骨骼肌中脂质液滴含量的纤维类型和亚细胞特异性分析
Summary
越来越多的证据表明,脂质在骨骼肌内的过度浸润会导致脂毒性和糖尿病。在这里,我们提出了一个完整的方案,包括组织处理,使用Bodypy染色,图像采集和分析,以纤维型特定方式量化脂质液滴的大小,密度和亚细胞分布。
Abstract
骨骼肌脂质浸润,称为肌质变性,随着肥胖和衰老而增加。肌层沉积症最近也被发现是心血管疾病和癌症等其他几种疾病的阴性预后因素。过量的脂质浸润会降低肌肉质量和力量。它还会导致脂毒性和胰岛素抵抗,这取决于总肌细胞内脂质含量,脂质液滴(LD)形态和亚细胞分布。纤维类型(氧化性与糖酵解)也很重要,因为氧化纤维具有更大的利用脂质的能力。由于它们在病理生理学中的重要意义,有必要以纤维类型特异性方式对LD动力学和功能进行深入研究。
本文提出了一个完整的方案,用于以纤维类型特异性方式定量肌细胞内脂质含量并分析LD形态和亚细胞分布。为此,用荧光染料Bodypy和针对肌球蛋白重链亚型的抗体染色连续肌肉冷冻切片。该协议可以同时处理不同的肌肉,节省时间并避免可能的伪影,并且由于在斐济创建的个性化宏,LD分析的自动化也是可能的。
Introduction
骨骼肌脂质浸润,称为肌质变性,随着肥胖和衰老而增加。肌层沉积与肌肉质量和力量以及胰岛素敏感性1呈负相关。此外,最近的研究表明,肌层沉积的程度可用作其他疾病的预后因素,如心血管疾病2,非酒精性脂肪性肝病3或癌症4。脂质可以作为肌细胞外脂质积聚在肌肉纤维之间的骨骼肌中,也可以作为肌细胞内脂质(IMCLs)积聚在纤维内。IMCL主要作为甘油三酯储存在脂质飞沫(LD)中,在体育锻炼期间用作代谢燃料5,6。然而,当脂质供应超过需求时,或当线粒体变得功能失调时,IMCLs将与肌肉胰岛素抵抗有关,如代谢不健康的个体,肥胖的个体和2型糖尿病患者7所示。有趣的是,耐力运动员的IMCL水平与肥胖的2型糖尿病患者相似,如果不是更高,同时保持高胰岛素敏感性。这种现象被描述为“运动员悖论”8,9,并通过对肌肉LD的更细致入微的评估来解释,这些评估与它们的大小,密度,定位,动力学和脂质物种组成有关。
首先,LD大小与胰岛素敏感性和体能10,11成反比。事实上,较小的LD表现出相对较大的脂肪酶作用表面积,因此可能具有更大的动员脂质12的能力。其次,LD密度(数/表面)在胰岛素作用8,10中起着有争议的作用;然而,它似乎在运动员中有所增加。第三,LD的亚细胞定位很重要,因为位于表面膜(肌层下或外周)正下方的LD对胰岛素敏感性的危害性比中心膜8,9,13更有害。后者为中枢线粒体提供燃料,中枢线粒体具有更大的呼吸活动,并且更专门地满足收缩14所需的高能量需求。相比之下,外周LD提供肌肉下线粒体,其参与膜相关过程8。最后,除了甘油三酯之外,肌肉内的特定复杂脂质可能比其他脂质更有害。例如,当甘油三酯周转率低时,二酰基甘油,长链酰基辅酶A和神经酰胺可能积聚在肌肉中,从而损害胰岛素信号传导9,15。回到“运动员悖论”,耐力运动员有大量较小的中央LD,I型(氧化性)纤维的周转率较高,而肥胖和糖尿病患者具有较大的外周LD,II型(糖酵解)纤维的周转率低8,15,16。除了在能量储存和释放中的作用外, 通过 衍生脂肪酸(FA)和涂层蛋白(周蛋白5)的LD还可以作为参与FA氧化和线粒体生物发生的转录调节的关键参与者8。由于它们在生理学和病理生理学中的关键意义,因此有必要对LD的动力学和功能进行深入研究。
虽然有几种技术可以研究IMCL,但它们并不适合以纤维特异性方式准确量化LD尺寸,密度和分布。例如,通过磁共振波谱对IMCL的评估虽然是非侵入性的,但提供的分辨率水平不足以研究光纤中LD的大小和精确位置,并且它不是光纤类型特异性的17,18。同样,在全肌肉匀浆19上进行的生化技术不能评估脂质的位置和大小。因此,分析LD形态和位置的最适当方法是定量透射电子显微镜13,但这种技术昂贵且耗时。因此,与油红O(ORO)20,21,单单苯基戊烷(MDH)22或Bodypy 23,24,25等染料制剂的共聚焦荧光成像已成为这些研究的最佳工具。
这里描述了一个完整的方案,包括组织采样和处理,Bodipy染色以及共聚焦图像采集和分析,以量化小鼠肌肉冷冻切片中的LD大小,数量和定位。由于IMCL在氧化纤维和糖酵解纤维之间分布不均匀,并且每种纤维类型调节LD动力学不同,因此IMCL的研究必须是纤维型特异性的16,25,26,27。因此,该方案在连续切片上使用免疫荧光来鉴定每根纤维表达的肌球蛋白重链(MyHC)亚型。该协议的另一个优点是在冷冻前同时处理糖酵解(长指伸肌,EDL)和并排放置的氧化(比目鱼)肌肉(图1)。这种同步处理不仅节省了时间,而且避免了由于样品的单独处理而导致的可变性。
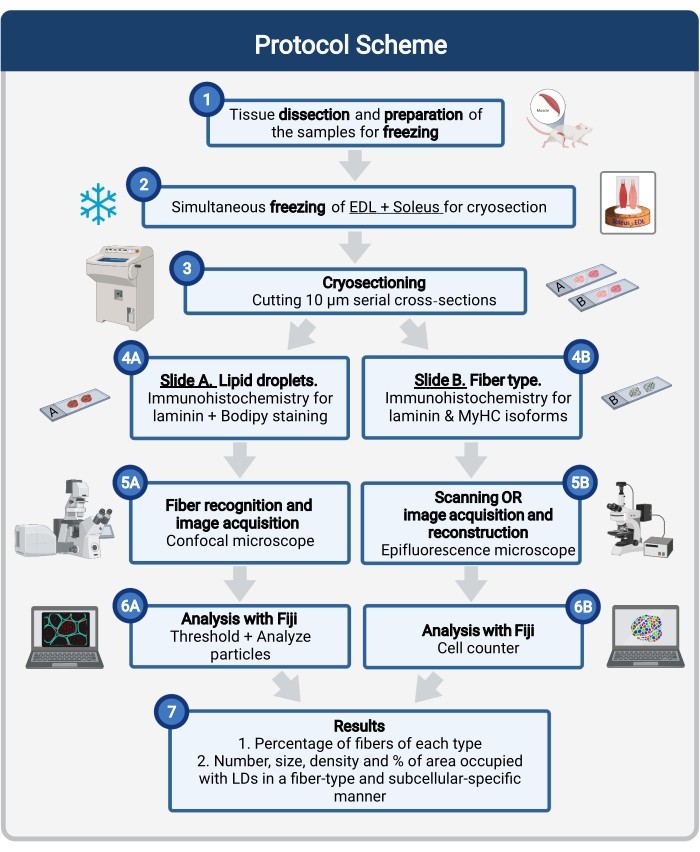
图 1:该过程的示意图。 肌肉解剖(1)后,准备相似大小的选定肌肉并一起冷冻(2)。使用低温恒温器获得10μm的连续横截面,并直接安装在粘附载玻片上(3)。从两张连续载玻片中,第一张(4A)被免疫标记为层粘连蛋白,并用Bodyy染色以识别LD,第二张(4B)用针对MyHC的抗体进行免疫染色,以识别肌肉纤维类型。使用共聚焦显微镜(5A)和肌肉纤维类型(5B)的落射荧光显微镜获取图像。在斐济,通过应用阈值并量化颗粒(6A)来分析图像,以获得LD(7)或计数细胞(6B)所占据的总面积的数量,平均尺寸,密度和百分比,以获得该部分(7)中每种类型纤维的百分比。缩写:LD =脂质液滴;EDL = 长指伸肌;肌HCs=肌球蛋白重链亚型。 请点击此处查看此图的大图。
Protocol
Representative Results
Discussion
这里详细介绍的方案描述了一种在纤维类型和亚细胞特异性基础上量化用Body标记的LD的有效方法。近年来,经典的脂质染料,如ORO或苏丹黑B,已被一系列新的细胞渗透性亲脂性荧光染料取代,这些染料与中性脂质(例如Bodypy)结合。作为不同的偶联物,Bodipy已被证明在标记LD以研究其形态,动力学和与其他细胞器的相互作用方面非常有效,不仅在不同的固定组织和细胞23,</s…
Disclosures
The authors have nothing to disclose.
Acknowledgements
这项工作得到了 国家科学研究基金会 (FNRS-Crédit de Recherche J.0022.20)和 法语国家糖尿病护理协会 (SFD-Roche糖尿病护理)的资助。M.A.D.-L.D.C.获得了瓦隆-布鲁塞尔国际卓越计划的研究金。
作者感谢爱丽丝·莫尼尔对该协议开发的贡献,并感谢卡罗琳·布津在图像获取过程中的专业知识和技术帮助。我们还感谢2IP-IREC成像平台访问低温恒温器和显微镜(2IP-IREC成像平台,鲁汶天主教大学实验和临床研究所,比利时布鲁塞尔1200)。最后,作者要感谢尼古拉斯·杜比松、罗曼·维塞尔和米歇尔·阿布-萨姆拉对手稿的建设性批评。这些文章的一些数字是用 BioRender.com 创建的。
Materials
| Equipment | |||
| AxioCam 506 mono 6 Mpix camera | Zeiss | ||
| AxioCam MRm 1.4MPix CCD camera | Zeiss | ||
| Chemical hood | Potteau Labo | EN-14175 | |
| Confocal microscope | Zeiss | LSM800 | |
| Cork discs (ø 20 mm, 3 mm thick) | Electron Microscopy Sciences | 63305 | |
| Cryo-Gloves | Tempshield | 16072252 | |
| Cryostat | Thermo Scientific | Microm Cryo Star HM 560 | |
| Dissecting Stereo Microscope SMZ745 | Nikon | ||
| Dry Ice | |||
| Dumont Forceps | F.S.T | 11295-10 | |
| Epifluorescence microscope | Zeiss | AxioImage-Apotome Z1 | |
| Extra Fine Bonn Scissors | F.S.T | 14084-08 | |
| FisherBrand Disposable Base Molds (0.7 x 0.7 cm) | ThermoFisher | 22-363-552 | Used to cut a piece to hold the muscle on the cork for freezing |
| Glass petri dish (H 25 mm, ø 150 mm) | BRAND Petri dish, MERK | BR455751 | Used to place the muscles on ice during dissection |
| ImmEdge Hydrophobic barrier PAP Pen | Vector Labs | H-4000 | Used to create an hidrophobic barrier around the muscle sections |
| Incubator | MMM Medcenter | Incucell 707 | |
| Microscope Cover Glasses (24×50 mm) | Assistent | 40990151 | |
| Microscope Slide Boxes | Kartell | 278 | Used as humid chambers for immunohistochemistry |
| Neck holder | Linie zwo | SB-035X-02 | Used as strap to hold the stainless steel tumbler |
| No 15 Sterile Carbon Steel Scalpel Blade | Swann-Morton | 0205 | |
| Paint brushes | Van Bleiswijck | Amazon B07W7KJQ2X | Used to handle cryosections |
| Permanent Marker Pen Black | Klinipath/VWR | 98307-R | Used to label slides |
| Pierce Fixation Forceps | F.S.T | 18155-13 | |
| Polystyrene Box | H 12 cm x L 25 cm x W 18 cm, used as a liquid nitrogen container and to transport the samples to the cryostat | ||
| Scalpel Handle, 125 mm (5"), No. 3 | Aesculap | BB073R | |
| Stainless Steel Cup 10oz | Eboxer | B07GFCBPFH | Tumbler to fill with isopentene for muscle freezing |
| Superfrost Ultra Plus slides | ThermoFisher | J1800AMNZ | |
| Surgical tweezers 1/2 teeth | Medische Vakhandel | 1303152 | Also called "Rat teeth tweezers" |
| Vannas Spring Scissors – 3 mm Cutting Edge | F.S.T | 15000-00 | |
| Weighing boats | VWR international | 611-2249 | |
| Whole-Slide Scanner for Fluorescence | Zeiss | Axio Scan.Z1 | |
| Reagents | |||
| Alexa Fluor 405 Goat Anti-Mouse IgG2b | Sigma-Aldrich | SAB4600477 | Used at a final concentration of 1:500 |
| Alexa Fluor 488 Goat Anti-Mouse IgG1 | ThermoFisher | A-21121 | Used at a final concentration of 1:500 |
| Alexa Fluor 568 Goat Anti-Mouse IgM | Abcam | ab175702 | Used at a final concentration of 1:1,000 |
| Alexa Fluor 647 goat anti rat-IgG (H+L) secondary antibody | ThermoFisher | A-21247 | Used at a final concentration of 1:500 |
| BODIPY-493/503 (4,4-difluoro-1,3,5,7,8-pentametil-4-bora-3a,4a-diaza-s-indaceno) | ThermoFisher | D3922 | Used at a final concentration of 1 µg/mL |
| BODIPY-558/568 C12 (4,4-Difluoro-5-(2-Thienyl)-4-Bora-3a,4a-Diaza-s-Indacene-3-Dodecanoic Acid) | ThermoFisher | D3835 | Used at a final concentration of 1 µg/mL |
| DAPI (4',6-diamidino-2-phenylindole) | ThermoFisher | D1306 | Used at a final concentration of 0.5 µg/mL |
| Dimethyl Sulfoxide (DMSO) | Sigma-Aldrich | D-8418 | Used to solve Bodipy for the 1 mg/mL stock solution. CAUTION: Toxic and flammable. Vapors may cause irritation. Manipulate in a fume hood. Avoid direct contact with skin. Wear rubber gloves, protective eye goggles. |
| Formaldehyde solution 4%, buffered, pH 6.9 | Sigma-Aldrich | 1004969011 | CAUTION: May cause an allergic skin reaction. Suspected of causing genetic defects. May cause cancer. Manipulate in a fume hood. Avoid direct contact with skin. Wear rubber gloves, protective eye goggles. |
| Isopentane GPR RectaPur | VWR international | 24872.298 | CAUTION: Extremely flammable liquid and vapor. May be fatal if swallowed and enters airways. May cause drowsiness or dizziness. Repeated exposure may cause skin dryness or cracking. Wear protective gloves/protective clothing/eye protection/face protection. |
| Liquid Nitrogen | CAUTION: Extremely cold. Wear gloves. Handle slowly to minimize boiling and splashing and in well ventilated areas. Use containers designed for low-temperature liquids. | ||
| Mouse on mouse Blocking Reagent | Vector Labs | MKB-2213-1 | Used at concentration of 1:30 |
| Myosin heavy chain Type I (BA-D5-s Primary Antibody) Gene: MYH7, monoclonal bovine anti mouse IgG2b | DSHB University of Iowa | BA-D5-supernatant | Used at a final concentration of 1:10 |
| Myosin heavy chain Type IIA (SC-71-s Primary Antibody) Gene: MYH2, Monoclonal bovine anti mouse IgG1 | DSHB University of Iowa | SC-71-supernatant | Used at a final concentration of 1:10 |
| Myosin heavy chain Type IIX (6H1-s Primary Antibody), Gene: MYH1, Monoclonal rabbit anti mouse IgM | Developmental Studies Hybridoma Bank, University of Iowa | 6H1-supernatant | Used at a final concentration of 1:5 |
| Normal Goat Serum (NGS) | Vector Labs | S-1000 | |
| PBS 0.1 M | Commonly used on histology laboratories | ||
| ProLong Gold Antifade Mountant | Invitrogen | P36930 | |
| Rat anti-Laminin-2 (α-2 Chain) primary antibody (monoclonal) | Sigma-Aldrich | L0663 | Used at a final concentration of 1:1,000 |
| Tissue-Tek O.C.T compound | Sakura | 4583 | |
| Software | |||
| Adobe Illustrator CC | Adobe Inc. | Used to design the figures | |
| Adobe Photoshop | Adobe Inc. | Confocal software | |
| BioRender | https://biorender.com/ | Used to design the figures | |
| Fiji/ImageJ | https://imagej.net/software/fiji/ | Used to analyse the acquired images | |
| Microsoft PowerPoint | Microsoft | Used to reconstruct the histology of the whole muscle after scanning the fiber types | |
| Zen Blue 2.6 | Zeiss | Used to reconstruct the histology of the whole muscle after scanning the fiber types |
References
- Correa-de-Araujo, R., et al. Myosteatosis in the context of skeletal muscle function deficit: an interdisciplinary workshop at the National Institute on Aging. Frontiers in Physiology. 11, 963 (2020).
- Miljkovic, I., et al. Greater skeletal muscle fat infiltration is associated with higher all-cause and cardiovascular mortality in older men. Journals of Gerontology Series A: Biomedical Sciences and Medical Sciences. 70 (9), 1133-1140 (2015).
- Nachit, M., et al. Myosteatosis rather than sarcopenia associates with non-alcoholic steatohepatitis in non-alcoholic fatty liver disease preclinical models. Journal of Cachexia, Sarcopenia, and Muscle. 12 (1), 144-158 (2021).
- Aleixo, G. F. P., et al. Myosteatosis and prognosis in cancer: Systematic review and meta-analysis. Critical Reviews in Oncology/Hematolgoy. 145, 102839 (2020).
- Gemmink, A., Schrauwen, P., Hesselink, M. K. C. Exercising your fat (metabolism) into shape: a muscle-centred view. Diabetologia. 63 (8), 1453-1463 (2020).
- van Loon, L. J. Use of intramuscular triacylglycerol as a substrate source during exercise in humans. Journal of Applied Physiology. 97 (4), 1170-1187 (2004).
- Coen, P. M., Goodpaster, B. H. Role of intramyocelluar lipids in human health. Trends in Endocrinology and Metabolism. 23 (8), 391-398 (2012).
- Seibert, J. T., Najt, C. P., Heden, T. D., Mashek, D. G., Chow, L. S. Muscle lipid droplets: cellular signaling to exercise physiology and beyond. Trends in Endocrinology and Metabolism. 31 (12), 928-938 (2020).
- Bergman, B. C., Goodpaster, B. H. Exercise and muscle lipid content, composition, and localization: influence on muscle insulin sensitivity. Diabetes. 69 (5), 848-858 (2020).
- Nielsen, J., Christensen, A. E., Nellemann, B., Christensen, B. Lipid droplet size and location in human skeletal muscle fibers are associated with insulin sensitivity. American Journal of Physiology-Endocrinology and Metabolism. 313 (6), 721-730 (2017).
- Covington, J. D., et al. Intramyocellular lipid droplet size rather than total lipid content is related to insulin sensitivity after 8 weeks of overfeeding. Obesity (Silver Spring). 25 (12), 2079-2087 (2017).
- Bosma, M. Lipid droplet dynamics in skeletal muscle). Experimental Cell Research. 340 (2), 180-186 (2016).
- Nielsen, J., et al. Increased subsarcolemmal lipids in type 2 diabetes: effect of training on localization of lipids, mitochondria, and glycogen in sedentary human skeletal muscle. American Journal of Physiology-Endocrinology and Metabolism. 298 (3), 706-713 (2010).
- Ferreira, R., et al. Subsarcolemmal and intermyofibrillar mitochondria proteome differences disclose functional specializations in skeletal muscle. Proteomics. 10 (17), 3142-3154 (2010).
- Barrett, J. S., Whytock, K. L., Strauss, J. A., Wagenmakers, A. J. M., Shepherd, S. O. High intramuscular triglyceride turnover rates and the link to insulin sensitivity: influence of obesity, type 2 diabetes and physical activity. Applied Physiology, Nutrition and Metabolism. , 1-14 (2022).
- Daemen, S., et al. Distinct lipid droplet characteristics and distribution unmask the apparent contradiction of the athlete’s paradox. Molecular Metabolism. 17, 71-81 (2018).
- Bredella, M. A., Ghomi, R. H., Thomas, B. J., Miller, K. K., Torriani, M. Comparison of 3.0 T proton magnetic resonance spectroscopy short and long echo-time measures of intramyocellular lipids in obese and normal-weight women. Journal of Magnetic Resonance Imaging. 32 (2), 388-393 (2010).
- Schrauwen-Hinderling, V. B., Hesselink, M. K., Schrauwen, P., Kooi, M. E. Intramyocellular lipid content in human skeletal muscle. Obesity (Silver Spring). 14 (3), 357-367 (2006).
- De Bock, K., et al. Evaluation of intramyocellular lipid breakdown during exercise by biochemical assay, NMR spectroscopy, and Oil Red O staining. American Journal of Physiology-Endocrinology and Metabolism. 293 (1), 428-434 (2007).
- Koopman, R., Schaart, G., Hesselink, M. K. Optimisation of oil red O staining permits combination with immunofluorescence and automated quantification of lipids. Histochemistry and Cell Biology. 116 (1), 63-68 (2001).
- Gueugneau, M., et al. Skeletal muscle lipid content and oxidative activity in relation to muscle fiber type in aging and metabolic syndrome. Journal of Gerontology Series A: Biomedical Sciences and Medical Sciences. 70 (5), 566-576 (2015).
- Gemmink, A., et al. Super-resolution microscopy localizes perilipin 5 at lipid droplet-mitochondria interaction sites and at lipid droplets juxtaposing to perilipin 2. Biochimica et Biophysica Acta-Molecular and Cell Biology of Lipids. 1863 (11), 1423-1432 (2018).
- Spangenburg, E. E., Pratt, S. J. P., Wohlers, L. M., Lovering, R. M. Use of BODIPY (493/503) to visualize intramuscular lipid droplets in skeletal muscle. Journal of Biomedicine and Biotechnology. 598358, (2011).
- Prats, C., et al. An optimized histochemical method to assess skeletal muscle glycogen and lipid stores reveals two metabolically distinct populations of type I muscle fibers. PLoS One. 8 (10), 77774 (2013).
- Strauss, J. A., Shepherd, D. A., Macey, M., Jevons, E. F. P., Shepherd, S. O. Divergence exists in the subcellular distribution of intramuscular triglyceride in human skeletal muscle dependent on the choice of lipid dye. Histochemistry and Cell Biology. 154 (4), 369-382 (2020).
- Shepherd, S. O., et al. Sprint interval and traditional endurance training increase net intramuscular triglyceride breakdown and expression of perilipin 2 and 5. Journal of Physiology. 591 (3), 657-675 (2013).
- Whytock, K. L., et al. A 7-day high-fat, high-calorie diet induces fibre-specific increases in intramuscular triglyceride and perilipin protein expression in human skeletal muscle. Journal of Physiology. 598 (6), 1151-1167 (2020).
- Wang, C., Yue, F., Kuang, S. Muscle histology characterization using h&e staining and muscle fiber type classification using immunofluorescence staining. Bio-Protocol. 7 (10), (2017).
- Meng, H., et al. Tissue triage and freezing for models of skeletal muscle disease. Journal of Visualized Experiments: JoVE. (89), e51586 (2014).
- Kumar, A., Accorsi, A., Rhee, Y., Girgenrath, M. Do’s and don’ts in the preparation of muscle cryosections for histological analysis. Journal of Visualized Experiments: JoVE. (99), e52793 (2015).
- Leiva-Cepas, F., et al. Laboratory methodology for the histological study of skeletal muscle. Archivos de Medicina del Deporte. 35 (186), 254-262 (2018).
- Schindelin, J., et al. Fiji: an open-source platform for biological-image analysis. Nature Methods. 9 (7), 676-682 (2012).
- Schiaffino, S., et al. Three myosin heavy chain isoforms in type 2 skeletal muscle fibres. Journal of Muscle Research & Cell Motility. 10 (3), 197-205 (1989).
- Komiya, Y., et al. Mouse soleus (slow) muscle shows greater intramyocellular lipid droplet accumulation than EDL (fast) muscle: fiber type-specific analysis. Journal of Muscle Research & Cell Motility. 38 (2), 163-173 (2017).
- Andrich, D. E., et al. Altered lipid metabolism impairs skeletal muscle force in young rats submitted to a short-term high-fat diet. Frontiers in Physiology. 9, 1327 (2018).
- Schiaffino, S. Fibre types in skeletal muscle: a personal account. Acta Physiologica. 199 (4), 451-463 (2010).
- Bloemberg, D., Quadrilatero, J. Rapid determination of myosin heavy chain expression in rat, mouse, and human skeletal muscle using multicolor immunofluorescence analysis. PLoS One. 7 (4), 35273 (2012).
- Gemmink, A., et al. Decoration of intramyocellular lipid droplets with PLIN5 modulates fasting-induced insulin resistance and lipotoxicity in humans. Diabetologia. 59 (5), 1040-1048 (2016).
- Askinas, C., et al. . Biophotonics Congress: Biomedical Optics Congress 2018 (Microscopy/Translational/Brain/OTS). , (2018).
- Morén, B., et al. EHD2 regulates adipocyte function and is enriched at cell surface-associated lipid droplets in primary human adipocytes. Molecular Biology of the Cell. 30 (10), 1147-1159 (2019).
- Benador, I. Y., et al. Mitochondria bound to lipid droplets have unique bioenergetics, composition, and dynamics that support lipid droplet expansion. Cell Metabolism. 27 (4), 869-885 (2018).
- de la Rosa Rodriguez, M. A., et al. Hypoxia-inducible lipid droplet-associated induces DGAT1 and promotes lipid storage in hepatocytes. Molecular Metabolism. 47, 101168 (2021).
- Jevons, E. F. P., Gejl, K. D., Strauss, J. A., Ørtenblad, N., Shepherd, S. O. Skeletal muscle lipid droplets are resynthesized before being coated with perilipin proteins following prolonged exercise in elite male triathletes. American Journal of Physiology-Endocrinology and Metabolism. 318 (3), 357-370 (2020).
- Ohsaki, Y., Maeda, T., Fujimoto, T. Fixation and permeabilization protocol is critical for the immunolabeling of lipid droplet proteins. Histochemistry and Cell Biology. 124 (5), 445-452 (2005).
- Prats, C., et al. Decrease in intramuscular lipid droplets and translocation of HSL in response to muscle contraction and epinephrine. Journal of Lipid Research. 47 (11), 2392-2399 (2006).
- Listenberger, L. L., Brown, D. A. Fluorescent detection of lipid droplets and associated proteins. Current Protocols in Cell Biology. , (2007).
- Xue, Y., Lim, S., Bråkenhielm, E., Cao, Y. Adipose angiogenesis: quantitative methods to study microvessel growth, regression and remodeling in vivo. Nature Protocols. 5 (5), 912-920 (2010).
- Muliyil, S., et al. ADAM17-triggered TNF signalling protects the ageing Drosophila retina from lipid droplet-mediated degeneration. The EMBO Journal. 39 (17), 104415 (2020).
- Yan, Q., et al. Autophagy activation contributes to lipid accumulation in tubular epithelial cells during kidney fibrosis. Cell Death Discovery. 4, 2 (2018).
- Coassin, S., et al. Investigation and functional characterization of rare genetic variants in the adipose triglyceride lipase in a large healthy working population. PLoS Genetics. 6 (12), 1001239 (2010).
- Daemen, S., van Zandvoort, M., Parekh, S. H., Hesselink, M. K. C. Microscopy tools for the investigation of intracellular lipid storage and dynamics. Molecular Metabolism. 5 (3), 153-163 (2016).
- Chen, Q., et al. Rab8a deficiency in skeletal muscle causes hyperlipidemia and hepatosteatosis by impairing muscle lipid uptake and storage. Diabetes. 66 (9), 2387-2399 (2017).
- Gemmink, A., et al. Decoration of myocellular lipid droplets with perilipins as a marker for in vivo lipid droplet dynamics: A super-resolution microscopy study in trained athletes and insulin resistant individuals. Biochimica et Biophysica Acta (BBA)-Molecular and Cell Biology of Lipids. 1866 (2), 158852 (2021).
- Bergman, B. C., Hunerdosse, D. M., Kerege, A., Playdon, M. C., Perreault, L. Localisation and composition of skeletal muscle diacylglycerol predicts insulin resistance in humans. Diabetologia. 55 (4), 1140-1150 (2012).

