Leukodepletion Filters-Derived CD34+ Cells As a Cell Source to Study Megakaryocyte Differentiation and Platelet Formation
Summary
This protocol describes in detail all the steps involved in obtaining leukofilter-derived CD34+ hematopoietic progenitors and their in vitro differentiation and maturation into proplatelet-bearing megakaryocytes that are able to release platelets in the culture medium. This procedure is useful for in-depth analysis of cellular and molecular mechanisms controlling megakaryopoiesis.
Abstract
The in vitro expansion and differentiation of human hematopoietic progenitors into megakaryocytes capable of elongating proplatelets and releasing platelets allows an in-depth study of the mechanisms underlying platelet biogenesis. Available culture protocols are mostly based on hematopoietic progenitors derived from bone marrow or cord blood raising a number of ethical, technical, and economic concerns. If there are already available protocols for obtaining CD34 cells from peripheral blood, this manuscript proposes a straightforward and optimized protocol for obtaining CD34+ cells from leukodepletion filters readily available in blood centers. These cells are isolated from leukodepletion filters used in the preparation of blood transfusion products, corresponding to eight blood donations. These filters are meant to be discarded. A detailed procedure to collect hematopoietic progenitors identified as CD34+ cells from these filters is described. The method to obtain mature megakaryocytes extending proplatelets while discussing their phenotypic evolution is also detailed. Finally, the protocol present a calibrated pipetting method, to efficiently release platelets that are morphologically and functionally similar to native ones. This protocol can serve as a basis for evaluating pharmacological compounds acting at various steps of the process to dissect the underlying mechanisms and approach the in vivo platelet yields.
Introduction
Blood platelets come from specialized large polyploid cells, the megakaryocytes (MK), that originate from a constant and fine-tuned production process known as megakaryopoiesis (MKP). At the apex of this process are hematopoietic stem cells which, in contact with the bone marrow environment (cytokines, transcription factors, hematopoietic niche), will be able to proliferate and differentiate into hematopoietic progenitors (HP) able to commit toward the megakaryocytic pathway, giving rise to immature MKs1. Under the influence of various cytokines, and in particular thrombopoietin (TPO), which is the major cytokine of MKP; the MK will then undergo two major stages of maturation: endomitosis and the development of demarcation membranes (DMS). This fully mature MK then appears close to a sinusoid vessel in which it can emit cytoplasmic extensions, the proplatelets, which will be released under the blood flow and subsequently remodeled into functional platelets2. The cloning of TPO in 19943 provided a boost in the study of MKP by accelerating the development of in vitro culture techniques allowing HP differentiation and MK maturation.
There are many pathologies affecting blood platelets, both in terms of platelet number (increase or decrease) and function4,5. Being able to recapitulate MKP in vitro from human HP could improve understanding of the molecular and cellular mechanisms underlying this process and ultimately the therapeutic management of patients.
Various sources of human HP are suitable: cord blood, bone marrow, and peripheral blood6,7,8. Harvesting HP from peripheral blood raises less logistical and ethical problems than their recovery from cord blood or the bone marrow. HP can be recovered from leukapheresis or buffy coat, but these sources are expensive and not always available in blood centers. Other protocols, less expensive and easier to perform, allow direct recovery of human peripheral blood mononuclear cells (PBMCs) without the need for prior CD34 driven isolation4,8. However, the purity of megakaryocytes is not satisfactory with this method and a selection of CD34+ cells from PBMC is recommended for optimal differentiation into MK. This led us to implement a HP purification from leukoreduction filters (LRF), routinely used in blood banks to remove white blood cells and thus avoid adverse immunological reactions9. Indeed, since 1998, platelet concentrates have been automatically leukodepleted in France. At the end of this process, LRF are discarded and all the cells retained in the LRF are destroyed. Cells in LRFs are, therefore, readily available at no additional cost. LRFs have a cellular content close to that obtained by leukapheresis or in buffy coats, notably in their composition of CD34+ HP making them a remarkably attractive source10. LRF as a human HP source has already been demonstrated to provide cells with intact functional capacities11. This source has the advantage of being abundant and affordable for laboratory research. In this context, this article describes successively: i) the extraction and selection of CD34+ HP from LRFs; ii) a two-phase optimized culture, which recapitulates the commitment of HP into the megakaryocytic pathway and the maturation of MK capable of emitting proplatelets; iii) a method for efficiently releasing platelets from these MK; and iv) a procedure for phenotyping MK and cultured platelets.
Protocol
Control human samples were obtained from volonteer blood donors who gave written informed consent recruited by the blood transfusion center where the research was performed (Etablissement Français du Sang-Grand Est). All procedures were registered and approved by the French Ministry of Higher Education and Research and registered under the number AC_2015_2371.The donors gave their approval in the CODHECO number AC- 2008 – 562 consent form, in order for the samples to be used for research purposes. Human studies were performed according to Helsinki declaration.
1. Extraction and selection of CD34+ cells (HP) from LRF
- Reagent's preparation (for one LRF)
- Prepare 25 mL of filtered elution buffer: 21.25 mL of Phosphate Buffered Saline (PBS), 2.5 mL of Acid-Citrate-Dextrose (ACD) and 1.25 mL of decomplemented Fetal Bovine Serum (FBS). Filter on 0.22 µm and place at 37 °C.
- Prepare 500 mL of PBS with 2 mM of ethylenediaminetetraacetic-acid (EDTA).
- Dispose 25 mL of density gradient medium (DGM) (1.077 g/mL) in two 50 mL tubes.
- LRF back-flushing modalities (Figure 1)
NOTE: This step requires a sterile tube welding machine, allowing sterile connection of thermoplastic tubing.- First, connect the LRF to an empty 600 mL transfer bag and the LRF to a tubing set (Figure 1A). Under the biosafety cabinet, inject the total volume of filtered and prepared elution buffer corresponding to the number of LRFs handled (xLRF x 25 mL) into the empty bag. Then, using a 30 mL syringe to gently aspirate the entire contents of the bag through the LRF, backflush and transfer the cells into a new 50 mL tube (Figure 1B).
- To the red blood cells sedimentation, dilute the cell suspension by half with Dextran 2% and mix well to aggregate red blood cells. Wait for 30 min at room temperature (RT).
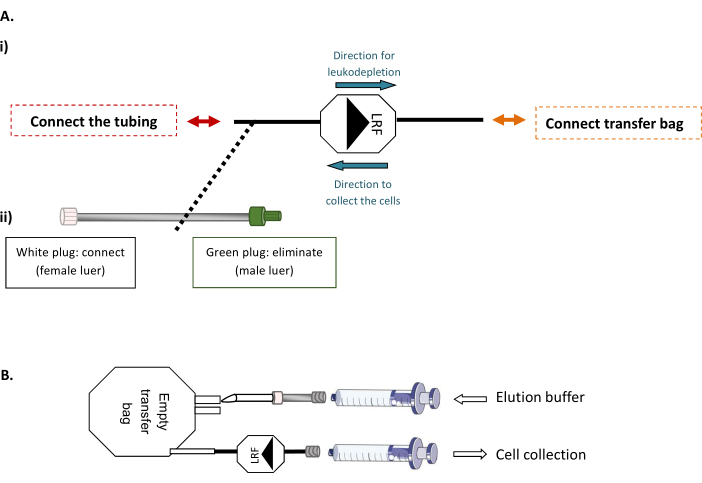
Figure 1: LRF back flushing modalities. (A) Representative scheme of (i) the sterile connection of the transfer bag to the LRF and (ii) the tubing set to the LRF. (B) Representative scheme of the connection of the syringes for cell collection. Please click here to view a larger version of this figure.
- PBMC collection
- Following the red blood cells sedimentation, remove and transfer the supernatant into a 50 mL tube and fill it with PBS-EDTA 2 mM. Gently overlay the supernatant onto the above-prepared DGM. Let the supernatant flow gently without breaking the surface plane of the density gradient. Centrifuge at 400 x g at RT for 30 min in brake off mode.
- Collect the PBMC layer with a disposable transfer pipette. Transfer the cells from each DGM tube into a new sterile 50 mL tube. Fill each tube with PBS-EDTA 2 mM and wash twice in 50 mL of PBS-EDTA 2 mM at 200 x g for 10 min at RT in brake on mode.
- Pipette off and pool the cell pellet with 50 mL PBS-EDTA 2 mM.
NOTE: There is a possibility to stop the procedure by maintaining the collected cells under agitation at 4 °C during the night. Then, filter the suspension with a 40 µm cell strainer to remove the aggregates formed.
- CD34+ cells selection
- Determine the cell number and centrifuge at 400 x g at room temperature for 10 min with the break on.
- Aspirate the supernatant completely and resuspend in the appropriate volume of PBS-EDTA 2 mM detailed by the manufacturer of the CD34 selection kit (300 µL of PBS-EDTA for 108 cells). Add the FcR blocking Reagent and the CD34 Microbeads in the appropriate concentration (50 µL for 108 cells).
- After 30 min at 4 °C, wash the cell suspension and resuspend in the appropriate volume of PBS-EDTA 2 mM detailed by the manufacturer of the CD34 selection kit (500 µL per 108 cells).
NOTE: The selection is made on sorting columns for the passage of maximum 2 x 109 cells per column. - Pass the sample over the wet column of the magnet. Wash twice with 3 mL of PBS-EDTA 2 mM and elute the cells with 5 mL of PBS-EDTA 2 mM. A second run on a new column following the same procedure is necessary to improve the purity of the sample.
NOTE: An expected number of 6.1 x 105 cells/LRF (Figure 2A). For higher LRF numbers, scale up reagents and methods accordingly.
- Evaluation of CD34+ cell purity
- Add to an aliquot of 100 µL of suspension obtained after the CD34+ selection, 2 µL of human CD34-PE antibody or 2 µL of IgG – PE (control). Mix well and incubate for 15 min at 4 °C.
- Wash cells by adding 2 mL of PBS and centrifuge at 400 x g for 5 min. Aspirate supernatant completely and resuspend in 200 µL of PBS.
- Analyze the purity by flow cytometry as shown in Figure 2A and in the Discussion.
NOTE: A purity of CD34+ cells above 90% is expected (Figure 2Bii). - Use the CD34+ cells directly or freeze for further use.
- CD34+ cells freezing
NOTE: CD34+ cells freezing is done at a density of 106 cells per mL.- Following the CD34+ cell number determination, prepare the following cryopreservation media: (1) 60% Stemspan + 40% FBS, (2) 40% Stemspan + 40% FBS + 20% Dimethyl Sulfoxide (DMSO) and allow cooling at 4 °C.
- Centrifuge the CD34+ cells at 400 x g at room temperature for 5 min and resuspend the pellet in cold solution 1 and then immediately add to the cold solution 2 (v/v).
- Place the cryotubes immediately into a -80 °C freezer for 24 h and then transfer cryotubes into the liquid nitrogen tank.
2. Culture and differentiation of CD34+ cells to produce mature proplatelet-bearing megakaryocytes
NOTE: Cell culture protocol (Figure 3A), representative scheme of the cell culture procedure are detailed in this section.
- CD34+ cells thawing (if required)
- Prepare the thawing solution: 13 mL of PBS-20% FBS and place at 37 °C for 15 min. Quickly transfer the cryotubes to a 37 °C water bath until only one small ice crystal. Under the biological culture cabinet, pipette the whole content, and slowly transfer in 13 mL of prewarmed thawing solution.
- Determine the cell number and cell viability.
- Culture protocol, step 1: from day 0 to day 7
NOTE: Usually cultures are made in 24-well plates with 1 mL of medium per well at a density of 40,000 viable cells/mL, corresponding to 20,000 viable cells/cm2. It is crucial to respect this density if any scale up is planned.- Growth medium preparation : In serum-free hematopoietic cell expansion media (previously heated to 37 °C) add Penicillin-Streptomycin-Glutamine (PSG) 1x, human Low-Density Lipoprotein (hLDL) at 20 µg/mL, cytokine cocktail of megakaryocyte expansion 1x and Stemregenin 1 (SR1) at 1 µM.
- Cell seeding : Centrifuge the thawed CD34+ cells at 400 x g at room temperature for 5 min. Thoroughly remove the supernatant and resuspend the cell pellet in 1 mL of culture media and performa cell numeration and viability to determine the appropriate volume to seed the cells.
- Centrifuge the cells 400 x g at room temperature for 5 min and resuspend the pellet in the appropriate volume of the warm growth medium. Incubate the cells at 37 °C with 5% CO2 for 7 days.
- Culture protocol, step 2: from day 7 to day 13 (Figure 3B, representative images at day 13)
NOTE: Usually cultures are made in 24-well plates with 1 mL of medium per well at a density of 50,000 viable cells/mL, corresponding to 25,000 viable cells/cm². It is crucial to respect this density if any scale up is planned.- Maturation medium preparation: In serum-free hematopoietic cell expansion media (previously heated to 37 °C) add PSG 1x, hLDL at 20 µg/mL, TPO at 50 ng/mL, and SR1 at 1 µM.
- Examine the cells under the microscope. At day 7, cells display a round and homogeneous appearance by filling the wells or the flasks without being too confluent.
- Under a biosafety cabinet, gently transfer the cells in a 15 mL tube. Wash wells with PBS. Then, determine the number of cells and their viability to calculate the appropriate volume to seed the cells.
- Centrifuge the cells at 400 x g at room temperature for 5 min. Remove the supernatant and resuspend the cells in the appropriate volume of warm media calculated in the previous step. Incubate the cells at 37 °C, 5% CO2 for 6 days.
- Cultured platelet release at day 13
- Add 0.5 µM of prostaglandine I2 (PGI2) and 0.02 U/mL of apyrase to the culture and perform successive pipetting five times with a 1 mL pipette.
NOTE: Platelets are now released into the medium.
- Add 0.5 µM of prostaglandine I2 (PGI2) and 0.02 U/mL of apyrase to the culture and perform successive pipetting five times with a 1 mL pipette.
3. Flow cytometry analysis (MK phenotyping and cultured platelets count)
NOTE: This protocol can be applied to the phenotyping of the cells on the selected culture days. It also allows the determination of the number of the cultured platelet release (Figure 4A,B).
- Preparation for MK analysis
- Label four sets of microcentrifuge tubes as follows: Unlabeled cells as control, cells + 5 µL CD41 – Alexa Fluor 488, Cells + 5 µL CD34 – PECy7, Cells + 5 µL CD41 – Alexa Fluor 488 + 5 µL CD34 – PECy7. Use a minimum of 1.105 cells per tube, do not exceed 1.106 cells per tube. Add to 100 µL of cell suspension per cytometry tube and the different antibodies. Incubate in the dark for 30 min at 4 °C.
- Then, add 2 mL of PBS-EDTA 2 mM per tube and centrifuge at 400 x g for 5 min at room temperature. During centrifugation, prepare a solution of PBS-EDTA 2 mM + 7-Aminoactinomycin-D (7AAD) (1/100), allow for 300 µL solution per tube.
- Remove the supernatant and take up the pellet in 300 µL of PBS-EDTA 2 mM with 7AAD. Run samples through the flow cytometer within 30 min.
NOTE: Analysis strategy for flow cytometry is shown in the Figure 3C and in the Discussion.
- Tube preparation for cultured platelet analysis
- Label four sets of microcentrifuge tubes as follows: Unlabeled cells as control, Cells + 5 µL CD41 – Alexa Fluor 488, Cells + 20 µL CD42a – PE, Cells + 5 µL CD41 – Alexa Fluor 488 + 20 µL CD42a – PE. Following five successive pipetting in culture well, transfer 300 µL of the suspension in tube for cytometry containing a calibrated number of fluorescent beads.
- Add antibodies and incubate in the dark at RT for 30 min.
- Run the samples through the flow cytometer within 30 min and set the acquisition for the passage of 5,000 beads.
NOTE: Analysis strategy for flow cytometry is shown in the Figure 4B and in the Discussion.
Representative Results
Extraction and selection of CD34+ cells from LRFs
Here, the method, derived from Peytour et al.9, describes the extraction and selection of CD34+ cells from discarded LRFs available in blood banks after leukocyte removal. Following the backflush procedure, usually 1.03 x 109 ± 2.45 x 108 cells/LRF (Mean±SEM; n = 155) are recovered with a viability of 94.88 ± 0.10% (Figure 2A i). After the CD34 positive selection, an average of 615.54 x 103 ± 12.28 cells/LRF is obtained (n = 155) (Figure 2A ii). If the number of cells is less than 300,000, it must be concluded that the procedure has not been carried out correctly and must be stopped. To evaluate the success of the CD34 selection, the purity of CD34+ cells is assessed by flow cytometry (Figure 2B). Routinely, a purity above 90% (91.88 ± 0.79%) is expected (Figure 2B). A purity below 75% could mean that there has been a problem in conducting the protocol and in particular the elution of the columns. Below a 75% purity the cells are not conserved for culture experiments.
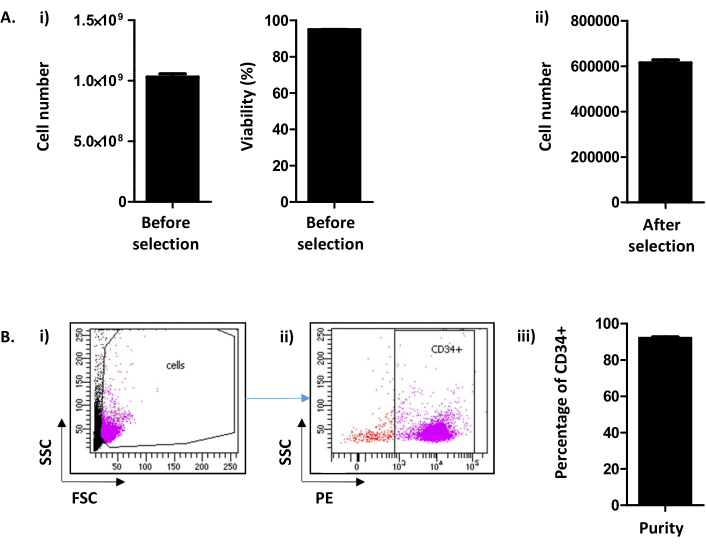
Figure 2: CD34+ cell number/LRF and CD34 purity analysis. (A) (i) An analysis, by cell counter, of the number of cells and their viability obtained following the procedure including both PBMC collection and CD34 selection is performed ((1.03.109 ± 2.45.108 cells/LRF (Mean ± SEM; n=155) with a viability of 94.88 ± 0.10% (n = 155)). (ii) An analysis, by cell counter, is also performed after CD34 selection (615.54 x 103 ± 12.28 cells/LRF (n = 155)). (B) CD34 Purity is analyzed by flow cytometry. (i) Cells were stained with a CD34-PE antibody and identified on their FSC/SSC parameters. (ii) Based on the scatter signal and CD34 expression analysis, the purity is determined. A pre-gate of CD34 positivity was used based on the negative control for the CD34 marker. (iii) As can be seen on the bar graph, a purity of CD34+ cells above 90% (91.88 ± 0.79% (n = 17)) is expected. Please click here to view a larger version of this figure.
Differenciation and maturation of MK-bearing proplatelets
The cell culture procedure described is divided in two steps. The first one, from day (D) 0 to D7, is dedicated to HP proliferation and commitment into the megakaryocytic pathway in response to a combination of cytokines and the addition of a chemical compound SR1. The second one, from D7 to D13, is focused on MK maturation and proplatelet extension following the addition of TPO and SR1 (Figure 3A). As quality control of the culture, cell counting, determination of cell viability, and phenotyping of cells, are perfomed at D7 and D10. These stages have been chosen because they are crucial for HP commitment, D7 and MK maturation, D10, respectively (personal data). At D7 and 10, proliferation is routinely of x4.16 ± 0.25 (n = 34) and x2.30 ± 0.16 (n = 5), respectively, with a cell viability comprised between 86.38 ± 0.73% (n = 34); and 84.80 ± 2.67% (n = 5) (Figure 3B). Concerning cell phenotyping, as shown in Figure 3C, at D0, more than 90% of the cells are positive for CD34. Then, CD34+ cells become committed toward the megakaryocytic lineage, as witnessed by the apparition of CD41, a specific and early marker of MKP. Indeed, at D7, 50.20 ± 2.90% of the cells are positive for both CD34 and CD41 (Figure 3Bii). Then, MK improves their maturation. At D10, a majority of MK are mature, with less than 15.60 ± 4.70% of the cells being negative for CD41, 47.90 ± 8.90% being CD34+CD41+ and 36.40 ± 12.40% being CD34–CD41+ (Figure 3Biii).
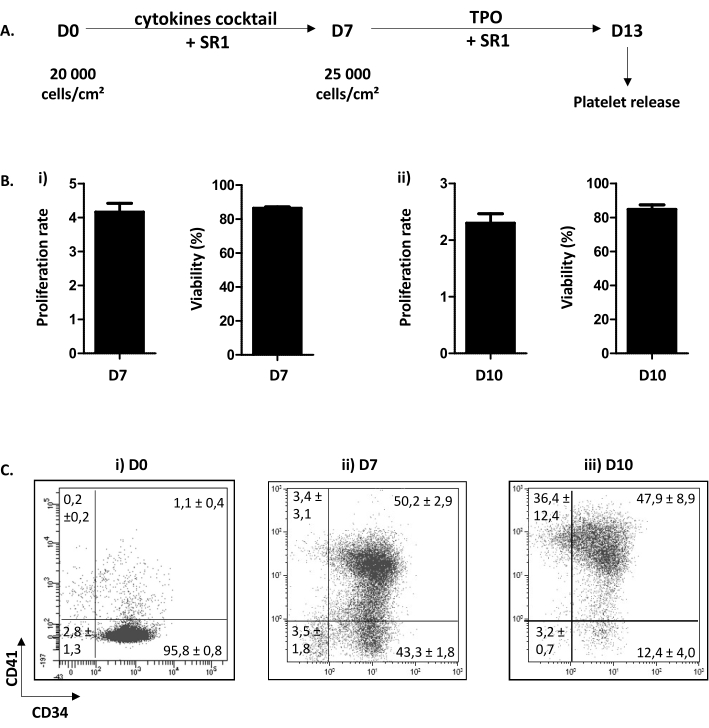
Figure 3: Differenciation and maturation of MK-bearing proplatelets. (A) Representative scheme of the cell culture procedure. A two-step method is used: a proliferation step from D0 to D7 (SR1 and cocktail of cytokines) and a maturation step from D7 to D13 (SR1 and TPO). At D13, cultured platelets can be released following five successive pipetting. (B) (i) Proliferation rate at D7 (x4.16 ± 0.25 (n = 34)) and cell viability (86.38 ± 0.73% (n = 34)). (ii) Proliferation rate at D10 (x2.30 ± 0.16 (n = 5)) and cell viability (84.80 ± 2.67% (n = 5)). (C) Flow cytometry analysis stategy of the phenotypic evolution of MK in culture. At D0, 95.80 ± 0.80% of the cells are CD34 positive (n = 3). At D7, 50.20 ± 2.90% of the cells are positive for CD34 and CD41. At D10, less than 15.60 ± 4.70% of the cells are negative for CD41. Please click here to view a larger version of this figure.
Cultured platelets release, day 13
Examination of the wells at D13 shows round MK and proplatelet-bearing MK (Figure 4A). An average of 35% of cultured MK extend proplatelets12. Of note, D13 represents the optimal day for proplatelet extension and platelet release. If the required level of MK capable of emitting proplatelets is not reached, something must have gone wrong along the culture process and the results should not be taken into account.
Although the exact mechanisms promoting platelet release from mature MK are still poorly understood, it is well known that hemodynamic forces are indispensable.To mimic these forces in vitro, the suspension containing proplatelet-bearing MK is aspirated and repelled five times with a P1000 cone and then analyzed by flow cytometry. For this purpose, tubes containing a calibrated number of fluorescent beads are used. First, beads present into the tube are gated on the CD41-Alexa-fluor 488/PErcP-Cy5 window (Figure 4Bi, in red). Then, cultured platelets are visualized in a pre-gate (platelet-like elements), determined on the forward scatter (FSC) and side scatter (SSC) parameters of native platelets (Figure 4Bii). The number of platelets is then determined on their CD41/CD42a positivity (Figure 4Biii). Cell counting is stopped in this protocol at 5,000 beads but another fixed number may be used depending on the supplier's recommendation. From the data acquired, the number of platelets counted per 5,000 beads is routinely at an average of 24.01 ± 92 (n = 15) (Figure 4C). Knowing the volume aspirated by the flow cytometer to count 5,000 beads (to be calculated for each cytometer) and the total volume of culture, it is possible to obtain an approximation of the total number of cultured platelets released.

Figure 4: Cultured platelets release at day 13. (A) Representative light microscopy image of MK emitting proplatelets at D13. (B) Strategy to quantify cultured platelet release. (i) Beads are gated on the CD41-Alexa-fluor 488/PErcP-Cy5 window (in red). (ii) Platelet-like elements are visualized in a gate determined on the FSC/SSC parameters of native platelets (grey dots). (iii) Cultured platelets are determined on their CD41/CD42 positivity (purple). (C) Number of platelets counted per 5,000 beads can be obtained, routinely an average of 24,011 ± 919 (n = 15). Please click here to view a larger version of this figure.
The procedure at a glance
To better summarize the method and understand each step, a poster summarizing the protocol step by step is presented in Figure 5. This summary sheet can be displayed in the culture room and serve as a memo. Of note, the success of the experiments is only guaranteed with the product references indicated in the table provided.
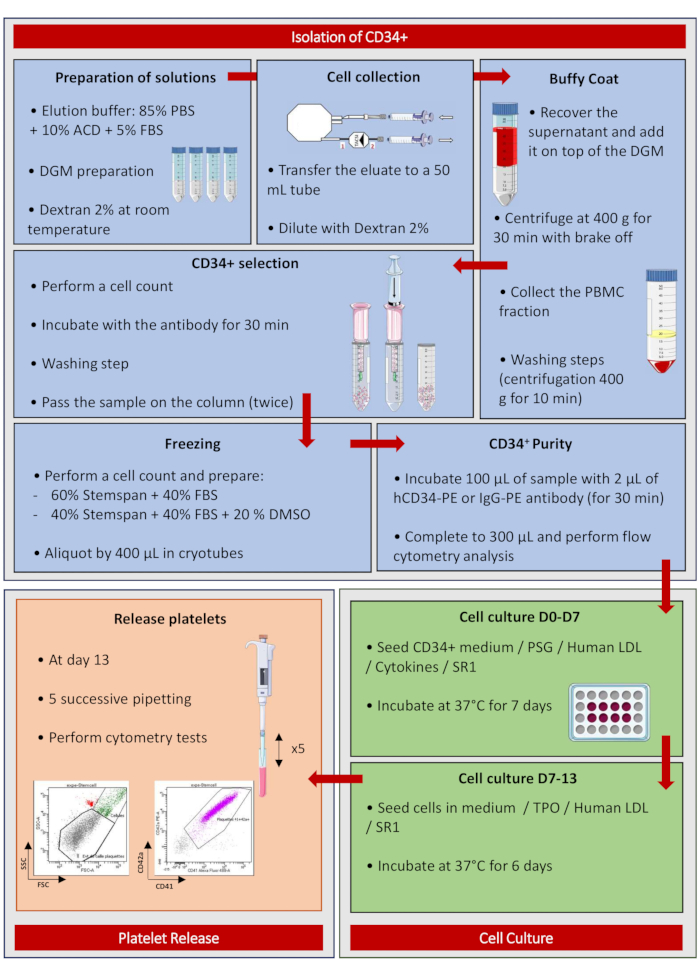
Figure 5: Isolation of CD34+. Poster summarizing the protocol step by step. Please click here to view a larger version of this figure.
Discussion
This protocol describes a method for producing MK capable of emitting proplatelets from blood-derived HP and to release platelets from the culture medium. HP are obtained from LRF, a by-product of the blood banks, used to remove contaminating leukocytes from cellular blood products and avoid adverse reactions. Although this method is relatively simple, a few points deserve special attention.
Deposition of the cell suspension on the density gradient medium (step 1.3.1) has to be performed gently to avoid mixture (red content). If this step is not carried out carefully, the protocol should stop at this point. Similarly, also in step 1.3.1, the brake has to be on the off mode to avoid mixing the fractions. If not, the HP selection must be suspended. As indicated in the protocol, section 1.4, the procedure can be interrupted following the PBMC collection. In this case, cells can be maintained under agitation overnight at 4 °C. Then, use a 40 µm cell strainer to remove the aggregates formed, which can impact on the subsequent CD34 selection. Of note, interrupting the procedure does not affect the yield and purity of CD34+ cells. At the end of the CD34 selection, the purity must be greater than 75% to seed the cells since, in a previous study, differentiation and maturation of MK were poor below this purity12 (Figure 2).
Special attention must be paid to the thawing of the CD34+ cells, which must be carried out quickly to avoid affecting the viability of the cells. In addition, washing steps must be carried out carefully to leave no trace of serum. The cell seeding density must be respected as it has been rigorously chosen for optimal CD34 commitment to the MKP pathway and MK maturation (Figure 3A).
A cell phenotyping protocol is proposed to follow the differentiation and maturation of MK. This protocol is relatively basic, but it is important to have all the control tubes, both unlabeled and single labeling tubes, available for each day of analysis to ensure reliable settings on the cytometer. To ensure that the culture runs smoothly, it is important to collect information of proliferation along the procedure. At D7, the average proliferation is between 2 to 4 fold13. This proliferation varies little between experiments, since each LRF comprises cells from 8 donors. To smooth out the variations further, it is possible to combine cells obtained in parallel from 4 to 8 LRFs.
It is possible to look at the morphology of the cells by light microscopy but the cells should not be observed every day because they are sensitive to temperature variations. When removing the cells from the incubator, ensure to make slow movements to avoid breaking the proplatelets.
Concerning the platelet release, five times successive pipetting are required. Doing less does not ensure optimal platelet release and doing more is detrimental to their functionality12. The most important aspect in this step is to use precise and regular movements, to generate a regular flow required for the platelet release14,15,16. The method of five successive pipetting is, therefore, simple and easy to perform with satisfactory performance results based on the yields described in the literature. The number of released platelets can be determined as mentioned in the section 3.3 by using the strategy of flow cytometry analysis shown in Figure 4B. The quality of the released platelets has been well documented in Do Sacramento et al. in terms of ultrastructure (morphology, size, granule content) and function (hemostasis), demonstrating that these cultured platelets are very similar to the native ones13.
The protocol described here is particularly suitable for small-volume cultures but is not applicable to large-scale culture. It is therefore an optimal method for the study of platelet biogenesis in order to better understand the molecular and cellular mechanisms governing platelet production by adding small molecules, agonists, or antagonists, for example. In addition, and to further explore the mechanisms that regulate MK commitment, MK maturation, and platelet production, it is now possible to genetically manipulate the CD34+ HP using a CRISPR-Cas9 genome editing method.
Disclosures
The authors have nothing to disclose.
Acknowledgements
This work has been supported by ANR (Agence National de la Recherche) Grant ANR- 17-CE14-0001-1.
Materials
| 7-AAD | Biolegend | 558819 | |
| ACD | EFS-Alsace | NA | |
| Anti-CD34-PE | Miltenyi biotec | 130-081-002 | |
| Anti-CD34-PECy7 | eBioscience | 25-0349-42 | |
| Anti-CD41-Alexa Fluor 488 | Biolegend | 303724 | |
| Anti-CD42a-PE | BD Bioscience | 559919 | |
| Apyrase | EFS-Alsace | NA | |
| BD Trucount Tubes | BD Bioscience | 340334 | |
| CD34 MicroBead Kit UltraPure, human | Miltenyi biotec | 130-100-453 | |
| Centrifuge | Heraeus | Megafuge 1.OR | Or equivalent material |
| Compteur ADAM | DiagitalBio | NA | Or equivalent material |
| Cryotubes | Dutscher | 55002 | Or equivalent material |
| Dextran from leuconostoc spp | Sigma | 31392-50g | Or equivalent material |
| DMSO Hybri-max | Sigma | D2650 | |
| EDTA 0.5 M | Gibco | 15575-039 | |
| Eppendorf 1,5 mL | Dutscher | 616201 | Or equivalent material |
| Filtration unit Steriflip PVDF | Merck Millipore Ltd | SE1M179M6 | |
| Flow Cytometer | BD Bioscience | Fortessa | |
| Human LDL | Stemcell technologies | #02698 | |
| ILOMEDINE 0,1 mg/1 mL | Bayer | MA038EX | |
| Inserts | Fenwal | R4R1401 | Or equivalent material |
| Laminar flow hood | Holten | NA | Archived product |
| LS Columms | Miltenyi Biotec | 130-042-401 | |
| Lymphoprep | Stemcell | 7861 | |
| Pen Strep Glutamine (100x) | Gibco | 10378-016 | |
| PBS (-) | Life Technologies | 14190-169 | Or equivalent material |
| PGi2 | Sigma | P6188 | |
| Poches de transferts 600ml | Macopharma | VSE4001XA | |
| Pre-Separation Filters (30µm) | Miltenyi Biotec | 130-041-407 | |
| StemRegenin 1 (SR1) | Stemcell technologies | #72344 | |
| StemSpan Expansion Supplement (100x) | Stemcell technologies | #02696 | |
| StemSpan-SFEM | Stemcell technologies | #09650 | |
| Stericup Durapore 0,22µm PVDF | Merck Millipore Ltd | SCGVU05RE | |
| SVF Hyclone | Thermos scientific | SH3007103 | |
| Syringues 30 mL | Terumo | SS*30ESE1 | Or equivalent material |
| Syringe filters Millex 0,22µM PVDF | Merck Millipore Ltd | SLGV033RB | |
| TPO | Stemcell technologies | #02822 | |
| Tubes 50 mL | Sarstedt | 62.548.004 PP | Or equivalent material |
| Tubes 15 mL | Sarstedt | 62.554.001 PP | Or equivalent material |
| Tubulures | B Braun | 4055137 | Or equivalent material |
References
- Deutsch, V. R., Tomer, A. Megakaryocyte development and platelet production. British Journal of Haematology. 134 (5), 453-466 (2006).
- Lefrancais, E., et al. The lung is a site of platelet biogenesis and a reservoir for haematopoietic progenitors. Nature. 544 (7648), 105-109 (2017).
- de Sauvage, F. J., et al. Stimulation of megakaryocytopoiesis and thrombopoiesis by the c-Mpl ligand. Nature. 369 (6481), 533-538 (1994).
- Almomani, M. H., Mangla, A. . StatPearls. , (2020).
- Strassel, C., Hechler, B., Bull, A., Gachet, C., Lanza, F. Studies of mice lacking the GPIb-V-IX complex question the role of this receptor in atherosclerosis. Journal of Thrombosis and Haemostasis. 7 (11), 1935-1938 (2009).
- Delalat, B., et al. Isolation and ex vivo expansion of human umbilical cord blood-derived CD34+ stem cells and their cotransplantation with or without mesenchymal stem cells. Hematology. 14 (3), 125-132 (2009).
- Yin, T., Li, L. The stem cell niches in bone. The Journal of Clinical Investigation. 116 (5), 1195-1201 (2006).
- Salunkhe, V., Papadopoulos, P., Gutiérrez, L. Culture of megakaryocytes from human peripheral blood mononuclear cells. Bio-protocol. 5 (21), 1639 (2015).
- Peytour, Y., Villacreces, A., Chevaleyre, J., Ivanovic, Z., Praloran, V. Discarded leukoreduction filters: a new source of stem cells for research, cell engineering and therapy. Stem Cell Research. 11 (2), 736-742 (2013).
- Lapostolle, V., et al. Repopulating hematopoietic stem cells from steady-state blood before and after ex vivo culture are enriched in the CD34(+)CD133(+)CXCR4(low) fraction. Haematologica. 103 (10), 1604-1615 (2018).
- Ivanovic, Z., et al. Whole-blood leuko-depletion filters as a source of CD 34+ progenitors potentially usable in cell therapy. Transfusion. 46 (1), 118-125 (2006).
- Strassel, C., et al. Aryl hydrocarbon receptor-dependent enrichment of a megakaryocytic precursor with a high potential to produce proplatelets. Blood. 127 (18), 2231-2240 (2016).
- Do Sacramento, V., et al. Functional properties of human platelets derived in vitro from CD34(+) cells. Scientific Reports. 10 (1), 914 (2020).
- Blin, A., et al. Microfluidic model of the platelet-generating organ: beyond bone marrow biomimetics. Scientific Reports. 6, 21700 (2016).
- Ito, Y., et al. Turbulence activates platelet biogenesis to enable clinical scale ex vivo production. Cell. 174 (3), 636-648 (2018).
- Pallotta, I., Lovett, M., Kaplan, D. L., Balduini, A. Three-dimensional system for the in vitro study of megakaryocytes and functional platelet production using silk-based vascular tubes. Tissue Engineering. Part C, Methods. 17 (12), 1223-1232 (2011).

