物5,25,26。
其中,介绍了一种将奇丁扩展到固体泡沫、增加其特定表面积和吸附能力以及保持其化学完整性的方法。该方法依赖于气体从奇廷凝胶内的快速演化,不需要专门的设备。扩大的辣椒素的吸附能力增加,用水Cu2+证明 – 当地地下水26中的常见污染物。
| 单位 | 整洁的弗莱克 | 烤泡沫 | 嗜血泡沫 | |
| 结晶 | % | 88 | 74 | 58 |
| 晶体大小 | 纳米 | 6.5 | 4.4 | 4.4 |
| 表面积 | m2/g | 12.6 ± 2.1 | 43.1 ± 0.2 | 73.9 ± 0.2 |
| 库塔克 | 毫克/克 | 13.8 ± 2.9 | 48.6 ± 1.9 | 73.1 ± 2.0 |
| 库塔克 | 原子/纳米2 | 10.5 ± 2.8 | 10.7 ± 0.4 | 9.4 ± 0.3 |
表1。材料属性摘要。 与整洁的奇丁片相比,奇廷泡沫的晶体度和晶体大小较低。然而,奇丁泡沫的特定表面积和Cu吸收量比整齐的奇丁片高。
Protocol
Representative Results
Discussion
建议的奇丁泡沫制造方法允许生产这种泡沫,而无需专门的设备或技术。奇廷泡沫的生产依赖于奇丁醇凝胶内氢化钠的悬浮。与大气中的水接触通过分解氢化钠诱导气质基质的凝胶和氢气的演化。因此,制备的关键步骤是(1)形成溶胶,(2)在无水条件下引入氢化钠,(3)大气水与奇丁醇凝胶和氢化钠悬浮剂的反应。
第三步有两个重要的限制。首先,该过程扩展得很差。?…
Disclosures
The authors have nothing to disclose.
Acknowledgements
这项研究由作战能力发展指挥部陆军研究实验室(合作协议编号W911NF-15-2-0020)赞助。本材料中表达的任何意见、发现和结论或建议都是作者的意见,并不一定反映陆军研究实验室的观点。
我们感谢蒙大拿理工大学高级材料处理中心 (CAMP) 使用本研究所需的一些专用设备。我们还感谢加里·怀斯、南希·奥耶、里克·拉杜瑟尔、约翰·柯特利和凯瑟琳·佐德罗的技术援助和有益的讨论。
Materials
| Ammonium bicarbonate | Sigma-Aldrich | 9830 | NH4HCO3, ≥99.5 % |
| Chitin | Sigma-Aldrich | C7170 | Pandalus borealis, practical grade |
| Colorimeter | Hanna Instruments | HI83399-01 | Photometer for wastewater analysis |
| Copper High Range Checker | Hanna Instruments | HI702 | Bicinchoninate colorimetric titration |
| Copper nitrate hydrate | Sigma-Aldrich | 223395 | Cu(NO3)2 · 2.5 H2O, 98 % |
| Dimethylacetamide (DMAc) | Sigma-Aldrich | 271012 | Anhydrous, 99.8 % |
| IR Spectrophotometer | Thermo Nicolet | Nexus 670 | Fitted with an ATR cell |
| Lithium chloride | Sigma-Aldrich | 310468 | LiCl, ≥99 % |
| N2 Physisorption Apparatus | Micromeritics | Tristar II | |
| Nitric acid | BDH | BDH7208-1 | HNO3, 0.1 N |
| Scanning electron microscope | Zeiss LEO | 1430 VP | 15 kV, secondary electron detector, 29-31 mm working distance |
| Sodium hydride | Sigma-Aldrich | 223441 | NaH, packed in mineral oil, 90 % |
| Thermogravimetric analyzer | TA Instruments | Q500 | 100 ml/min N2, 10 °C/min to 800 °C |
| Water Purification System | Millipore | Milli-Q | Type A water (18 MΩ) |
| X-Ray Diffractometer | Rigaku | Ultima IV | Cu K-α radiation, 8.04 keV |
References
- Rinaudo, M. Chitin and chitosan: Properties and applications. Progress in Polymer Science. 31 (7), 603-632 (2006).
- Percot, A., Viton, C., Domard, A. Optimization of chitin extraction from shrimp shells. Biomacromolecules. 4 (1), 12-18 (2003).
- Austin, P. R. Chitin solvents and solubility parameters. Chitin, Chitosan, and Related Enzymes. , 227-237 (1984).
- Deepthi, S., Venkatesan, J., Kim, S. K., Bumgardner, J. D., Jayakumar, R. An overview of chitin or chitosan/nano ceramic composite scaffolds for bone tissue engineering. International Journal of Biological Macromolecules. 93, 1338-1353 (2016).
- Tao, F., et al. Applications of chitin and chitosan nanofibers in bone regenerative engineering. Carbohydrate Polymers. 230, 115658 (2020).
- Zhao, L., et al. Regulation of the morphological and physical properties of a soft tissue scaffold by manipulating DD and DS of O-carboxymethyl chitin. ACS Applied Bio Materials. 3 (9), 6187-6195 (2020).
- Duan, Y., Freyburger, A., Kunz, W., Zollfrank, C. Cellulose and chitin composite materials from an ionic liquid and a green co-solvent. Carbohydrate Polymers. 192, 159-165 (2018).
- Kadokawa, J., Takegawa, A., Mine, S., Prasad, K. Preparation of chitin nanowhiskers using an ionic liquid and their composite materials with poly(vinyl alcohol). Carbohydrate Polymers. 84 (4), 1408-1412 (2011).
- Chen, Z., Wang, J., Qi, H. J., Wang, T., Naguib, H. E. Green and sustainable layered chitin-vitrimer composite with enhanced modulus, reprocessability, and smart actuator function. ACS Sustainable Chemistry and Engineering. 8 (40), 15168-15178 (2020).
- Zhang, Z., Lucia, L. A. Chitin-clay composite gels with enhanced thermal stability prepared in a green and facile approach. Journal of Materials Science. 56 (4), 3600-3611 (2021).
- Ahmed, M. J., Hameed, B. H., Hummadi, E. H. Review on recent progress in chitosan/chitin-carbonaceous material composites for the adsorption of water pollutants. Carbohydrate Polymers. 247, 116690 (2020).
- Matsuoka, A., et al. Hydration of nitriles to amides by a chitin-supported ruthenium catalyst. RSC Advances. 5 (16), 12152-12160 (2015).
- Wang, Y., Li, Y., Liu, S., Li, B. Fabrication of chitin microspheres and their multipurpose application as catalyst support and adsorbent. Carbohydrate Polymers. 120, 53-59 (2015).
- Anastopoulos, I., Bhatnagar, A., Bikiaris, D., Kyzas, G. Chitin Adsorbents for Toxic Metals: A Review. International Journal of Molecular Sciences. 18 (1), 114 (2017).
- Habiba, U., Afifi, A. M., Salleh, A., Ang, B. C. Chitosan/(polyvinyl alcohol)/zeolite electrospun composite nanofibrous membrane for adsorption of Cr6+, Fe3+ and Ni2+. Journal of Hazardous Materials. 322, 182-194 (2017).
- Kim, U. J., et al. Protein adsorption of dialdehyde cellulose-crosslinked chitosan with high amino group contents. Carbohydrate Polymers. 163, 34-42 (2017).
- He, Y., et al. Fabrication of PVA nanofibers grafted with octaamino-POSS and their application in heavy metal adsorption. Journal of Polymers and the Environment. , (2020).
- Tian, H., et al. Electrospinning of polyvinyl alcohol into crosslinked nanofibers: An approach to fabricate functional adsorbent for heavy metals. Journal of Hazardous Materials. 378, (2019).
- Meille, V. Review on methods to deposit catalysts on structured surfaces. Applied Catalysis A: General. 315, 1-17 (2006).
- Dotto, G. L., Cunha, J. M., Calgaro, C. O., Tanabe, E. H., Bertuol, D. A. Surface modification of chitin using ultrasound-assisted and supercritical CO2 technologies for cobalt adsorption. Journal of Hazardous Materials. 295, 29-36 (2015).
- Phongying, S., Aiba, S., Chirachanchai, S. Direct chitosan nanoscaffold formation via chitin whiskers. Polymer. 48 (1), 393-400 (2007).
- Tan, T. S., Chin, H. Y., Tsai, M. L., Liu, C. L. Structural alterations, pore generation, and deacetylation of α- and β-chitin submitted to steam explosion. Carbohydrate Polymers. 122, 321-328 (2015).
- Chang, F. S., Chin, H. Y., Tsai, M. L. Preparation of chitin with puffing pretreatment. Research on Chemical Intermediates. 44 (8), 4939-4955 (2018).
- Goodrich, J. D., Winter, W. T. α-Chitin Nanocrystals prepared from shrimp shells and their specific surface area measurement. Biomacromolecules. 8 (1), 252-257 (2007).
- Rolandi, M., Felts, J. . Naturally sourced chitin foam. , (2020).
- McDermott, S., Hailer, M. K., Lead, J. R. Meconium identifies high levels of metals in newborns from a mining community in the U.S. Science of the Total Environment. 707, 135528 (2020).
- Hach Handbook of Water Analysis. Copper, Bicinchoninate Method, Method 8506. Hach Handbook of Water Analysis. , (1979).
- Crittenden, J. C., Trusell, R. R., Hand, D. R., Howe, K. J., Tchbanoglous, G. Adsorption. MWH’s Water Treatment. , 1117 (2012).
- Focher, B., Beltrame, P. L., Naggi, A., Torri, G. Alkaline N-deacetylation of chitin enhanced by flash treatments. Reaction kinetics and structure modifications. Carbohydrate Polymers. 12 (4), 405-418 (1990).
- Scherrer, P. Determination of the size and the internal structure of colloidal particles by means of X-rays. News from the Society of Sciences in Göttingen, Mathematical- Physical Class. 2, 98-100 (1918).
- Brunauer, S., Emmett, P. H., Teller, E. Adsorption of gases in multimolecular layers. Journal of the American Chemical Society. 60 (2), 309-319 (1938).
- Sing, K. S. W. Adsorption methods for the characterization of porous materials. Advances in Colloid and Interface Science. 76-77, 3-11 (1998).
- Rouquerol, J., Llewellyn, P., Rouquerol, F. Is the bet equation applicable to microporous adsorbents. Studies in Surface Science and Catalysis. 160, 49-56 (2007).
- Vorokh, A. S. Scherrer formula: estimation of error in determining small nanoparticle size. Nanosystems: Physics, Chemistry, Mathematics. , 364-369 (2018).
- Labidi, A., Salaberria, A. M., Fernandes, S. C. M., Labidi, J., Abderrabba, M. Adsorption of copper on chitin-based materials: Kinetic and thermodynamic studies. Journal of the Taiwan Institute of Chemical Engineers. 65, 140-148 (2016).
- Tian, M., Zhao, T. Q., Chin, P. L., Liu, B. S., Cheung, A. S. -. C. Methane and propane co-conversion study over zinc, molybdenum and gallium modified HZSM-5 catalysts using time-of-flight mass-spectrometry. Chemical Physics Letters. 592, 36-40 (2014).

