Sample Preparation for Computed Tomography-based Three-dimensional Visualization of Murine Hind-limb Vessels
Summary
Here, we describe a visualization and quantification method for murine hind-limb vessels using micro-X-ray computed tomography.
Abstract
Blood vessels are complex networks with tree-like structures, and vascular networks are essential for maintaining both circulation and maintaining organ function. Clarifying the mechanism of blood vessel formation is therefore extremely useful for elucidating developmental processes and pathological mechanisms. Murine hind-limb vessels are often used as a model for physiological and pathological angiogenesis. Evaluation is mainly performed via a two-dimensional method using tissue sections. However, methods for evaluating three-dimensional (3D) vascular morphology are particularly limited. This paper introduces a method for visualizing murine hind-limbs using computed tomography (CT). Radiation-opaque resin is injected through the descending aorta, and whole vessels are filled with dye. By adjusting the time of dye injection, arterial-specific filling is also possible, and samples can be obtained with any micro-X-ray CT device. This contrast method provides a basic technique for the 3D evaluation of murine blood vessels in the lower extremities. Furthermore, this method can be used to visualize all blood vessels below the diaphragm and evaluate blood vessels in the abdominal organs.
Introduction
Blood vessels are complex networks with tree-like structures. Angiogenesis and new vascular formation play essential roles in the maintenance of organ homeostasis1. Angiogenesis is regulated for the treatment of ischemic and malignant diseases2. It is therefore essential to understand the underlying mechanisms of angiogenesis. Murine hind-limb vessels are often used as a useful model for vascular research3; ipsilateral ligation of the iliac or femoral artery is a known hind-limb ischemia model used to assess angiogenesis and vascular remodeling in physiological and pathological angiogenesis4. However, the evaluation of angiogenesis is mainly performed by section staining, and methods for evaluating 3D vascular morphology are particularly limited.
Compared with section staining, CT enables 3D visualization. Recently, Weyers et al. reported a sophisticated protocol suitable for CT imaging, enabling the visualization of the murine adult coronary circulatory system5. We modified their method to create a sample preparation method suitable for CT imaging of the lower extremity blood vessels6. Here, a radiation-opaque resin is injected through the descending aorta, and vessels in the lower extremities are filled with dye. By adjusting the time of dye injection, arterial-specific filling is also possible, and samples can be obtained with any micro-X-ray computed tomography device. This contrast method provides a basic technique for the 3D evaluation of murine blood vessels below the diaphragm and in abdominal organs and the lower extremities.
Protocol
All procedures were performed in accordance with the Kumamoto University animal care guidelines (approval reference no. M30-040/A2020-105), which conform to the US National Institutes of Health Guide for the Care and Use of Laboratory Animals (publication no. 85-23, revised 2011).
1. Preparation
- Prepare the perfusion apparatus and vasodilator buffer (4 mg/L papaverine hydrochloride, 4 g/L; adenosine, 1 g/L; heparin, 1 U/mL in phosphate-buffered saline (PBS)).
NOTE: The reflux device and vasodilator reagents are the same as those reported by Weyers et al.5. - Connect the 22 G catheter, 2 mL extension tube, and three-way stopcock (Figure 1A).
NOTE: Adjust the gauge according to the size of the animal. For adult C57BL/6 mice, 22 G is optimal. - Fill the pressure perfusion apparatus with the vasodilator buffer (Figure 1A).
NOTE: Avoid the formation of bubbles to prevent disruption in the filling of the contrast medium.
2. Perfusion
- Inject 1 U/g heparin in PBS into the intraperitoneal cavity 30 min before the operation.
- Completely anesthetize the mouse with isoflurane and euthanize it by cervical dislocation.
- After decapitation, make a midline incision in the sternum and fix the open thorax with pins.
NOTE: To avoid leakage of the contrast, avoid injuring the diaphragm. - Cut the ascending aorta and remove the heart.
- Remove the lung and expose the descending aorta.
NOTE: Do not injure the descending aorta. - Cut the descending aorta diagonally to expose the cross-section (Figure 1B).
NOTE: Do not peel off the aorta; a diagonal section is better for catheter insertion. - Insert the 22 G catheter into the descending aorta while running the vasodilation buffer.
NOTE: Insertion of the catheter while running the vasodilation buffer avoids air contamination. - Pin the root of the catheter (Figure 1C).
- Make a knot to prevent leakage due to backflow.
- Perfuse a warmed vasodilating solution (papaverine hydrochloride, 4 g/L; adenosine, 1 g/L; heparin, 1 U/mL) for 3 min at a fixed pressure between 13 and 15 kPa.
- Perfuse a solution of 4% paraformaldehyde in (PBS) for 3 min.
NOTE: The success of fixation can be confirmed by the movement of the foot (Figure 1D). - Prepare the contrast medium just before perfusion.
NOTE: Adjust the dilution rate according to the sample; for adult mice, mix the stain and diluent in a ratio of 1:1. - Stop the perfusion and fill the extension tube with 2 mL of diluted contrast medium (Figure 1E).
NOTE: The contrast medium should be injected slowly to avoid injury to the blood vessels. - Perfuse the contrast medium at a fixed pressure between 13 and 15 kPa.
- To visualize arteries, check the toenail to confirm that the contrast medium has reached the artery (Figure 1F).
- To visualize all vessels, check the inferior vena cava of the diaphragm to confirm complete circulation of the contrast medium.
NOTE: In the beginning, the contrast contains the vasodilating solution; hence, its proper circulation is essential.
- Close the three-way stopcock and remove the tube (Figure 1G).
NOTE: If the three-way stopcock is not closed, the contrast will flow backward. - Incubate the sample overnight at 4 °C.
- Remove the skin and fix it in 10% formaldehyde solution.
3. Visualization
NOTE: Visualization protocols differ depending on the CT scanner. A microfocus X-ray CT scanner was used in this protocol. It is necessary to optimize the imaging method according to each CT scanner.
- Fix the sample in a 50 mL tube containing PBS.
- Place the sample tube on the table.
- Scan the sample with a voltage of 50 kV and a current of 600 µA, ensuring a focus-to-center distance of 75.2 mm.
NOTE: A dimension of 1 voxel was 28.7 µm x 28.7 µm x 28.7 µm in this setting. - Load the acquired image data with Fiji, an open-source platform for biological-image analysis.
- Determine the muscle voxel value using the gastrocnemius muscle.
- Choose the gastrocnemius muscle by using the rectangle tool.
- Check the mean and standard deviation (SD) from the histogram (Analyze | Histogram).
- Define the muscle voxel density as mean + 2SD of the gastrocnemius muscle.
- Set the muscle voxel density as the lower threshold level (Image | adjust | Threshold | Set | lower threshold level).
NOTE: The vascular area and bone area remain in the binarized data after the threshold is set.
Representative Results
All vessels in the lower extremities can be visualized if this protocol is correctly performed (Figure 2A). In a hind-limb ischemia model, the non-ligated femoral artery runs parallel to the femoral vein (Figure 2B), and a ligated femoral artery can be confirmed by the interruption of contrast media (Figure 2C). The results revealed the development of collateral vessels (Figure 2D). Collateral circulation is formed between the arteries proximal to the ligated artery and the artery in the lower leg region and on the ventral and dorsal sides of the femoral artery. The inferior gluteal artery-beginning on the dorsal side of the pelvis and running on the lateral side of the thigh-expands robustly on the ischemic side.
Contrast-filled vessels are filled with the contrast medium (Figure 2E); disruption in the contrast indicates mixing of noncontrast media (e.g., blood, vasodilator buffer, or bubbles) or insufficient perfusion of the contrast (Figure 2F). Vasodilatation and fixation would not work well if the blood vessels have shrunk. Although CT imaging can only visualize the contrast medium, it is possible to view the arteries on the surface of the body by macroscopic or stereomicroscopic observation (Figure 2G). Thus, it is easier to assess defects using the contrast medium (Figure 2H).
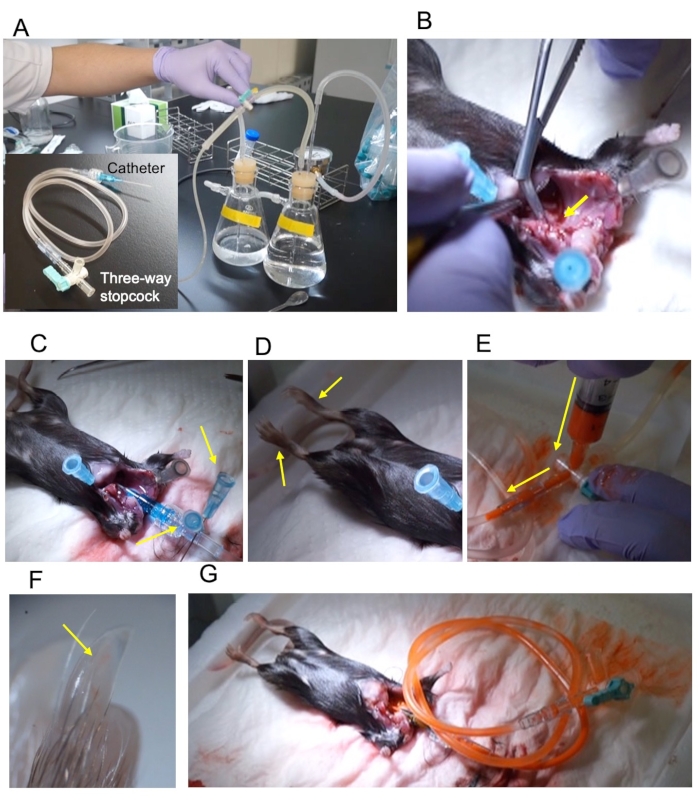
Figure 1: Outline of the procedure. (A) Pressure perfusion apparatus and the 22 G catheter connected via a 2 mL extension tube and three-way stopcock. (B) The ascending aorta is cut diagonally to expose the cross-section (yellow arrow). (C) The catheter was fixed using two pins (yellow arrows). (D) The fixed lower limbs extend upon fixation (yellow arrows). (E) Injection of contrast through the three-way stopcock. The injection direction is indicated by a yellow arrow. (F) The hind-limb nail is filled with the contrast (yellow arrow). (G) The stopcock is closed and removed from the perfusion apparatus. Please click here to view a larger version of this figure.
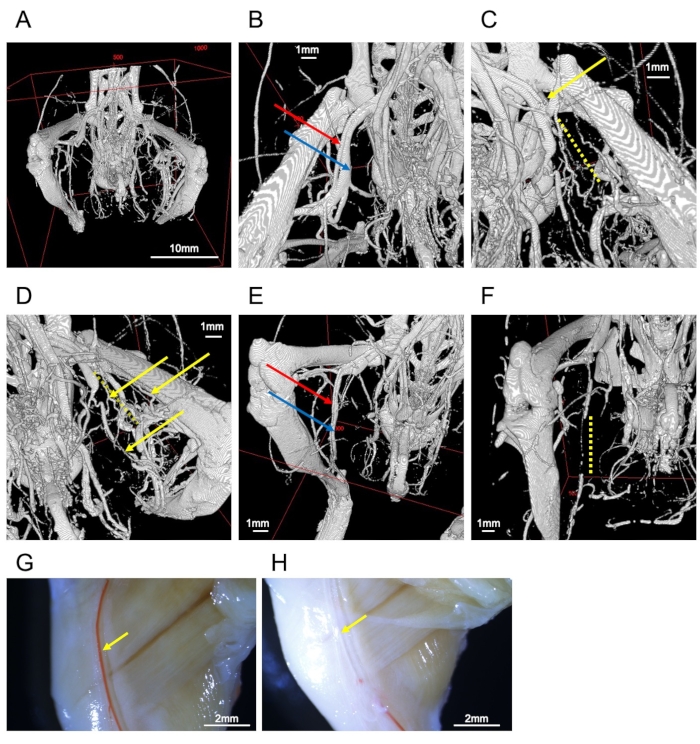
Figure 2: Images of vessels. (A) Whole image of the hind-limb bones and vessels. (B) Femoral artery (red arrow) and vein (blue arrow). (C) Ligated femoral artery (yellow arrow). The periphery is interrupted by the obstruction (yellow dotted line). (D) Collateral vessels in the ligated side (yellow arrows). The yellow dotted line represents the interrupted femoral artery. (E) A well-filled sample of the saphenous artery (red arrow) and vein (blue arrow). (F) Inadequate perfusion leads to interruption of saphenous vessels (yellow dotted line). (G) Stereomicroscopic observation of a representative sample. The right femoral artery (yellow arrow) is filled with contrast medium. (H) Stereomicroscope observation of a failed sample. The right femoral artery (yellow arrow) lacks contrast medium. Scale bars = 1 mm (B–F), 2 mm (G, H), 10 mm (A). Please click here to view a larger version of this figure.
Discussion
This report introduces a sophisticated method to visualize blood vessels in the lower body. There are several critical steps in this process: the first is preperfusion before the injection of contrast medium. If enough blood is not removed, the contrast will not fill the system. Additionally, the inclusion of air bubbles disturbs the filling of the contrast; thus, the air in the circuit must be completely removed. Moreover, because the contrast medium does not solidify immediately after injection, the sample should not be excessively moved.
This method is useful to evaluate the increased formation of blood vessels and circulation, such as collateral circulation. Conversely, as a limitation, it is difficult to evaluate narrowed blood vessels, as distinguishing between stenosis and an artificial reduction in contrast medium is difficult. Additionally, it is challenging to evaluate blood vessels in bones, as the separation of blood and bone is difficult.
An alternative method for 3D visualization is immunostaining. Using the tissue clearing technique, several methods are available for 3D imaging7. Immunostaining is advantageous as it allows the staining of specific proteins using antibodies. A recent report challenges whole-body imaging based on immunostaining8; however, CT-based imaging does not require any tissue-clearing pretreatment.
This method enables the visualization of all vessels below the diaphragm, including the abdominal organs. Angiogenesis in the abdominal organs has a strong impact on maintaining homeostasis and developing diseases9,10. As this protocol was optimized for the evaluation of the lower extremity vessels, organ-specific priming would enable the visualization of angiogenesis associated with any factor, such as inflammation or tumors.
Disclosures
The authors have nothing to disclose.
Acknowledgements
We thank Yasuyo Kimura, Megumi Nagahiro, and Saeko Tokunaga for the excellent technical support in animal experiments.
Materials
| 1 mL syringe | TERUMO | SS-01T | |
| 10% Formalin Solution | Fujifilm-Wako | 068-03841 | |
| 10x phosphate-buffered saline (-) (PBS) | Fujifilm-Wako | 163-25265 | Prepare 1x PBS |
| 22 G catheter (22 G S5 x 1" V(F)) | MEDIKIT | HP2140 | Only catheter is used. |
| 23 G needle | TERUMO | NN-2325R | Use as a pin |
| 4% paraformaldehyde in PBS | Fujifilm-Wako | 163-20145 | |
| 5 mL syringe | |||
| 5-0 Suture with needle | Alfresa Pharma Corporation | ER1205SB45 | |
| Adenosine | Sigma-aldrich | A9251-5G | For vasodilating solution |
| Dumont #55 Forceps | FST | No.11255-20 | |
| Extension tube | TOP | X2-FL50 | |
| Falcon 50 mL tube | CORNING | 352098 | |
| Graefe Forceps | FST | No.11051-10 | |
| Heparin Sodium 5,000 units/5 mL | Mochida Co. Ltd. | 224122458 | |
| Isoflurane | Fujifilm-Wako | 099-06571 | |
| Microfil Injection Compounds | Flow Tech Inc. | MV-117 | Mix liquid MV-Compound (stain) and MV-Diluent 1: 1 |
| Papaverine hydrochloride | Fujifilm | 164-18002 | For vasodilating solution |
| Small Animal Anesthetizer | Muromachi Kikai Co. Ltd. | MK-A100ecoW-ST | |
| Spring Scissors – Angled to Side | FST | No.15006-09 | |
| Surgical Scissors – Sharp-Blunt | FST | No.14001-12 | |
| three-way cock | TERUMO | TS-TR1K | |
| Transfer pipette | SAMCO SCIENTIFIC | SM262-1S | Use for mixing contrast medium |
| X-ray CT scanner | Toshiba IT & Control Systems Corporation | TOSHIBA TOSCANNER 32300 FPD |
References
- Folkman, J. Angiogenesis. Annual Review of Medicine. 57, 1-18 (2006).
- Folkman, J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nature Medicine. 1 (1), 27-31 (1995).
- Kochi, T., et al. Characterization of the arterial anatomy of the murine hindlimb: functional role in the design and understanding of ischemia models. PLoS One. 8 (12), 84047 (2013).
- Limbourg, A., et al. Evaluation of postnatal arteriogenesis and angiogenesis in a mouse model of hind-limb ischemia. Nature Protocols. 4 (12), 1737-1746 (2009).
- Weyers, J. J., Carlson, D. D., Murry, C. E., Schwartz, S. M., Mahoney, W. M. Retrograde perfusion and filling of mouse coronary vasculature as preparation for micro computed tomography imaging. Journal of Visualized Experiments: JoVE. (60), e3740 (2012).
- Arima, Y., et al. Evaluation of collateral source characteristics with 3-dimensional analysis using micro-X-ray computed tomography. Journal of the American Heart Association. 7 (6), 007800 (2018).
- Tian, T., Yang, Z., Li, X. Tissue clearing technique: Recent progress and biomedical applications. Journal of Anatomy. 238 (2), 489-507 (2021).
- Susaki, E. A., et al. Advanced CUBIC protocols for whole-brain and whole-body clearing and imaging. Nature Protocols. 10 (11), 1709-1727 (2015).
- Fernandez, M., et al. Angiogenesis in liver disease. Journal of Hepatology. 50 (3), 604-620 (2009).
- Li, S., et al. Angiogenesis in pancreatic cancer: current research status and clinical implications. Angiogenesis. 22 (1), 15-36 (2019).

