The Isolation of Flowing Mesenteric Lymph in Mice to Quantify In Vivo Kinetics of Dietary Lipid Absorption and Chylomicron Secretion
Summary
The present protocol describes a detailed surgical protocol for isolating flowing intestinal lymph in response to intraduodenal nutrient infusions in mice. This allows for the physiological determination of total intestinal lipid absorption and chylomicron synthesis and secretion in response to various experimental nutrients.
Abstract
Intestinal lipoproteins, especially triglyceride-rich chylomicrons, are a major driver of metabolism, inflammation, and cardiovascular diseases. However, isolating intestinal lipoproteins is very difficult in vivo because they are first secreted from the small intestine into the mesenteric lymphatics. Chylomicron-containing lymph then empties into the subclavian vein from the thoracic duct to deliver components of the meal to the heart, lungs, and, ultimately, whole-body circulation. Isolating naïve chylomicrons is impossible from blood since chylomicron triglyceride undergoes hydrolysis immediately upon interaction with lipoprotein lipase and other lipoprotein receptors in circulation. Therefore, the original 2-day lymph fistula procedure, described by Bollman et al. in rats, has historically been used to isolate fresh mesenteric lymph before its entry into the thoracic vein. That protocol has been improved upon and professionalized by the laboratory of Patrick Tso for the last 45 years, allowing for the analysis of these critical lipoproteins and secretions from the gut. The Tso lymph fistula procedure has now been updated and is presented here visually for the first time. This revised procedure is a single-day surgical technique for installing a duodenal feeding tube, cannulating the mesenteric lymph duct, and collecting lymph after a meal in conscious mice. The major benefits of these new techniques include the ability to reproducibly collect lymph from mice (which harnesses the power of genetic mouse models); the reduced anesthesia time for mice during the implantation of the duodenal infusion tube and the lymph cannula; the ability to continuously sample lymph throughout the feeding and post-prandial period; the ability to quantitatively measure hormones and cytokines before their dilution and enzymatic hydrolysis in blood; and the ability to collect large quantities of lymph for isolating intestinal lipoproteins. This technique is a powerful tool for directly and quantitatively measuring dietary nutrient absorption, intestinal lipoprotein synthesis, and chylomicron secretion.
Introduction
The physiological importance of the mesenteric lymphatic system
The mesenteric lymphatics have been described in some form since ~300 BC when Herophilos described the hepatic portal system and all the "absorptive veins in the intestines"1,2,3,4,5. For more than 1,700 years after this initial description, a defining characteristic of intestinal lymphatics was the presence of milky fluid in the mesenteric lymph shortly after a meal3. It is now known that milky, chylomicron-containing lymph ("chylous" lymph) does not drain into the portal vein and liver but instead travels through the cisterna chyli into the thoracic duct and, ultimately, joins the blood at the left subclavian vein6. Due to this anatomical arrangement, chylous lymph first travels through the heart and lungs before circulating through the rest of the body. This means that the heart and lungs get a "first-pass" at the secretions into the mesenteric lymph7.
A major role of mesenteric lymphatics is their transport of dietary lipids from the small intestine8,9,10. Anatomically, chylomicrons, intestinal immune cells11,12,13,14, gut hormones15,16,17,18, antigens19,20,21, non-chylomicron lipophilic compounds22,23, and excess fluid enter the lacteals underlying the enterocyte basement membrane and are then concentrated through the lymphatic capillaries to the mesenteric lymph nodes. There are likely many unknown mesenteric lymphatic components, including metabolites, antigens, environmental contaminants, nutrients, and signaling molecules.
The components of mesenteric lymph have not been systematically identified, largely due to the difficulty of isolating mesenteric lymph. Accessing the mesenteric lymph has always been a serious challenge because lymph ducts are colorless, and except for after a fatty meal when they become chylous and milky, the mesenteric lymphatics contain colorless lymphatic fluid6,8,9,10.
Current and commons methods for isolating intestinal lymph
Mesenteric lymph cannot be accessed in humans (except for rare and extreme circumstances where serious GI trauma has occurred)24. In vivo lymph collection is surgically complex and demanding. The original 2 day lymph fistula procedure was described by Bollman et al.25 and has been improved upon and professionalized by the laboratory of Patrick Tso for the last 45 years26,27. The lymph fistula procedure allows investigators to collect flowing lymph from conscious animals during a 6 h duodenal lipid infusion period.
The lymph fistula model has mainly been used in rats to measure lymph flow rate, the output of triglyceride and cholesterol (or other duodenal-infused compounds), chylomicron composition, and intestinal hormone concentrations. To a lesser extent, this technique can also be used in mice, though surgical survival and lymph volume suffer. Due to the difficulties in seeing mesenteric lymph ducts, historical methods have included cannulating mesenteric lymph in larger animals like mini-pigs28, sheep29, pigs30, dogs31, and rats17. Working with these larger models is resource intensive and does not allow for studies in knock-in or knock-out models.
Alternative approaches have also been used. Chylomicrons can be isolated from blood in the post-prandial state (though these will be partially hydrolyzed by plasma lipoprotein lipase)32,33,34,35. The thoracic duct can also be cannulated, though the lymph collected there contains an admixture of mesenteric lymph and extra-intestinal lymph drainage26,36. In vitro, Caco-2 cells secrete a chylomicron-like particle in response to fatty acid treatment, and these cells can be co-cultured with relevant lymphatic endothelial or vascular cells37,38. Human and mouse intestinal organoid cultures have been shown to process apical lipids and secrete chylomicrons39,40,41,42. These models are highly advantageous and enable mechanistic insights into small intestinal physiology, but they cannot replicate the complexity, physio-chemical gradients, or dynamic lymph flow of in situ mesenteric lymphatics.
Advantages of the 1 day mouse lymph fistula model presented here
With respect to these other methods of isolating intestinal lipoproteins, the Tso Lab lymph fistula technique has traditionally been considered the gold-standard technique for measuring the absorption of dietary nutrients into mesenteric lymph. This in vivo technique has the advantage of capturing key physiological aspects of dietary lipid absorption-the dynamic appearance of compounds over the absorption period, which requires the repeated sampling of flowing mesenteric lymph in live animals with duodenal nutrients. This surgical technique also measures gut hormones and cytokines directly in their physiological compartment rather than in blood, where they are diluted and enzymatically degraded17,43.
If the experimental question requires an understanding of lipid secretion dynamics or the dynamic absorption and metabolism of any hydrophobic GI compound or drug, then this technique is not only appropriate but is also the only approach that interpolates the movement of luminal contents from the proximal to the distal gut (stomach to colon) and from the apical to the basolateral surface (luminal contents through enterocyte to lacteals and portal circulation). As this technique employs the luminal delivery of nutrients through the intraduodenal catheter, and because the flowing mesenteric lymph is diverted and collected, the entire absorption apparatus is under experimental control and can be used to qualitatively assess small intestinal absorption profiles.
Presented visually here for the first time is a major update to the Tso Lab lymph fistula model, which (1) reduces the experimental time to a 1 day surgical implantation and experimental collection period; (2) improves mouse survival and animal welfare considerations; and (3) increases the reproducibility of the approach in mice to harness the power of mouse genetic models. This technique must be considered a gold standard for all experimental questions of intestinal secretions, lipoproteins, or dietary lipid absorption and is the best technique for the high-fidelity determination of lipid absorption kinetics and chylomicrons.
Protocol
All surgical procedures were approved by the University of Pittsburgh Internal Animal Care and Use Committee [Protocol # 20047008] and comply with the NIH Guide for the Care and Use of Laboratory Animals. C57BL6/J male mice, 8-14 weeks of age, were used for the present study. The mice were obtained from a commercial source (see Table of Materials). All mice were housed on a 12 h light/dark cycle with ad libitum access to standard chow and water.
1. Animal preparation
- Depending on the experimental design, feed the mice or allow them to fast.
NOTE: An overnight fast is unnecessary unless there are concerns about differences in the rate of stomach emptying. - Induce anesthesia by 5% isoflurane gas and ensure proper anesthetization by tail and toe pinch. Once anesthetized, keep the mice at a proper anesthetic plane with 2%-3% isoflurane gas and position them with tape on the surgical platform.
- Keep the mice warm on a heated surgical platform that utilizes circulating warm water (see Table of Materials).
2. Surgery and experimental design
- Start the surgery by applying vet ointment to the eyes, shaving the abdomen, and applying antiseptic surgical scrub (see Table of Materials) to the surgical site. This sterilizes the incision and reduces the generation of airborne fur during the midline incision.
- Maintain a sterile working area by utilizing sterile instruments, drapes, and other equipment and supplies needed.
- Inject the first dose of carprofen (see Table of Materials) for pain relief (i.p.) at a dose of 5 mg/kg.
- Grasp the skin with tweezers and make a midline abdominal incision with small scissors. Cut toward the sternum (never above) and cut down to the inguinal fat. Then, cut through the muscle layer separately using the scissors.
NOTE: Sterilize all equipment before use. - Using the retractor, move the peritoneal viscera out of the way until the lymph duct is visible.
- Using a saline-soaked Q-tip (see Table of Materials), move the liver toward the top-right side of the body and the intestines and stomach to the left of the animal.
- Stretch the duodenum toward the left transversely to expose the superior mesenteric artery and the intestinal lymph duct.
- Prepare a 30-40 cm length of cannula tubing by inserting a blunt needle in the cannula tubing (see Table of Materials) and flush a small amount of heparin (1,000 U/L) through the tube using a 1 mL syringe.
NOTE: Lymph flow is assisted by gravity to flow down and into the microcentrifuge collection tubes on ice. Depending on where the collection ice bucket is located adjacent to the surgical setup, the length of the cannula tubing may need to be adjusted. - Use scissors to cut a bevel at the tip of the cannula tubing.
- Make a shallow incision with iris scissors (see Table of Materials) in the lymph duct approximately 5 mm from its appearance in the small intestine.
- Hold the cannula tubing with a pair of fine forceps and gently insert the tip bevel up into the duct.
NOTE: It is important not to push the cannula too far into the duct because this may prevent lymph flow when the retracted organs are returned to their original position. - The lymph duct is much too fragile for the manipulations associated with tying the catheter in with a suture. Instead, use a drop of cyanoacrylate glue (see Table of Materials) to glue the lymph cannula into the mesenteric lymph duct.
- Check for the milky, white lymph (if pre-surgery olive oil gavage was used) or clear lymph (if mice have fasted pre-surgery) that begins to flow immediately through the lymph fistula cannula.
NOTE: Check that the lymph cannula is successfully placed and lymph is flowing; continue with the intraduodenal cannula placement. - Using an 18 G needle, puncture a hole through the pyloric region of the stomach posterior to the pyloric sphincter.
- Insert the duodenal infusion tube (see Table of Materials) through that hole in the stomach to approximately 1-2 mm beyond the pyloric sphincter into the duodenum.
- Secure by a purse-string ligature using silk 5-0 suture (see Table of Materials) with a needle to the stomach and seal with a drop of cyanoacrylate glue to prevent leakage.
- Start the intraduodenal infusion of 5% glucose-0.9% saline at 0.3 mL/h.
NOTE: When using mice with small variances in body weight who are roughly 25 g (~8 weeks in age), the 0.3 mL/h infusion of glucose/saline is appropriate. If mice are dramatically larger, the infusion rate must be adjusted upward to account for the change in body weight and blood volume. - Replace the organs in the body cavity and suture (5-0) the muscle and skin tissues separately.
NOTE: The lymph cannula and the intraduodenal feeding tube are both exteriorized through the same incision. There is no precedence for separating the tubes with a suture and no preference for the angle of the exteriorization of the tubes. - After surgery but prior to the withdrawal of anesthetic, place the mice gently in Snuggle restraints (see Table of Materials) to limit motility.
NOTE: The snuggle restraints prevent the mice from grasping or rotating the head to chew on the stitches and tubings. - Then, place the mice in a clear plexiglass box on a rotator with gentle rocking, and warm using a temperature-controlled commercially available amphibian/reptile heating pad adhered to the side of the containment box. Provide humidification also in the form of sterile deionized water containers at the corners of the containment box (see Table of Materials).
- At 30 min before the isoflurane is withdrawn, administer the mice their second pain relief dose (Buprenex, 0.1 mg/kg, i.p.).
- Depending on the experiment, provide the mice with a continuous intraduodenal infusion of 5% glucose-0.9% saline at a rate of 0.3 mL/h for 1 h.
NOTE: The 5% glucose-0.9% saline solution is prepared using a sterile saline bag (for human use, pH 7.4) from the pharmacy and a sterile bottle for human use of 50% dextrose, following strict guidelines for sterility. The solution must be made fresh and discarded if past the expiration dates provided on the saline bag or the dextrose bottle. - Administer the mice with one of the lipids listed in Table 1 as an experimental lipid via an intraduodenal cannula (see Table of Materials).
NOTE: For all data shown in Figure 1, SMOFlipid (20% lipid injectable emulsion, USP, see Table of Materials) was intraduodenally infused. This infusion both pre- and post-intraduodenal lipid bolus replaces lost fluid draining through the lymph duct and is an absolute requirement. The mice are now ready to be infused with an experimental lipid dose. - Perform the classic lipid infusion of a 0.3 mL lipid emulsion bolus (SMOFlipid 20% lipid injectable emulsion, USP) via the intra-duodenal infusion tube (see Table of Materials).
- Following the bolus infusion of intraduodenal lipids, switch the infusion back to 5% glucose-0.9% saline at 0.3 mL/h continuously through to the end of the experiment.
- Collect the lymph samples in pre-weighed microcentrifuge tubes for 60 min and keep the lymph on ice. Weigh the tubes a second time to establish the weight of lymph that is secreted each 60 min period.
NOTE: Expect to collect approximately 50-300 µL of lymph per hour, per mouse in the ~6 h after a 0.3 mL lipid bolus. - Lymph can flow for up to 6 h after the lipid infusion. At the 6 h time point, euthanize the mice via isoflurane and cervical dislocation.
NOTE: A surgeon and/or lab technician should be present to monitor the animals throughout the experiment. During the observation, look out for clots in the lymphatic drainage and changes in animal behavior that indicate pain/distress. If the lymph duct is clogged at any point during the experiment, the experiment is terminated, and the animal is euthanized. The animal can technically be kept alive for up to 24 h after surgery (as per IACUC survival surgery rules). However, survival rates decrease over time, and 6 h is a reproducible survival period where lipid absorption still mimics the in vivo physiology, and the animal is not struggling with survival. - Perform terminal tissue collection after euthanasia (step 3.1).
NOTE: The difference in dietary lipid absorption profiles in mice kept for 6-h or 24-h post-surgery was recently tested44. We found that the 24-h procedure reduces the animal survival rates and the excursion of dietary lipids into the lymph. For these reasons, we strongly recommend the 6-h approach. This updated surgical design reduces unnecessary animal death and the potential for animal distress in the postsurgical period. This supports a major goal of the American Association for Accreditation of Laboratory Animal Care, which is animal “Replacement, Reduction, Refinement” [ref PMID: 21595115].
3. Collection of luminal contents and mucosal tissue
- At the end of the 6 h lipid infusion period, sacrifice the mice via isoflurane and cervical dislocation. Tie both ends of the stomach, small intestine, and cecum with 5-0 sutures to avoid leakage of the luminal contents.
- Collect the stomach, cecum, and colon, and place each into a 15 mL glass tube. Divide the small intestine into three or four segments, and collect the luminal contents by rinsing the tissue in 2-3 mL of cold PBS after cutting open longitudinally.
- Remove the intestinal mucosa comprising epithelial cells and associated lamina propria from the muscular layer by scraping each section in 2-3 mL of cold PBS with a curved tweezer.
- Place all tissues, the luminal and mucosal isolates, and the muscular layer in glass tubes and add 8 mL of 2:1 vol/vol CHCl3:MeOH to each tube.
- Determine the radioactivity and/or lipid concentrations by liquid scintillation counting and/or a triglyceride assay kit (see Table of Materials) after Folch extraction45.
4. Thin-layer chromatography (TLC)
- Extract the total lipids of the luminal and mucosal isolates via a modified Folch extraction45.
- Dry the extracted lipids in the solvent under a nitrogen evaporator and then resuspend them in 2:1 vol/vol of CHCl3:MeOH before loading them onto a TLC silica gel plate (see Table of Materials).
- Separate the lipids using a solvent system of petroleum ether, ethyl ether, and glacial acetic acid (25:5:1 vol/vol/vol). Use iodine vapor for the visualization of different lipids, including standards.
- Scrape the spots on the TLC plate corresponding to monoglyceride/phospholipid, diglycerides, fatty acids, triglyceride, and cholesterol ester into individual scintillation vials before the addition of 4 mL of the scintillation fluid (see Table of Materials) for scintillation counting.
- Express the data as either a percent of the total bolus lipid dose or as a fraction of the total lipid detected for each sample.
5. Chylomicron isolation and characterization
- Transfer the combined lymph samples from the 6 h post-lipid bolus (step 2.29) to ultracentrifuge tubes, mixed with 0.9% NaCl (300-500 µL), and then carefully overlay with an appropriate volume of 0.87% NaCl (300-500 µL).
- Ultracentrifuge (see Table of Materials) the samples at 110,000 x g at 4 °C for 16 h. Collect the top fraction containing isolated chylomicrons and transfer them to a new microcentrifuge tube.
- Determine chylomicron triglyceride, cholesterol, and apoB concentrations following the steps below.
- Determine triglyceride and cholesterol concentrations using a triglyceride and cholesterol assay kit (see Table of Materials).
- Briefly, incubate 2 µL of the samples with 200 µL of enzyme reagent at 37 °C for 5 min in a 96-well plate. Read the plate using a plate reader at 500 nm for triglyceride and 600 nm for cholesterol.
NOTE: Standards and blanks were used for the calculation of concentrations. ApoB concentrations were determined using Mouse ApoB ELISA Kit (see Table of Materials).
- Determine the chylomicron particle size.
- Use fresh chylomicron samples at triglyceride concentrations of around 40 mg/dL for TEM imaging. Briefly, place 5-10 µL of each sample on a TEM grid and dry.
- Examine the grid with a transmission electron microscope and capture images. Measure the lipoprotein particle sizes and analyze them using ImageJ software (see Table of Materials).
Representative Results
Figure 2 shows the triglyceride secretion in mesenteric lymph and the average lymph flow rates in the most recent n = 17 wild-type (WT) mice who underwent the single-day lymph fistula procedure described here. As shown in Figure 2A, the triglyceride concentration in lymph increases in response to an intraduodenal bolus of 300 µL of SMOFLipid. Peak triglyceride concentration is reached at ~2-3 h post-bolus and decreases steadily through the 6 h time (immediately before euthanasia). In parallel with triglyceride secretion, lymph flow rate increases from Time 0 bolus infusion through the end of the experiment (Figure 2B).
These results show that the surgical implantation of both the mesenteric lymph duct and intraduodenal cannula have occurred and are representative of a positive control to which other lymphatic contents (hormones, peptides, nutrients) could be benchmarked. If there is no change in triglyceride concentration in lymph in response to bolus intraduodenal lipids, this signals that the surgery has caused significant damage to the small intestine so that lipid absorption capacity is absent, or, in previously unphenotyped genetic models, this would suggest that the mouse has a defect in lipid absorption that is physiologically meaningful.
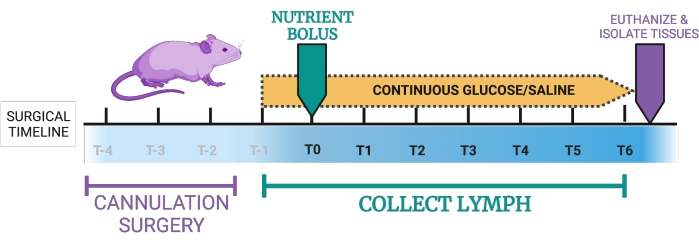
Figure 1: Proposed timeline for the lymph fistula mouse model. T-4, T-3, T-2: Double-cannulation takes approximately 2-4 h, followed by placement of the mice in recovery chambers. T-1: Once the mice are in the recovery period, they receive a continuous duodenal infusion of 5% glucose-0.9% saline. T0: the continuous intraduodenal infusion is switched from 5% glucose-0.9% saline to an infusion of experimental nutrients. T0-T6: Mice receive continuous intraduodenal 5% glucose in saline or, alternatively, continuous nutrients. Lymph is collected hourly during this period. Endpoint: Mice are euthanized, and tissues can be collected. The figure was created with BioRender.com. Please click here to view a larger version of this figure.
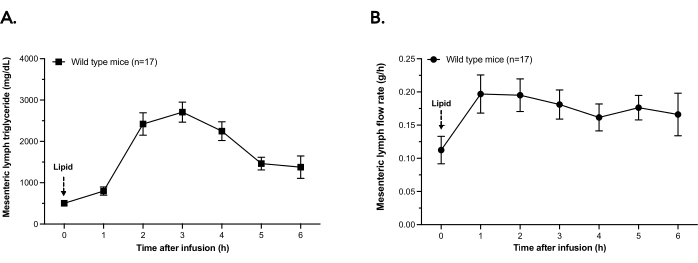
Figure 2: Mesenteric lymph triglyceride concentration and flow rate. Wild-type mice were fitted with an intraduodenal feeding tube and a mesenteric lymph cannula. Mice received a bolus infusion of 300 µL of lipid. Lymph was collected for 6 h in hourly aliquots and kept on ice. (A) Lymph triglyceride content was determined by a chemical assay in each hourly aliquot of lymph. (B) In the 6 h after bolus infusion, the lymph flow is plotted as grams of lymph secreted per hour. Points are means ± SEM. Please click here to view a larger version of this figure.
| Lipid Delivery Methods | Recommendations/Instructions | |||||
| Mixed lipid emulsion bolus | A 0.3 mL dose of either (A) Liposyn III 20% lipid injectable or (B) SMOFlipid 20% lipid injectable emulsion, USP, via intra-duodenal infusion tube can be used. These emulsified lipids are useful because they do not need to be prepared in advance, are liquid at room temperature, are sterile, and because they contain an “easily digestible” mix of free fatty acids, triglyceride, phospholipids, and sodium taurocholate. This is a good starter infusion for mice that may have pancreatic or biliary insufficiencies. | |||||
| Triglyceride bolus | 0.3 mL gently warmed olive oil (or purified triolein) as a neutral lipid that reflects a diet rich in unsaturated fat, lard (reflective of a diet with high saturated fat content) or coconut oil (reflective of a diet with high medium chain triglyceride content) can all be given by intra-duodenal infusion tube or oral gavage. If the experimental triglyceride is saturated, it must be warmed to be liquid at room temperature. | |||||
| Mixed meal bolus | 0.3 mL Ensure with the formulation containing 0.075 g fat (21.6%), 0.5 g carbohydrate (64.0%), and 0.1125 g protein (14.4%) and reflects a low-fat mixed meal, can be administered via intra-duodenal infusion. | |||||
| Radiolabeled lipid bolus | 5.0 µCi 3H-labeled glycerol trioleate and/or 1.0 µCi 14C-cholesterol in 0.3 mL of olive oil can be given via intra-duodenal infusion. 14C-cholesterol: Cholesterol-[4-14C] with specific activity of 55Ci/mmol and a concentration of 0.1mCi/ mL. 3H-TG Triolein [9,10-3H(N)] (3H-TG) with specific activity of 60Ci/mmol and concentration of 1mCi/mL | |||||
| Continuous dose | The infusion of any of the above experimental nutrient formulations at a rate of 0.3 mL/h (rather than 0.3 mL total bolus), will result in a steady output of triglyceride into the lymph. This differs from a bolus infusion, where triglyceride concentration in lymph peaks at ~2–3 h, and then returns to baseline concentrations at ~6–8 h. | |||||
Table 1: Table of lipid infusions.
Discussion
The original 2 day lymph fistula procedure was described by Bollman et al.25 and practiced by the laboratory of Patrick Tso for the last 45 years26,27. The protocol presented here is a powerful addition to this classic, gold-standard method for identifying, quantifying, and understanding the unique chylous secretions of the small intestine, as well as the in vivo dynamics of dietary nutrient absorption and metabolism, gut hormones, and intestinal immunity.
The advantages of this model include (1) the ability to continuously sample mesenteric lymph throughout the feeding and post-prandial period rather than static sampling at one time point during either absorption, digestion, or secretion; (2) the measurement of gut hormones and cytokines directly in their physiological compartment rather than in blood, where they are diluted and enzymatically degraded17,44; (3) the ability to isolate, quantitate, and characterize the lipoproteins secreted by the small intestine following the ingestion of a lipid meal and the absence of endothelial lipases in the mesenteric lymph, which preserves chylomicron triglyceride concentrations and native chylomicron structure46,47; (4) the ability to directly measure lymph flow rate, the output of triglyceride and cholesterol (or other duodenal-infused compounds), chylomicron composition, and intestinal hormone concentrations. Finally, this protocol allows for collecting relatively large quantities of >50 µL of lymph every hour over a 6 h period. As the fluid is replenished with intra-duodenal saline and glucose infusion, the lymph fistula model is significantly improved over other lymph sampling techniques and results in flowing rather than static pools of mesenteric lymph. As volume is a major hurdle for lipidomic, proteomic, and metabolomic approaches, this is a major strength.
The 1 day mouse lymph fistula protocol described here has several advantages over the original lymph fistula protocol, including a reduction in total experimental animal number from previously described 2 day lymph fistula protocols26,27 because of a higher survival rate after surgery; a reduction in the overall experimental time from 2 days to a single day; and, finally, a reduction in the recovery period for mice from overnight (>18 h), where breakthrough pain or poor post-surgical outcomes may occur, to a more manageable ~6 h post-surgery period.
A feature of this 1 day protocol is the focus on humane considerations and endpoints. These must take the highest priority: (1) animals must receive intraduodenal or IV replacement fluids; (2) they must be kept warm and as pain-free as possible (with post-operative Buprenex and/or carprofen, depending upon the experimental design and the need for avoiding anti-inflammatory effects); (3) bleeding, shaking, diarrhea, or signs of distress are all compelling reasons for a humane endpoint. Per strict IACUC guidelines, isoflurane followed by cervical dislocation is a good endpoint. Surgical survival rates are ~70% for a single day (compared to ~40% for the original 2 day surgery), but investigators should not hesitate to end the experiment at a sign of distress. This should be taken into consideration when planning animal numbers.
In terms of troubleshooting this technique, successful placement of the lymph cannula is the major bottleneck in this surgical procedure. While practicing the surgery, it helps to gavage the mouse with 0.3 mL olive oil approximately 2 h prior to surgery. This will cause the secretion of chylomicrons into the mesenteric lymph duct, making it appear "milky" and more visible. Methylene blue can also be used but is often less obvious than the milky lymph duct. If after the placement of the lymph cannula and its placement with glue the mesenteric lymph is successfully flowing through the cannula into a collection vessel, then one can proceed with the placement of a duodenal infusion tube. Occasionally, the lymph may not flow continuously but may start flowing again when the animal is placed on the rotating table. Critically, watch out for clots within the lymph tubing. These should be massaged out of the tubes to prevent backflow pressure on the lymph duct.
In addition to triglyceride secretion and lymph flow rate, this technique can be used to determine the following lipid absorption kinetics and chylomicron characteristics:
Chylomicron secretion45,48,49
Immediately following a meal containing fat, there is a transient rise in circulating plasma triglyceride. As triglycerides are inherently hydrophobic, they must first be emulsified to be soluble in blood50. Small intestinal enterocytes carry out this role and package dietary triglyceride into chylomicron emulsion particles51. Chylomicrons contain cholesterol and dietary triglyceride in their core, surrounded by phospholipids and apolipoproteins, including apoB-48, apoA-I, apoA-IV, and apoC-III52,53,54,55. ApoB-48 is the essential structural protein, and the other apolipoproteins have various functions required for chylomicron metabolism and clearance from the blood. To determine the key characteristics of chylomicrons, including their triglyceride and apolipoprotein content, the 1 day lymph fistula technique shown here should be used. The chylomicron secretion rate is the percent of infused 3H-triglyceride that is secreted into the lymph and measured by scintillation by counting hourly lymph samples. This can be combined with detailed chylomicron characterization48,49,56,57,58. Hourly lymph samples can be combined or kept separately. Lymph is transferred to ultracentrifuge tubes, mixed with 0.9% NaCl, and then carefully overlaid with 300-500 µL of 0.87% NaCl. Samples are then ultra-centrifuged at 110,000 x g at 4 °C for 16 h. The top fractions containing isolated chylomicrons are collected and tested for triglyceride concentrations using the triglyceride assay kit. Briefly, 2 µL of the chylomicron (1:10 dilution) is incubated with 200 µL of enzyme reagent at room temperature for 10 min in a 96-well plate. The plate is read by a plate reader at 500 nm, and the standards and blanks are used for the calculation of triglyceride concentrations. Chylomicron size can then be determined by negative staining and transmission electron microscopy (TEM)14,29. Triglyceride and cholesterol can be quantified by chemical assay and apolipoprotein content (apoB-48, A-I, C-II, C-III) by ELISA Kits or Western blot.
Determining the primary site of lipid absorption48,59
By isolating the luminal and epithelial cell compartments of the duodenum, jejunum, and ileum at 6 h after the infusion of 3H-triglyceride or a radio-labeled mixed meal, the contents are Folch extracted to determine how much of the 3H-triglyceride is absorbed across the epithelial cell membrane (normal) or is retained in the lumen (abnormal) along the length of the small intestine48. These studies are particularly impactful if there are potential differences in GI motility60,61,62, if there is a hypothesis regarding bile acids (highly active throughout lipid absorption and themselves reabsorbed in the ileum)63,64,65,66, or if there is a concern that nutrients are being absorbed in the wrong anatomical location (ileum or even colon)67,68,69,70.
Identifying mechanisms of 3H-triglyceride trafficking into absorptive epithelial cells48
This is performed by calculating the percent of 3H-triglyceride hydrolyzed to 3H-free fatty acid in the intestinal lumen, absorbed into the mucosa, and re-esterified into intracellular 3H-triglyceride prior to secretion as chylomicrons. This is a powerful marker of absorption/secretion defects since it can be traced to show the movement of dietary triglyceride into its breakdown products and subsequent packaging into chylomicrons. mRNA expression of the fatty acid absorption machinery (CD36, FABPs, ACSLs), re-esterification pathway (MGAT, DGAT, MTTP, apoB), and apolipoproteins (apoC-III, B-48, C-II, A-I, A-IV) can be further quantitated by RT-PCR.
Future applications of this technique are only limited by interest in gut-organ crosstalk, metabolism, immunity, nutrient absorption, environmental dietary contaminants, or any other disease with a role in the GI system. It is likely that many compelling experiments and hypotheses have been stalled because of the difficulty in accessing the critical mesenteric lymph system, and the goal of this visualized protocol is to make this technique more readily available. Isolating chylomicrons and the mesenteric lymph in which they initially reside is a critical part of understanding whole-body metabolism; the 1 day mouse lymph fistula model is a powerful physiological model for studying these events.
Disclosures
The authors have nothing to disclose.
Acknowledgements
We are extremely grateful to the Cystic Fibrosis Foundation (Pilot and Feasibility Award 1810, to ABK), the Rainin Foundation (Synergy Award, to PI GJ Randolph and Co-I ABK), and the National Institutes of Health (R01DK118239, R03DK116011 to ABK) for their support of these studies.
Materials
| 0.9% Sodium Chloride Injection, USP sterile | Hospira, Lake Forest, IL, US | NDC 0409-4888-06 | |
| 1.7 mL Eppendorf tubes | Fisher Scientific, Waltham, MA, US | 7200184 | |
| 14C-cholesterol: Cholesterol-[4-14C] (0.1mCi/ mL) | American Radiolabeled Chemicals Inc., St. Louis, MO, US | ARC 0857 | |
| 18 G needle | Becton Dickinson, Franklin Lakes, NJ, US | 305199 | |
| 2 Dumont micro-dissecting forceps | Fine Science Tools, Foster City, CA, US | 11251-35 | |
| 2 Forceps | ROBOZ Surgical Instrument Co., Gaithersburg, MD, US | RS-5130 | |
| 3H-TAG: Triolein [9,10-3H(N)] (3H-TG) (1mCi/ mL) | American Radiolabeled Chemicals Inc., St. Louis, MO, US | ART0199 | |
| 3H-TG Triolein [9,10-3H(N)] (3H-TG) | American Radiolabeled Chemicals, INC. St. Louis | MO 63146 | |
| 50% Dextrode Injection, USP 25grams/50 mL sterile | Hospira, Lake Forest, IL, US | NDC-0409-6648-16 | |
| Analtech TLC Uniplates: silica gel matrix Z265500-1PAK | Fisher Scientific, Waltham, MA, US | 11-101-0007 | |
| BD CareFusion ChloraPrep Swabstick | Fisher Scientific, Waltham, MA, US | 14-910-501 | |
| Betadine surgical scrub | Dynarex Corp., Orangeburg, NY, US | 1201 | |
| Bevel-cut cannula | Braintree Scientific., Braintree , MA, US | MRE025 | |
| Buprenorphine HCl Injection Carpuject PFS 0.3mg/mL 10/Bx (Buprenex) | HenrySchein, Warrendale, PA, US | 1278184 | |
| C57BL6/J male mice | Jackson Laboratory, Bar Harbor, Maine | ||
| ChloraPrep | Becton Dickinson, Franklin Lakes, NJ, US | 260100 | |
| Cholesterol Assay Kit | FujiFilm Healthcare, Lexington, MA, US | 99902601 | |
| Cholesterol-[4-14C] | American Radiolabeled Chemicals, INC. St. Louis | MO 63146 | |
| Clear plexiglass box L43cm X W26 X H 21 with temperature-controlled heating pad and humidification | our own design and modifications | ||
| commercially available amphibian/reptile heating pad | ShenZhen XingHongChang Electric CO., LTD. ShenZhen, China | XHC-F035D | |
| Cotton tip applicators | Fisher Scientific, Waltham, MA, US | 22363156 | |
| Duodenal infusion tube – canuala | Braintree Scientific, Braintree , MA, US | MRE037 | |
| Ensure | Abbott Nutrition, Columbus, OH | ||
| Heating pad surgical platform with circulating warm water pump combination | Patterson Scientific, Waukesha, WI, US | Gaymar T/Pump Classic | |
| Hetarin Sodium Injection, USP 1,000 units/mL sterile | Mylan, Morgantown, WV, US | NDC-67457-384-31 | |
| Image J Software | National Institute of Health, Bethesda, Maryland, | ||
| Iris scissors | ROBOZ Surgical Instrument Co., Gaithersburg, MD, US | RS-5602 | |
| Isoflurane | Piramal Pharma Solutions, Riverview, MI, US | NDC 66794-017-25 | |
| Isoflurane induction apparatus and Anesthesia Apparatus | Patterson Scientific, Waukesha, WI, US | mouse induction chamber | |
| Krazy glue | Elmer's products Inc., Columbus, OH, US | KG484 | |
| Liposyn III 20% lipid injectable | Hospira Inc. Lake Forest, Illinois, USA | ||
| LS 6500 Multi-Purpose Scintillation Counter | Beckman Coulter, Brea, CA | ||
| Micro-dissecting Spring Scissors | ROBOZ Surgical Instrument Co., Gaithersburg, MD, US | RS-5602 | |
| Mouse apoB ELISA Kit | ABCAM Inc., Waltham, MA, US | ab230932-1 | |
| Needle Holder | Fine Science Tools, Foster City, CA, US | 12002-12 | |
| Retractors | Kent Scientific Co., Torrington, CT, US | SURGI-5001 | |
| Rimadyl (Carprofen) | Zoetis Inc., Kalamazoo MI, US | 4019449 | |
| Rotating table Barnstead Thermolyne | Labquake, Zürich, Switzerland | Barnstead Thermolyne | |
| SMOFlipid 20% lipid injectable emulsion, USP | Fresenius Kabi, Warrendale, PA, US | NDC-63323-820-01 | |
| Snuggle | Lomir Biomedical Inc., Notre-Dame-de-l'Île-Perrot, QC J7V 7M4, Canada | MS 02.5PM | |
| Surgical Scissors | ROBOZ Surgical Instrument Co., Gaithersburg, MD, US | RS-5912SC | |
| Suture (5-0 silk with needle) | DemeTECH, Miami Lakes, FL, US | DT-719 | |
| Transmission Electron Microscope (JEOL 1400-FLASH 120KV ) | JEOL, Peabody, MA | ||
| Triglyceride Assay Kit | Randox Laboratories, Crumlin, United Kingdom | TR210 | |
| ULTIMA GOLD XR Scintillation Fluid | Perkin Elmer, Hebron, KY, US | 6013119 | |
| Ultracentrifuge, rotor S100AT4-497 | SORVALL RC M120 GX |
References
- Natale, G., Bocci, G., Ribatti, D. Scholars and Scientists in the History of the Lymphatic System. Journal of Anatomy. 231 (3), 417-429 (2017).
- Loukas, M., et al. The lymphatic system: A historical perspective. Clinical Anatomy. 24 (7), 807-816 (2011).
- Suy, R., Thomis, S., Fourneau, I. The discovery of the lymphatic system in the seventeenth century. Part II: The discovery of Chyle vessels. Acta Chirurgica Belgica. 116 (5), 325-331 (2016).
- Azzali, G. Historical notes on the lymphatic vascular system. Acta Bio-medica de L’Ateneo Parmense. 61 (3-4), 113-125 (1990).
- Irschick, R., Siemon, C., Brenner, E. The history of anatomical research of lymphatics – From the ancient times to the end of the European Renaissance. Annals of Anatomy. 223, 49-69 (2019).
- Swartz, M. A. The physiology of the lymphatic system. Advanced Drug Delivery Reviews. 50 (1-2), 3-20 (2001).
- Deitch, E. A. Gut lymph and lymphatics: A source of factors leading to organ injury and dysfunction. Annals of the New York Academy of Sciences. 1207, 103-111 (2010).
- Tso, P., Gollamudi, S. R. Pluronic L-81: A potent inhibitor of the transport of intestinal chylomicrons. American Journal of Physiology. 247, 32-36 (1984).
- Tso, P., Karlstad, M. D., Bistrian, B. R., DeMichele, S. J. Intestinal digestion, absorption, and transport of structured triglycerides and cholesterol in rats. American Journal of Physiology. 268, 568-577 (1995).
- Mansbach, C. M., Dowell, R. F., Pritchett, D. Portal transport of absorbed lipids in rats. American Journal of Physiology. 261, 530-538 (1991).
- Bogunovic, M., et al. Origin of the lamina propria dendritic cell network. Immunity. 31 (3), 513-525 (2009).
- Balmer, M. L., et al. The liver may act as a firewall mediating mutualism between the host and its gut commensal microbiota. Science Translational Medicine. 6 (237), (2014).
- Ji, Y., Sakata, Y., Tso, P. Nutrient-induced inflammation in the intestine. Current Opinion in Clinical Nutrition & Metabolic Care. 14 (4), 315-321 (2011).
- Ji, Y., Sakata, Y., Yang, Q., Tso, P. Intestinal mucosal mast cells is activated by fat absorption. Gastroenterology. 140 (5), (2011).
- Wang, F., et al. Chronic high-fat feeding increases mixed meal-induced incretin secretion in Sprague-Dawley rats. American Journal of Physiology-Gastrointestinal and Liver Physiology. 309 (10), 807-815 (2015).
- Kindel, T. L., Yoder, S. M., D’Alessio, D. A., Tso, P. The effect of duodenal-jejunal bypass on glucose-dependent insulinotropic polypeptide secretion in Wistar rats. Obesity Surgery. 20 (6), 768-775 (2010).
- Kohan, A. B., Yoder, S. M., Tso, P. Using the lymphatics to study nutrient absorption and the secretion of gastrointestinal hormones. Physiology & Behavior. 105 (1), 82-88 (2011).
- Yoder, S. M., Yang, Q., Kindel, T. L., Tso, P. Stimulation of incretin secretion by dietary lipid: Is it dose dependent. American Journal of Physiology-Gastrointestinal and Liver Physiology. 297 (2), 299-305 (2009).
- Wang, Y., et al. Chylomicrons promote intestinal absorption and systemic dissemination of dietary antigen (ovalbumin) in mice. PLoS One. 4 (12), 8442 (2009).
- Vors, C., et al. Postprandial endotoxemia linked with chylomicrons and lipopolysaccharides handling in obese versus lean men: A lipid dose-effect trial. Journal of Clinical Endocrinology and Metabolism. 100 (9), 3427-3435 (2015).
- Ghoshal, S., Witta, J., Zhong, J., de Villiers, W., Eckhardt, E. Chylomicrons promote intestinal absorption of lipopolysaccharides. Journal of Lipid Research. 50 (1), 90-97 (2009).
- Jandacek, R. J., Rider, T., Keller, E. R., Tso, P. The effect of olestra on the absorption, excretion and storage of 2,2′,5,5′ tetrachlorobiphenyl; 3,3′,4,4′ tetrachlorobiphenyl; and perfluorooctanoic acid. Environment International. 36 (8), 880-883 (2010).
- Jandacek, R. J., Tso, P. Organochlorine compounds are absorbed via lymph and portal circulation. The FASEB Journal. 22, (2008).
- Utermann, G., Beisiegel, U. Apolipoprotein A-IV: A protein occurring in human mesenteric lymph chylomicrons and free in plasma. Isolation and quantification. European Journal of Biochemistry. 99 (2), 333-344 (1979).
- Bollman, J. L., Cain, J. C., Grindlay, J. H. Techniques for the collection of lymph from the liver, small intestine, or thoracic duct of the rat. Journal of Laboratory and Clinical Medicine. 33 (10), 1349-1352 (1948).
- Tso, P., Balint, J. A., Rodgers, J. B. Effect of hydrophobic surfactant (pluronic L-81) on lymphatic lipid transport in the rat. American Journal of Physiology. 239 (5), 348-353 (1980).
- Liu, M., et al. Sexual dimorphism in intestinal absorption and lymphatic transport of dietary lipids. Journal of Physiology. 599 (22), 5015-5030 (2021).
- Manolas, K. J., et al. Lymph, pancreatic and gastrointestinal hormones in response to feeding in the conscious pig. European Surgical Research. 17 (5), 324-332 (1985).
- Lascelles, A. K., Morris, B. Surgical techniques for the collection of lymph from unanaesthetized sheep. Quarterly Journal of Experimental Physiology and Cognate Medical Sciences. 46, 199-205 (1961).
- Jensen, L. T., Olesen, H. P., Risteli, J., Lorenzen, I. External thoracic duct-venous shunt in conscious pigs for long term studies of connective tissue metabolites in lymph. Laboratory Animal Science. 40 (6), 620-624 (1990).
- Edwards, G. A., Porter, C. J., Caliph, S. M., Khoo, S. M., Charman, W. N. Animal models for the study of intestinal lymphatic drug transport. Advanced Drug Delivery Reviews. 50 (1-2), 45-60 (2001).
- Yasunaga, K., Saito, S., Zhang, Y. -. L., Hernandez-Ono, A., Ginsberg, H. N. Effects of triacylglycerol and diacylglycerol oils on blood clearance, tissue uptake, and hepatic apolipoprotein B secretion in mice. Journal of Lipid Research. 48 (5), 1108-1121 (2007).
- Curtin, A., et al. Elevated triglyceride-rich lipoproteins in diabetes. A study of apolipoprotein B-48. Acta Diabetologica. 33 (3), 205-210 (1996).
- Gordts, P. L. S. M., et al. ApoC-III inhibits clearance of triglyceride-rich lipoproteins through LDL family receptors. Journal of Clinical Investigation. 126 (8), 2855-2866 (2016).
- Ginsberg, H. N., et al. Apolipoprotein B metabolism in subjects with deficiency of apolipoproteins CIII and AI. Evidence that apolipoprotein CIII inhibits catabolism of triglyceride-rich lipoproteins by lipoprotein lipase in vivo. The Journal of Clinical Investigation. 78 (5), 1287-1295 (1986).
- Ormai, S., Palkovits, M. Size distribution of lymphocytes in the thoracic duct lymph in rat after lymphocyte mobilization induced by polymethacrylic acid. Blut Zeitschrift für die Gesamte Blutforschung. 24 (3), 161-165 (1972).
- Luchoomun, J., Hussain, M. M. Assembly and secretion of chylomicrons by differentiated Caco-2 cells. Nascent triglycerides and preformed phospholipids are preferentially used for lipoprotein assembly. Journal of Biological Chemistry. 274 (28), 19565-19572 (1999).
- Nollevaux, G., et al. Development of a serum-free co-culture of human intestinal epithelium cell-lines (Caco-2/HT29-5M21). BMC Cell Biology. 7 (1), 20 (2006).
- Li, D., Dong, H., Kohan, A. B. The Isolation, Culture, and Propagation of Murine Intestinal Enteroids for the Study of Dietary Lipid Metabolism. Organoids. Methods in Molecular Biology. 1576, 195-204 (2019).
- Jattan, J. J., et al. Using murine-derived primary intestinal enteroids for studies of dietary triglyceride absorption and lipoprotein synthesis, and to determine the role of intestine-specific ApoC-III in the intestine. Journal of Lipid Research. 58 (5), 853-865 (2017).
- Haring, E., et al. Bile acids regulate intestinal antigen presentation and reduce graft-versus-host disease without impairing the graft-versus-leukemia effect. Haematologica. 106 (8), 2131-2146 (2021).
- Date, S., Sato, T. Mini-gut organoids: Reconstitution of the stem cell niche. Annual Review of Cell and Developmental Biology. 31 (1), 269-289 (2015).
- Deacon, C. F. Circulation and degradation of GIP and GLP-1. Hormone and Metabolic Research. 36 (11-12), 761-765 (2004).
- Dedousis, N., Teng, L., Kanshana, J. S., Kohan, A. B. A single-day mouse mesenteric lymph surgery in mice: an updated approach to study dietary lipid absorption, chylomicron secretion, and lymphocyte dynamics. J Lipid Res. 63 (11), 100284 (2022).
- Li, D., et al. Intestinal basolateral lipid substrate transport is linked to chylomicron secretion and is regulated by ApoC-III. Journal of Lipid Research. 60 (9), 1503-1515 (2019).
- Glatzle, J., et al. Chylomicron components mediate intestinal lipid-induced inhibition of gastric motor function. American Journal of Physiology-Gastrointestinal and Liver Physiology. 282 (1), 86-91 (2002).
- Huang, J., Sloop, C. H., Roheim, P. S., Wong, L. Lipoprotein lipase and hepatic triacylglycerol lipase activities in peripheral and skeletal muscle lymph. Arteriosclerosis, Thrombosis, and Vascular Biology. 10 (5), 720-726 (1990).
- Wang, F., et al. Overexpression of apolipoprotein C-III decreases secretion of dietary triglyceride into lymph. Physiological Reports. 2 (3), 00247 (2014).
- Kohan, A. B., et al. Apolipoprotein A-IV regulates chylomicron metabolism-Mechanism and function. American Journal of Physiology. Gastrointestinal and Liver Physiology. 302 (6), 628-636 (2012).
- Hayashi, H., et al. Fat feeding increases size, but not number, of chylomicrons produced by small intestine. American Journal of Physiology. 259 (5), 709-719 (1990).
- Tso, P., Balint, J. A. Formation and transport of chylomicrons by enterocytes to the lymphatics. American Journal of Physiology. 250 (6), 715-726 (1986).
- Bhattacharya, S., Redgrave, T. G. The content of apolipoprotein B in chylomicron particles. Journal of Lipid Research. 22 (5), 820-828 (1981).
- Björkegren, J., Karpe, F., Milne, R. W., Hamsten, A. Differences in apolipoprotein and lipid composition between human chylomicron remnants and very low density lipoproteins isolated from fasting and postprandial plasma. Journal of Lipid Research. 39 (7), 1412-1420 (1998).
- Martins, I. J., Sainsbury, A. J., Mamo, J. C., Redgrave, T. G. Lipid and apolipoprotein B48 transport in mesenteric lymph and the effect of hyperphagia on the clearance of chylomicron-like emulsions in insulin-deficient rats. Diabetologia. 37 (3), 238-246 (1994).
- Mar, R., et al. Association of the APOLIPOPROTEIN A1/C3/A4/A5 gene cluster with triglyceride levels and LDL particle size in familial combined hyperlipidemia. Circulation Research. 94 (7), 993-999 (2004).
- Kassis, T., et al. Dual-channel in-situ optical imaging system for quantifying lipid uptake and lymphatic pump function. Journal of Biomedical Optics. 17 (8), 086005 (2012).
- Nauli, A. M., et al. Chylomicrons produced by Caco-2 cells contained ApoB-48 with diameter of 80-200 nm. Physiological Reports. 2 (6), 192-196 (2014).
- Drover, V. A., et al. CD36 deficiency impairs intestinal lipid secretion and clearance of chylomicrons from the blood. Journal of Clinical Investigation. 115 (5), 1290-1297 (2005).
- Kohan, A. B., et al. Is apolipoprotein A-IV rate limiting in the intestinal transport and absorption of triglyceride. American Journal of Physiology-Gastrointestinal and Liver Physiology. 304 (12), 1128-1135 (2013).
- De Lisle, R. C. Altered transit and bacterial overgrowth in the cystic fibrosis mouse small intestine. American Journal of Physiology-Gastrointestinal and Liver Physiology. 293 (1), 104-111 (2007).
- Debas, H. T., Farooq, O., Grossman, M. I. Inhibition of gastric emptying is a physiological action of cholecystokinin. Gastroenterology. 68 (5), 1211-1217 (1975).
- Spiller, R. C., et al. Further characterisation of the ‘ileal brake’ reflex in man–Effect of ileal infusion of partial digests of fat, protein, and starch on jejunal motility and release of neurotensin, enteroglucagon, and peptide YY. Gut. 29 (8), 1042-1051 (1988).
- Battle, M. A., et al. GATA4 is essential for jejunal function in mice. Gastroenterology. 135 (5), 1676-1686 (2008).
- Morgan, R. G., Borgström, B. The mechanism of fat absorption in the bile fistula rat. Quarterly Journal of Experimental Physiology and Cognate Medical Sciences. 54 (2), 228-243 (1969).
- Davidson, N. O., Kollmer, M. E., Glickman, R. M. Apolipoprotein B synthesis in rat small intestine: Regulation by dietary triglyceride and biliary lipid. Journal of Lipid Research. 27 (1), 30-39 (1986).
- Bouchi, R., et al. FOXO1 inhibition yields functional insulin-producing cells in human gut organoid cultures. Nature Communications. 5, 4242 (2014).
- Bijvelds, M. J. C., et al. Fat absorption in cystic fibrosis mice is impeded by defective lipolysis and post-lipolytic events. American Journal of Physiology-Gastrointestinal and Liver Physiology. 288 (4), 646-653 (2005).
- Struyvenberg, M. R., Martin, C. R., Freedman, S. D. Practical guide to exocrine pancreatic insufficiency – Breaking the myths. BMC Medicine. 15 (1), 29 (2017).
- Tickell, K. D., Atlas, H. E., Walson, J. L. Environmental enteric dysfunction: A review of potential mechanisms, consequences and management strategies. BMC Medicine. 17 (1), 181 (2019).
- Lo, C. M., et al. Cholecystokinin knockout mice are resistant to high-fat diet-induced obesity. Gastroenterology. 138 (5), 1997-2005 (2010).

