成纤维细胞衍生的人类工程结缔组织用于筛查应用
Summary
这里介绍的是一种方案,用于在具有双极的多孔板中为48个组织的平行培养生成工程结缔组织,适用于机制研究,疾病建模和筛选应用。该协议与来自不同器官和物种的成纤维细胞兼容,并且在这里以人类原发性心脏成纤维细胞为例。
Abstract
成纤维细胞是表型高度动态的细胞,它们响应生化和生物力学刺激迅速转入肌成纤维细胞。目前对纤维化过程(包括心脏纤维化)的理解仍然很差,这阻碍了新的抗纤维化疗法的发展。可控和可靠的人体模型系统对于更好地了解纤维化病理学至关重要。这是一种高度可重复和可扩展的方案,可在48孔铸造板中生成工程结缔组织(ECT),以促进在3D(3D)环境中研究成纤维细胞和纤维化组织的病理生理学。ECT在极点周围产生,刚度可调,允许在定义的生物力学载荷下进行研究。在定义的负载条件下,可以研究由细胞 – 基质相互作用控制的表型适应性。并行测试在48孔格式中是可行的,并有机会对多个参数进行时程分析,例如组织压实和针对负载的收缩。从这些参数中,可以研究组织刚度和弹性等生物力学特性。
Introduction
纤维化疾病研究的一个主要障碍是缺乏具有代表性的人类3D组织模型,这些模型可以深入了解成纤维细胞及其病理衍生物的行为。为了研究纤维化过程,标准2D培养系统不是最佳的,因为分离的成纤维细胞在不合规的2D底物上培养时迅速转入α平滑肌肌动蛋白(SMA)表达肌成纤维细胞1,2,3。因此,标准2D培养物中的成纤维细胞不反映规则的”健康”组织表型3,4,5,6。在柔韧的底物上引入培养物以模拟非纤维化(10 kPa)和纤维化(35 kPa)组织环境7,但这些缺乏第三维度,这在病理生理学方面非常重要。组织工程提供了克服这一限制的机会,允许在定义且实验上可调的细胞外基质(ECM)环境中进行成纤维细胞培养,例如,通过细胞性,ECM组成和ECM浓度的改变,所有这些都可以确定组织生物力学。
使用成纤维细胞生成了各种3D模型。漂浮的圆盘和微球是最早的,并证明胶原蛋白以时间依赖的方式重塑和压实。成纤维细胞对胶原原纤维施加牵引力,这一过程可以通过添加促纤维化剂(例如转化生长因子-β1(TGF-β1))来促进。8,9,10,11,12,13,14,15,16.然而,自由漂浮的培养物不允许受控的外部载荷,因此构成连续收缩或压实模型。片状工程组织开辟了研究组织生物力学性质的稳态调节的可能性,即通过单,双轴,多轴或循环应变测试17,18,19,20.例如,这些模型已被用于证明细胞数量对组织刚度的影响,发现其与细胞骨架完整性和肌球蛋白细胞骨架收缩力呈正相关。18,19.然而,重要的是要注意,力到应变的转换由于力传感器和锚点的夹紧点周围的组织变形不均匀而变得复杂。这种固有的限制可以通过狗骨或环形组织绕过,在锚点提供一些组织执法21,22,23.环形组织可以通过将细胞胶原水凝胶分配到环形模具中来制备。当水凝胶致密时,在模具的不可压缩内棒周围形成组织,这为进一步的组织收缩提供了阻力24,25,26,27.在初始和通常最大压实后,组织也可以转移到可调节的垫片上,以进一步限制在定义的组织长度处的圆形ECT3,24,25,26,27,28,29,30.生物物理特性可以在标准水平或垂直应变应力设备中使用适当的称重传感器在单向或动态应变下进行评估3.由于组织具有大致均匀的圆形结构,并且可以保持在杆/钩(锚固点和/或力传感器)上,尽管这些可能仍将压缩区域封闭在加载杆周围,但与夹紧相比,这种格式允许更均匀的应变变化3.此外,锚定的组织引发双极细胞形状,并且细胞通过沿着促进各向异性牵引的力线伸长来适应组织力。31,32,33,34,35,36.我们以前在功能应激应变实验中将来自大鼠和人心脏成纤维细胞(CF)的环形ECT应用于单个刚性极点,并使用病毒转导成纤维细胞进行功能的获得和丧失研究24,25,26 和药理学研究37.此外,我们可以在ECT模型中识别CF介导的纤维化的性别差异。27.
以下用于生成人ECT的协议,以从商业供应商处获得的作为冷冻保存CF的初级人CF为例(见 材料表),将环形组织的优势与为48孔平台生产宏观组织的简单快速方法相结合,该平台设计用于并行高内涵测试。
重要的是,ECT模型不限于特定的成纤维细胞类型,并记录了用于研究其他成纤维细胞,例如皮肤成纤维细胞38,39。此外,来自患者活检的成纤维细胞同样有效,成纤维细胞的选择最终取决于要解决的科学问题。
该方案中描述的用于生成ECT的平台是市售的48孔3D细胞/组织培养板(图1A)。描述了在48孔板的帮助下,在定义的几何形状和机械载荷下制备,培养和监测ECT形成和功能的方法。成型的ECT由集成的柔性杆固定,机械载荷可以根据最终用途通过使用具有不同硬度的极(邵氏A值36-89)进行微调,从而影响其弯曲刚度。建议使用岸边 A 值为 46 的杆子。此外,该协议还与前面描述的定制圆形模具兼容,其中ECT围绕单个刚性杆37保持。该模具的尺寸如图 1B所示。
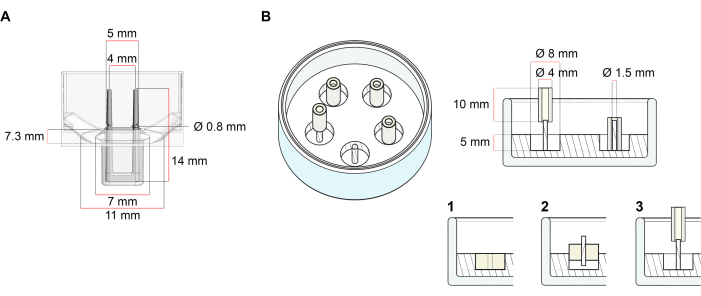
图1:铸造模具的示意图。 (A)具有两个柔性杆的铸造模具的技术图纸和尺寸。模具包括一个由短壁分隔的内周,该短壁在模具主体上固定双固定杆。柔性杆彼此之间具有自由的水平距离,并在底座处连接。该模具允许180 μL的浇注体积。每个模具的孔允许至少600μL培养基的体积容量。可以使用不同的材料成分来生产具有特定刚度的杆(例如,TM5MED-TM9MED)。(B)带有单个刚性杆的环形模具的技术图纸和尺寸。这是一种具有独特几何形状和机械环境的替代模具,可与ECT铸造协议一起使用37。环形模具装配方法改编自已发表的较大格式28,41。简而言之,该方法包括(1)将聚四氟乙烯(PTFE)模塑垫片(直径8毫米)倒入玻璃盘(直径60毫米)中的聚二甲基硅氧烷(PDMS,硅胶)中,以及(2)将PDMS杆架(直径1.5毫米)同心固定在形成的空腔内,其用于(3)保持可拆卸的极(直径4毫米硅胶管)。空心空间生成物允许180 μL的浇注体积。每个玻璃培养皿可以装入多个压印模具(例如,用5个模具显示),并且具有高达5 mL培养基的容量。 请点击此处查看此图的放大版本。
Protocol
Representative Results
Discussion
所提出的方案描述了从原发性人CF产生ECT,这允许研究这些细胞对其细胞外基质环境的机械影响,反之亦然。
成纤维细胞需要扩增以产生足够的细胞用于计划的ECT实验(0.75×106 细胞/ ECT)。为了获得最佳的可重复性,建议在2D单层培养物中预培养冷冻或组织衍生的成纤维细胞,标准化持续时间高达80%,每次传代内以及将其用于ECT生成之前(方案步骤3)。为了培养原代?…
Disclosures
The authors have nothing to disclose.
Acknowledgements
这项工作得到了德国心脏学会(GLS的DGK研究奖学金)和德国研究基金会(DFG通过GLS和AD的IRTG 1816项目;DFG 417880571和DFG TI 956/1-1用于MT;SFB 1002 TP C04 用于 MT 和 WHZ;SFB 1002 TP S01 用于 WHZ;和 EXC 2067/1-390729940J 用于 WHZ)。WHZ得到了德国联邦科学与教育部(BMBF通过IndiHEART项目)和Leducq基金会(20CVD04)的支持。MT,WHZ和SL由德国心血管研究中心(DZHK)提供支持。
Materials
| Cell culture reagents: | |||
| Accutase Solution | Merk Millipore | SCR005 | |
| Dissociation reagent – TrypLE Express | Gibco | 12604013 | |
| Dulbecco's Modified Eagle Medium (DMEM) powder, high glucose | Gibco | 12100061 | |
| Dulbecco’s phosphate buffered saline (DPBS), pH 7.2, -Ca2+, -Mg2+ | Gibco | 14190144 | |
| FGM-2 Fibroblast Growth Medium-2 BulletKit | Lonza | CC-3132 | |
| FBM Fibroblast Growth Basal Medium | Lonza | CC-3131 | |
| FGM-2 Fibroblast Growth Medium-2 SingleQuots, Supplements and Growth Factors | Lonza | CC-4126 | |
| Fibroblast Growth Medium 3 KIT | PromoCell | C-23130 | |
| Fibroblast Basal Medium 3 | PromoCell | C-23230 | |
| Growth Medium 3 SupplementPack | PromoCell | C-39350 | |
| Penicillin (10000 U/mL)/ Streptomycin (10000 μL/mL) | Gibco | 15140122 | |
| Sodium hydroxide solution (NaOH) 1.0 N | Sigma-Aldrich | S2770-100ML | |
| Cell sources: | |||
| Normal human cardiac fibroblasts from the ventricle (NHCF-V) | Lonza | CC-2904 | |
| Human Cardiac Fibroblasts (HCF-c) | PromoCell | C-12375 | |
| Human Cardiac Fibroblasts (HCF-p) | PromoCell | C-12377 | |
| Primary human foreskin fibroblasts-1 (HFF-1) | ATCC | SCRC- 1041 | |
| Collagen sourses: | |||
| Collagen Type I (bovine) in 0.01 M HCl | LLC Collagen Solutions | FS22024 | 6-7 mg/mL |
| Collagen Type I (rat tail) in 0.02 M HCl | Corning | 354236 | ~4 mg/mL |
| Drugs: | |||
| Latrunculin-A (Lat-A) | Enzo Life Sciences | BML-T119-0100 | |
| Plastic ware: | |||
| Cell culture plastic ware | Sarstedt and Starlab | ||
| Mesh cell strainer (Nylon, pore size 40 μm) | Falcon | 352340 | |
| myrPlate-uniform | myriamed GmbH | TM5 med | |
| Serological pipettes wide opening, sterile (10 mL) | Corning | 07-200-619 | |
| Specific instruments: | |||
| Bi-telecentric CORE lens for 1/2″ detectors | OptoEngineering | TCCR12096 | |
| Area scan camera Basler ace acA4024 | Basler | 107404 |
References
- Driesen, R. B., et al. Reversible and irreversible differentiation of cardiac fibroblasts. Cardiovascular Research. 101 (3), 411-422 (2014).
- Shi, X., et al. Elasticity of cardiac cells on the polymer substrates with different stiffness: an atomic force microscopy study. Physical Chemistry Chemical Physics. 13 (16), 7540-7545 (2011).
- Elson, E. L., Genin, G. M. Tissue constructs: platforms for basic research and drug discovery. Interface Focus. 6 (1), 20150095 (2016).
- Cho, N., Razipour, S. E., McCain, M. L. TGF-beta1 dominates extracellular matrix rigidity for inducing differentiation of human cardiac fibroblasts to myofibroblasts. Experimental Biology and Medicine. 243 (7), 601-612 (2018).
- Cucoranu, I., et al. NAD(P)H oxidase 4 mediates transforming growth factor-beta1-induced differentiation of cardiac fibroblasts into myofibroblasts. Circulation Research. 97 (9), 900-907 (2005).
- Peng, H., Carretero, O. A., Peterson, E. L., Rhaleb, N. E. Ac-SDKP inhibits transforming growth factor-beta1-induced differentiation of human cardiac fibroblasts into myofibroblasts. American Journal of Physiology-Heart and Circulatory Physiology. 298 (5), 1357-1364 (2010).
- Ribeiro, A. J., et al. Contractility of single cardiomyocytes differentiated from pluripotent stem cells depends on physiological shape and substrate stiffness. Proceedings of the National Academy of Sciences of the United States of America. 112 (41), 12705-12710 (2015).
- Tranquillo, R. T., Durrani, M. A., Moon, A. G. Tissue engineering science: consequences of cell traction force. Cytotechnology. 10 (3), 225-250 (1992).
- Barocas, V. H., Moon, A. G., Tranquillo, R. T. The fibroblast-populated collagen microsphere assay of cell traction force–Part 2: Measurement of the cell traction parameter. Journal of Biomechanical Engineering. 117 (2), 161-170 (1995).
- Lijnen, P., Petrov, V., Rumilla, K., Fagard, R. Stimulation of collagen gel contraction by angiotensin II and III in cardiac fibroblasts. Journal of the Renin-Angiotensin-Aldosterone System. 3 (3), 160-166 (2002).
- Baxter, S. C., Morales, M. O., Goldsmith, E. C. Adaptive changes in cardiac fibroblast morphology and collagen organization as a result of mechanical environment. Cell Biochemistry and Biophysics. 51 (1), 33-44 (2008).
- Zhou, Y., et al. Inhibition of mechanosensitive signaling in myofibroblasts ameliorates experimental pulmonary fibrosis. Journal of Clinical Investigation. 123 (3), 1096-1108 (2013).
- Lijnen, P., Petrov, V., Fagard, R. In vitro assay of collagen gel contraction by cardiac fibroblasts in serum-free conditions. Methods and Findings in Experimental and Clinical Pharmacology. 23 (7), 377-382 (2001).
- Burgess, M. L., et al. Integrin-mediated collagen gel contraction by cardiac fibroblasts. Effects of angiotensin II. Circulation Research. 74 (2), 291-298 (1994).
- Nunohiro, T., Ashizawa, N., Graf, K., Hsueh, W. A., Yano, K. Angiotensin II promotes integrin-mediated collagen gel contraction by adult rat cardiac fibroblasts. Japanese Heart Journal. 40 (4), 461-469 (1999).
- Ngu, J. M., et al. Human cardiac fibroblast extracellular matrix remodeling: Dual effects of tissue inhibitor of metalloproteinase-2. Cardiovascular Pathology. 23 (6), 335-343 (2014).
- Knezevic, V., Sim, A. J., Borg, T. K., Holmes, J. W. Isotonic biaxial loading of fibroblast-populated collagen gels: a versatile, low-cost system for the study of mechanobiology. Biomechanics and Modeling in Mechanobiology. 1 (1), 59-67 (2002).
- Delvoye, P., Wiliquet, P., Leveque, J. L., Nusgens, B. V., Lapiere, C. M. Measurement of mechanical forces generated by skin fibroblasts embedded in a three-dimensional collagen gel. Journal of Investigative Dermatology. 97 (5), 898-902 (1991).
- Kolodney, M. S., Elson, E. L. Correlation of myosin light chain phosphorylation with isometric contraction of fibroblasts. Journal of Biological Chemistry. 268 (32), 23850-23855 (1993).
- Bell, B. J., Nauman, E., Voytik-Harbin, S. L. Multiscale strain analysis of tissue equivalents using a custom-designed biaxial testing device. Biophysical Journal. 102 (6), 1303-1312 (2012).
- Wakatsuki, T., Kolodney, M. S., Zahalak, G. I., Elson, E. L. Cell mechanics studied by a reconstituted model tissue. Biophysical Journal. 79 (5), 2353-2368 (2000).
- Thomopoulos, S., et al. Fibrocartilage tissue engineering: The role of the stress environment on cell morphology and matrix expression. Tissue Engineering Part A. 17 (7-8), 1039-1053 (2011).
- Roeder, B. A., Kokini, K., Sturgis, J. E., Robinson, J. P., Voytik-Harbin, S. L. Tensile mechanical properties of three-dimensional type I collagen extracellular matrices with varied microstructure. Journal of Biomechanical Engineering. 124 (2), 214-222 (2002).
- Ongherth, A., et al. p63RhoGEF regulates auto- and paracrine signaling in cardiac fibroblasts. Journal of Molecular and Cellular Cardiology. 88, 39-54 (2015).
- Vettel, C., et al. PDE2-mediated cAMP hydrolysis accelerates cardiac fibroblast to myofibroblast conversion and is antagonized by exogenous activation of cGMP signaling pathways. American Journal of Physiology-Heart and Circulatory Physiology. 306 (8), 1246-1252 (2014).
- Jatho, A., et al. RhoA Ambivalently Controls Prominent Myofibroblast Characteritics by Involving Distinct Signaling Routes. PLoS One. 10 (10), 0137519 (2015).
- Dworatzek, E., et al. Sex-specific regulation of collagen I and III expression by 17beta-Estradiol in cardiac fibroblasts: role of estrogen receptors. Cardiovascular Research. 115 (2), 315-327 (2019).
- Tiburcy, M., Meyer, T., Soong, P. L., Zimmermann, W. H. Collagen-based engineered heart muscle. Methods in Molecular Biology. 1181, 167-176 (2014).
- Schlick, S. F., et al. Agonistic and antagonistic roles of fibroblasts and cardiomyocytes on viscoelastic stiffening of engineered human myocardium. Progress in Biophysics and Molecular Biology. 144, 51-60 (2019).
- Wille, J. J., Elson, E. L., Okamoto, R. J. Cellular and matrix mechanics of bioartificial tissues during continuous cyclic stretch. Annals of Biomedical Engineering. 34 (11), 1678-1690 (2006).
- Berry, C. C., Shelton, J. C., Bader, D. L., Lee, D. A. Influence of external uniaxial cyclic strain on oriented fibroblast-seeded collagen gels. Tissue Engineering. 9 (4), 613-624 (2003).
- Stopak, D., Harris, A. K. Connective tissue morphogenesis by fibroblast traction. I. Tissue culture observations. 발생학. 90 (2), 383-398 (1982).
- Bellows, C. G., Melcher, A. H., Aubin, J. E. Association between tension and orientation of periodontal ligament fibroblasts and exogenous collagen fibres in collagen gels in vitro. Journal of Cell Science. 58 (1), 125-138 (1982).
- Tranquillo, R. T. Self-organization of tissue-equivalents: the nature and role of contact guidance. Biochemical Society Symposia. 65, 27-42 (1999).
- Barocas, V. H., Tranquillo, R. T. An anisotropic biphasic theory of tissue-equivalent mechanics: the interplay among cell traction, fibrillar network deformation, fibril alignment, and cell contact guidance. Journal of Biomechanical Engineering. 119 (2), 137-145 (1997).
- Yip, A. K., et al. Anisotropic traction stresses and focal adhesion polarization mediates topography-induced cell elongation. Biomaterials. 181, 103-112 (2018).
- Santos, G. L., Hartmann, S., Zimmermann, W. H., Ridley, A., Lutz, S. Inhibition of Rho-associated kinases suppresses cardiac myofibroblast function in engineered connective and heart muscle tissues. Journal of Molecular and Cellular Cardiology. 134, 13-28 (2019).
- Kittana, N., et al. Modulating the biomechanical properties of engineered connective tissues by chitosan-coated multiwall carbon nanotubes. International Journal of Nanomedicine. 16, 989-1000 (2021).
- Kittana, N., et al. Enhancement of wound healing by single-wall/multi-wall carbon nanotubes complexed with chitosan. International Journal of Nanomedicine. 13, 7195-7206 (2018).
- Antoine, E. E., Vlachos, P. P., Rylander, M. N. Review of collagen I hydrogels for bioengineered tissue microenvironments: characterization of mechanics, structure, and transport. Tissue Engineering Part B: Reviews. 20 (6), 683-696 (2014).
- Holder, A. J., et al. Control of collagen gel mechanical properties through manipulation of gelation conditions near the sol-gel transition. Soft Matter. 14 (4), 574-580 (2018).
- Zimmermann, W. H., et al. Tissue engineering of a differentiated cardiac muscle construct. Circulation Research. 90 (2), 223-230 (2002).

