Coral Reef Arks: An In Situ Mesocosm and Toolkit for Assembling Reef Communities
Summary
Moored midwater geodesic structures called Coral Arks provide a modular, scalable, and vertically adjustable research platform that can be used to build, monitor, and perturb coral reef communities in previously inoperative areas, including offshore.
Abstract
Coral reefs thrive and provide maximal ecosystem services when they support a multi-level trophic structure and grow in favorable water quality conditions that include high light levels, rapid water flow, and low nutrient levels. Poor water quality and other anthropogenic stressors have caused coral mortality in recent decades, leading to trophic downgrading and the loss of biological complexity on many reefs. Solutions to reverse the causes of trophic downgrading remain elusive, in part because efforts to restore reefs are often attempted in the same diminished conditions that caused coral mortality in the first place.
Coral Arks, positively buoyant, midwater structures, are designed to provide improved water quality conditions and supportive cryptic biodiversity for translocated and naturally recruited corals to assemble healthy reef mesocosms for use as long-term research platforms. Autonomous Reef Monitoring Structures (ARMS), passive settlement devices, are used to translocate the cryptic reef biodiversity to the Coral Arks, thereby providing a “boost” to natural recruitment and contributing ecological support to the coral health. We modeled and experimentally tested two designs of Arks to evaluate the drag characteristics of the structures and assess their long-term stability in the midwater based on their response to hydrodynamic forces.
We then installed two designs of Arks structures at two Caribbean reef sites and measured several water quality metrics associated with the Arks environment over time. At deployment and 6 months after, the Coral Arks displayed enhanced metrics of reef function, including higher flow, light, and dissolved oxygen, higher survival of translocated corals, and reduced sedimentation and microbialization relative to nearby seafloor sites at the same depth. This method provides researchers with an adaptable, long-term platform for building reef communities where local water quality conditions can be adjusted by altering deployment parameters such as the depth and site.
Introduction
Across the globe, coral reef ecosystems are undergoing transitions from high-biodiversity, coral-dominated benthic communities to lower-diversity communities dominated by turf- and fleshy macroalgae1,2,3. Decades of progress in characterizing the mechanisms of coral reef degradation have revealed how links between microbial and macro-organismal communities enhance the pace and severity of these transitions. For example, the overfishing of reefs by human populations initiates a trophic cascade in which excess photosynthetically derived sugars from ungrazed algae shunt energy into the reef microbial communities, thus driving pathogenesis and causing coral decline4,5,6. This trophic downgrading is reinforced by the loss of biodiversity on reefs that results from water quality decline7,8. Mesocosm-level experiments can be used to better understand and mitigate the trophic downgrading of coral reef communities by enhancing biodiversity and improving water quality, but logistical challenges make these studies difficult to implement in situ.
A consequence of trophic downgrading on reefs is the widespread loss of cryptic biodiversity, much of which remains uncharacterized7,9. Corals rely on a diverse suite of cryptic reef organisms ("cryptobiota") that support their health by playing integral roles in predator defense10, cleaning11, grazing competing algae12,13, and the regulation of reef water chemistry14,15. Until recently and due to the methodological limitations of visual surveys, reef cryptobiota have been underrepresented and poorly understood in the context of reef ecology, and they are, thus, rarely considered in efforts to restore or rebuild reefs. In the past decade, the use of standardized settlement units called Autonomous Reef Monitoring Structures (ARMS) combined with high-throughput sequencing approaches has enabled the better collection and characterization of reef cryptobiota16,17. ARMS passively recruit representatives of almost all known coral reef biodiversity and have helped reveal numerous functional roles of cryptic organisms in reef-scale processes9,18,19,20,21,22,23. These settlement units, therefore, provide a mechanism to translocate cryptic reef biota alongside corals in order to assemble more intact reef communities with biologically mediated mechanisms, such as grazing, defense, and enhancement of local water quality, that are essential to maintaining the trophic structure.
Coral-dominated reefs thrive in high-light, low-nutrient, and well-oxygenated environments. Human activities such as urbanization, agriculture, and overfishing have reduced the water quality on many coral reefs by increasing the sediment, nutrients, metals, and other compounds in runoff24,25 and by altering biogeochemical cycling26. In turn, these activities degrade reef communities through smothering, energy depletion, the delivery of pollutants associated with sedimentation27,28, enhancing the growth of macroalgae that compete with corals29, increasing the abundance of microbial pathogens6,30,31, and creating hypoxic zones that kill cryptic invertebrates32,33. These and other "local impacts" are compounded by regional and global changes in ocean conditions, including increasing temperatures and decreasing pH, further worsening the conditions for corals and other reef organisms34,35. At the benthic-water interface, specifically, the respiratory and photosynthetic dynamics of benthic communities cause diel fluctuations in the pH and dissolved oxygen, which become more pronounced on highly degraded reefs, thus creating conditions that benthic invertebrates cannot tolerate32,36,37,38. Providing appropriate water quality conditions is, therefore, essential for assembling functioning reef communities, but this remains challenging because an increasing number of reefs are trapped in various states of degradation.
Many of the challenges faced by corals and foundational cryptic taxa on the benthos may be overcome via relocation to the midwater, defined here as the water column setting between the ocean surface and the seafloor. In the midwater environment, water quality is improved39,40, sedimentation is reduced, and the distance from the seafloor dampens fluctuations in the parameters associated with benthic metabolism. These characteristics are improved further by moving offshore, where land-based anthropogenic impacts, such as terrestrially derived runoff, become increasingly diluted with distance from the coast. Here, we introduce and provide protocols to build, deploy, and monitor Coral Reef Arks, an approach that leverages improved water quality conditions in the midwater and incorporates cryptic biodiversity on anchored, positively buoyant structures for the assembly of coral reef communities.
Coral Reef Arks systems, or "Arks," are comprised of two primary components: (1) a suspended rigid geodesic platform elevated above the benthos and (2) organism-covered or "seeded" ARMS that translocate reef cryptobiota from nearby benthic areas, thereby supplementing the natural recruitment processes to provide the translocated corals with a more diverse and functional reef community. A geodesic structure was selected to maximize the strength and minimize the building material (and, thus, the weight), as well as to create an internal, turbulent flow environment analogous to the reef matrix.
Two designs of Arks were successfully installed at two Caribbean field sites and are currently being used for research into reef community establishment and ecological succession (Figure 1). Coral Arks structures are intended to be long-term research platforms, and as such, a primary focus of this manuscript is to describe protocols to site, install, monitor, and maintain these structures to maximize their stability and longevity in the midwater environment. A combination of modeling and in-water testing was used to evaluate the drag characteristics of the structures and adjust the design to withstand the anticipated hydrodynamic forces. After installation, reef communities were established on the Arks and on nearby benthic control sites at the same depth through a combination of active translocation (corals and seeded ARMS units) and natural recruitment. Water quality conditions, microbial community dynamics, and coral survival on the Arks were documented at several time points throughout the early successional period and compared against the benthic control sites. To date, the conditions associated with the midwater Coral Arks environment have been consistently more favorable for corals and their associated cryptic consortia relative to the neighboring benthic control sites at the same depths. The methods below describe the steps required to replicate the Coral Arks approach, including how to select sites and design and deploy Coral Arks structures. Suggested approaches for monitoring Coral Arks are included in Supplemental File 1.
Protocol
NOTE: Detailed information regarding the manufacture, deployment, and monitoring of ARMS and Coral Arks structures, including technical drawings, diagrams, and photos, are provided in Supplemental File 1. Sections of the protocol involving underwater work, including the installation of Arks and ARMS structures, are recommended to be conducted by a team of three divers (on SCUBA) and two surface support personnel.
1. ARMS assembly and deployment
NOTE: ARMS are approximately 1 ft3 (30 cm3) structures made from PVC or limestone base materials that mimic the three-dimensional complexity of reef hardbottom substrates. Table 1 discusses two designs for ARMS given different project considerations. ARMS are recommended to be deployed for 1-2 years prior to transfer to Arks to maximize the colonization by cryptic biota.
- PVC ARMS
NOTE: The off-the-shelf components referred to in this protocol (and listed in the Table of Materials) are described using imperial units. The fabricated materials are described using metric units. Detailed fabrication instructions, including technical drawings for the manufacture of the components, are provided in Section 1 of Supplemental File 1.- Assembly
- Insert four 1/4 in-20, 8 in long, hex-head bolts through the center holes on a 1/2 in thick PVC baseplate; then, invert it such that the bolts face up vertically.
- Add a nylon spacer to each bolt, and then add a 1/4 in thick, PVC 9 in x 9 in plate. This creates an open layer between the baseplate and the first stacking plate.
- Add a long cross spacer onto two bolts in opposite corners, and then add two short cross spacers onto the remaining bolts such that an "X" is formed. Add another PVC stacking plate to create a closed layer.
- Repeat step 1.1.1.2 and step 1.1.1.3, alternating between open and closed layers, until seven to nine plate layers have been added to the bolts (Supplemental File 1-Figure S5).
- Add a washer, a hex nut, and a nylon insert locknut to the top of each bolt, and tighten down securely.
- For deployment, transport the assembled PVC ARMS to the target deployment site, covering the ARMS with 100 μm mesh during the transfer to retain small mobile invertebrates (Supplemental File 1-Figure S6). Locate a patch of reef hardbottom substrate in close proximity to healthy coral reef communities.
NOTE: The specific deployment sites should be selected with consideration of the local regulations and permit stipulations, such as avoiding the critical habitats for Endangered Species Act listed species in US waters.- Using 3 in lengths of 1/2 in rebar and a mallet, secure the ARMS to the benthos at all four corners by pounding the rebar, slightly angled outward, into the base limestone such that the rebar generates tension against the edge of the baseplate (Figure 2A, B).
- Alternatively, connect the chains of the ARMS using heavy-duty cable ties, and anchor the ends of the chains with hardened concrete bags (Figure 2C and Supplemental File 1-Figure S6).
- Assembly
- Limestone ARMS
- For assembly, begin with 12 in x 12 in unfinished limestone or travertine tiles (Figure 2). Identify the desired complexity of the limestone ARMS interior.
NOTE: It is recommended to use 2 cm3 cubes. Alternative designs and considerations are provided in Section 2 of Supplemental File 1.- Using a wet tile saw, cut several unfinished tiles into 2 cm2 square spacers (~250).
- Cut travertine tiles to the desired shape for the ARMS layers. Similar to the PVC ARMS, use 12 in x 12 in squares, and layer them with spacers to form 1 ft3 cubes (Supplemental File 1-Figure S8).
- Using a two-part, non-toxic marine grade epoxy, glue the smaller travertine pieces to a larger travertine layering plate along a pre-drawn grid pattern.
- Prepare several layers that, when stacked together, achieve the desired ARMS height. Allow the epoxy to cure based on the manufacturer's recommendations.
- Assemble the ARMS stacking plates using epoxy to glue each layer to the one above it.
NOTE: The ARMS height will vary based on the desired weight and internal complexity. A final size of approximately 1 ft3 is recommended. - Allow the epoxy to cure out of direct sunlight for 24 h before deployment.
- For deployment, transport the assembled Limestone ARMS to the target deployment site. Locate a patch of reef hardbottom substrate in close proximity to healthy coral reef communities.
NOTE: The specific deployment sites should be selected with consideration of the local regulations and permit stipulations, such as avoiding the critical habitats of Endangered Species Act listed species in US waters.- Transport the ARMS to the benthos using a milk crate and lift bag. Wedge the Limestone ARMS into dead reef matrix (live rock). Avoid sandy bottom habitats and those heavily colonized by turf algae or benthic cyanobacterial mats.
- Place the Limestone ARMS next to rocky overhangs and outcrops to protect them from wave action and storm surges.
- For assembly, begin with 12 in x 12 in unfinished limestone or travertine tiles (Figure 2). Identify the desired complexity of the limestone ARMS interior.
2. Coral Arks assembly and deployment
NOTE: Table 2 discusses the design considerations of Coral Arks given different project parameters. The dimensions of the sub-elements (struts, hubs, platforms, mooring components, and positive buoyancy) can be modified depending on the desired size and weight of the final Coral Ark structures.
- Installation of the anchoring system
NOTE: Select the anchoring system based on site- and project-specific considerations such as Ark design, storm frequency, bottom type, site exposure, duration of the project, and anticipated forces due to drag, currents, and buoyancy. See PADI41 for insights into mooring system selection.- Use sand screws in sandy bottom and loose rubble habitats.
- Transport the sand screws to the benthos. Standing the sand screw upright, twist and bury the sand screw until the first disk has been covered in sand or loose rubble.
- Place a 5 feet long metal turning bar through the eye of the anchor such that the majority of the turning bar sticks out of one side of the eye.
- Walking or swimming in circles on the benthos, screw the sand screw into the substrate until only the eye remains sticking out of the benthos (Supplemental File 1-Figure S20).
- Install three sand screws in a triangular pattern, connected by a chain bridle, for increased holding power (Supplemental File 1-Figure S20).
- Use Halas anchors in hardbottom and carbonate base rock habitats.
- Transport 9-12 in eyebolts and a submersible drill (electric or pneumatic) to the anchor site.
- Use the submersible drill and a 1 in diameter masonry hole saw to drill a 9 in deep and 1 in wide hole into the base rock. Periodically clean out excess substrate from the hole using a turkey baster.
- Fill the hole with Portland cement or marine-grade epoxy. Push the eyebolt shaft into the hole, and fill the remaining gaps with cement or epoxy.
- Let the cement/epoxy cure for 5 days.
- For increased holding power, install three Halas anchors in a triangular pattern, connected by a chain bridle.
- Use block-type mooring at sites with existing mooring blocks or heavy debris elements.
NOTE: The installation of a new mooring block requires commercial-grade installation equipment such as a barge-mounted crane and is not recommended for projects with a smaller scope.- Attach the mooring system to existing heavy debris elements (sunken vessels, engine blocks) or to existing mooring block eyes via hardware and tackle.
- Ensure the metal mooring components are made from similar metals and protected against galvanic corrosion using sacrificial anodes.
- Use sand screws in sandy bottom and loose rubble habitats.
- The 1V frequency structure (Two Platform)
NOTE: Detailed fabrication instructions, including technical drawings for the manufacture of the components, are provided in Section 4 of Supplemental File 1. The off-the-shelf components referred to in this protocol (and listed in the Table of Materials) are described using imperial units.- Assembly of the 1V geodesic frame
- Screw a 1/4-20 stainless steel hex nut onto a 1/4-20 2.5 in stainless steel bolt 3/4 of the way to the top of the bolt. Insert the bolt into one of the inside-facing holes on the strut.
- Secure a locknut onto the other side of the screw, tightening it down until it mates securely with the PVC to prevent the hub from sliding down the length of the strut.
- Repeat for the opposite side of the strut and for the remaining 29 struts.
- Push the end of each strut through one of the holes in the hubs and fasten another bolt through the outer hole on the strut, finishing with a locknut to prevent the strut from sliding out of the hub (Supplemental File 1-Figure S24).
- Repeat for all five struts in one hub, and then continue to add hubs and struts until the geodesic sphere is assembled (Supplemental File 1-Figure S24).
- Unspool the 1/8 in stainless steel wire rope and begin threading it through the struts. Create 12 loops, about the size of a silver dollar, out of nylon cable ties-one for each hub. As the wire rope is threaded through the struts, pass the rope through the zip tie loop at the hub, and then continue to the next strut.
NOTE: Some struts will be repeated. - Continue threading until the wire rope has been threaded through all the struts, connected in the middle of each vertex by the zip tie loop.
- Thread the cable back to the starting point. Using pliers, pull the zip tie loops to shrink them to the smallest size possible, bringing the lengths of wire rope close together. Fit a 1/2 in stainless steel cable clamp onto all the wire rope lengths and tighten down securely.
- Repeat for all the vertices of the structure.
- Mate the beginning length of the wire rope with the end length, and clamp these together using three 1/2 in cable clamps.
NOTE: The wire rope (breaking strength: 2,000 lb) should now support most of the load placed on the structure, strengthening it considerably. - Add the rigging system, which is composed of two lengths of 3/8 in stainless steel cable hydraulically swaged onto an eye at each end. Fit the PVC endcaps between the swages such that the cable passes through the entire Ark length, with eyes at the top and bottom for the mooring/buoy line attachments. A turnbuckle system in the middle connects the two lengths of stainless cable.
- Pass the bottom ends of the cable through the top and bottom of the Ark, fitting the endcaps onto the top and bottom hubs using a mallet. Screw the eyebolts into the turnbuckle and tighten until there is sufficient tension on the structure to make the system rigid (Supplemental File 1-Figure S24).
- Add each molded fiberglass grating, cut into two half-pentagons, into the Ark interior using heavy-duty 250 lb zip ties to anchor the sides of the platform to the Ark struts (Supplemental File 1-Figure S24).
- Underneath the structure, place one length of fiberglass I-beam so that it joins both halves of the fiberglass platform. Secure to the underside of the platform using two 1/4 in-20 stainless steel U-bolts.
- Repeat for the other four I-beams, equally distributing them down the length of the platform. This joins and supports the two halves of the platform, creating a full pentagon.
- Tighten the heavy-duty zip ties at the edges of the platform, and clip off the excess. At the end of this step, the internal platform is firmly integrated into the Ark structure (Supplemental File 1-Figure S24).
- Use stainless steel mousing wire to mouse the ends of the turnbuckle and all the shackles. At the end of this step, the Ark will have two integrated platforms, top and bottom attachments for hardware attachment, and a central cable that bears the bulk of the tension force placed on the structures via anchoring and positive buoyancy.
- Attachment of the mooring line to the geodesic frame
NOTE: Mooring systems should be designed such that the breaking strength of all the individual mooring components exceeds the maximum load expected due to ambient and extreme environmental conditions. See the representative results for a description of the use of hydrodynamic modeling in mooring system design. It is recommended to distribute the load across multiple attachment points on the Ark and on the seafloor anchoring system, as this adds redundancy to the system in case of the failure of individual elements.- Design the mooring lines and hardware to ensure secure connections between the Ark base and the anchor system (see Figure 1 for an example).
NOTE: It is recommended to design the mooring system such that the midline of the Ark structure is positioned at a 30 m depth. - Connect the top of a double-spliced line to the base eye of the Ark with a shackle. Connect a high-strength, stainless steel swivel shackle to the base of this line (Figure 1 and Supplemental File 1-Figure S25).
- Connect the top of a double-spliced line to the base of the swivel shackle. The bottom of this line will connect to the anchor system (Figure 1 and Supplemental File 1-Figure S25).
- Design the mooring lines and hardware to ensure secure connections between the Ark base and the anchor system (see Figure 1 for an example).
- Transportation of the Ark to the deployment site
- Transport the Ark via a flatbed truck to a beach adjacent to the deployment site (nearshore deployment with sand entry) or to a boat launch site (vessel deployment).
- Attach a 220 lb lift bag to the top stainless eye of the Ark using a 1/2 in shackle.
- Attach a mooring line, including the hardware for attaching to the seafloor anchor, to the base of the Ark.
- For deployment from a vessel lacking an A-frame or davit, load the Ark onto the vessel such that it can be easily rolled off the boat and into the water (avoiding bows with high gunnels or sterns with outboard engines).
- For deployment from the shore, roll the Ark into the water until a sufficient depth at which the lift bag can be filled with air (Figure 3).
- Swim, tow, or transport the Ark to the anchoring site at the surface (Figure 3).
- Attachment of the Arks to the mooring system
NOTE: At this stage, the Ark system is floating at the surface above the anchoring site with a lift bag. The following tasks are performed underwater on SCUBA and require a team of at least three divers.- Slowly venting the air from the lift bag, perform a controlled descent to the anchoring system.
- Attach the mooring hardware at the base of the Ark to the anchoring system.
- Increase the positive buoyancy of the Arks system by filling the lift bag with air, and inspect the monitoring components for structural integrity. Ensure the shackles are seated properly and that the anchors are firmly in place. Use mousing wire to mouse all the shackles.
- Connect the eye of a short, double-spliced length of line to the top eye of the Arks system with a shackle. Connect a polyform, inflatable mooring buoy to the other end of this line with a shackle (Supplemental File 1-Figure S25).
- Fill the mooring buoy with air using a standard low-pressure air nozzle adapter attached to a pony bottle of compressed air until it is approximately 75% full of air.
- Slowly vent the air from the lift bag, and remove it from the system.
- Add larger or more numerous mooring buoys for Arks systems utilizing limestone ARMS or to compensate for biological mass accumulation.
- Attachment of the ARMS to the Arks
- Retrieve the ARMS from the seeding location, and place into milk crates lined with 100 µm mesh to prevent the loss of small mobile invertebrates living within the ARMS.
- Transfer the ARMS to the Arks sites in tubs of shaded, cool seawater.
- Place the ARMS on the top or bottom platform of the Arks, evenly distributing the weight across the platform.
- Pass heavy-duty cable ties through both the molded fiberglass platform and the base of the PVC or Limestone ARMS and tighten to secure the ARMS to the Ark frame (Supplemental File 1-Figure S25).
- Assembly of the 1V geodesic frame
- The 2V frequency structure (Shell)
NOTE: Detailed fabrication instructions, including technical drawings for the manufacture of the components, are provided in Section 3 of Supplemental File 1.- Assembly of the 2V geodesic frame
- Assemble the Ark mounting framework according to the provided guide from VikingDome (Supplemental File 1-Figure S11).
- Add a washer to a 2.5 in long, 10/32 stainless bolt. Insert the bolt through one of the two holes at the end of a strut, adding a STAR connector to the inside face (hole specific to S1 or S2 struts), and fasten with a locknut.
- Repeat for the second bolt hole. Continue without tightening the locknuts until the structure is fully assembled (Supplemental File 1-Figure S12).
- Tighten down the Ark mounting framework. At the end of step 2.3.1.1, the strut-STAR connections will be loose and malleable. Begin tightening the locknuts using a socket wrench (10 mm or 3/8 in socket) and a Philips head screwdriver.
- Continue throughout the structure until all the locknuts have been tightened, with the nylon insert of the locknut fully engaged on the threads of the bolts.
- Add pad eyes for the attachment of the mooring bridle. Add a pad eye to the stainless S1 strut at the base of the Ark, and secure with four 3 in pan head stainless steel bolts.
- Add 1/4 in-20 locknuts and tighten down. Repeat for a total of five mooring connection points (Supplemental File 1-Figure S17).
- Mount 10 ARMS baseplates to the middle-facing N2 STAR connectors. Place a 3 in pan head bolt through the center hole on the ARMS baseplate. Add a grey PVC standoff to the bolt shaft and place it through the center hole of the N2 STAR connector, with the baseplate inside the structure. Add a washer and a locknut and tighten down.
- Add two brackets and use four 3 1/4 in hex head bolts and locknuts to secure the ARMS baseplate to the struts. Tighten down all the locknuts. Maintain the same orientation for all the ARMS baseplates (Supplemental File 1-Figure S15).
- Mount 20 coral plate baseplates to the top-facing struts. Place four 3 in hex head bolts through the holes on the coral plate baseplate and fasten to the strut using a bracket and a locknut. Repeat for the other side. Tighten the locknuts to secure (Supplemental File 1-Figure S15).
- Add a central rod and trawl float to the central spine of the Ark. Insert an 8 feet long, unthreaded fiberglass rod into the STAR connectors modified with a welded pipe segment at the base of the Ark. Add a 1 in washer and an unmodified trawl float onto the unthreaded fiberglass rod inside the structure. Finish inserting the rod through the top STAR connector of the Ark.
- Fit the bolts through the metal tube on the modified STAR connectors and the locknuts to the lock rod inside the Ark. Add a green tube clamp snugly below the trawl float (top of the Ark), and tighten down.
- Mount modified trawl floats inside the top facing N2 and N1 STAR connectors modified with a 1 in center hole. Add a fiberglass washer to the longer end of the exposed threaded fiberglass rod.
- Secure through the modified STAR connector hole so that trawl float faces inside the structure. Add another fiberglass washer and a fiberglass hex nut. Tighten down using a wrench and by twisting the floats (Supplemental File 1-Figure S16).
- Attachment of the mooring system to the geodesic frame
- Design the mooring lines and hardware to ensure secure connections between the Ark base and the anchor system (see Figure 1 for example).
NOTE: It is recommended to design the mooring system such that the midline of the Ark structure is positioned at a 10 m depth. - Connect each pad eye at the base of the Ark structure to the spliced eye at the end of a double-spliced length of a 3/4 in spectra line with a high-strength, 7/16 in stainless steel shackle (Supplemental File 1-Figure S17).
- Using a 1/2 in screw pin shackle, connect the other end of each spectra line to one of the two stainless steel Masterlinks, such that each link has two or three connections.
- Attach the 3/4 in swivel shackle to the bottom of the Masterlink and the eye of a 1 in nylon line spliced with a stainless-steel thimble.
- Attach a 3/4 in shackle to the eye and thimble at the other end of the nylon line. This shackle will connect to the anchor system (Supplemental File 1-Figure S17).
- Design the mooring lines and hardware to ensure secure connections between the Ark base and the anchor system (see Figure 1 for example).
- Transportation of the 2V Ark to the deployment site
NOTE: The deployment of the Shell Ark requires a vessel with a flat stern and inboard engines, such that the Ark can be rolled off the boat deck and into the water, or a vessel with a large davit or A-frame.- Transport the Ark via a flatbed truck to the dock or marina.
- Load the Ark onto the vessel using an appropriately sized forklift (Supplemental File 1-Figure S21).
- Attach the mooring lines and hardware, including the downlines and hardware for attaching to the seafloor anchor system, to the base of the Ark.
- Transport the Ark to the anchor site (Figure 3). Prepare a line approximately the same length as the depth of the anchoring system with a shackle at one end and a buoy at the other end.
- Attach the shackle end of the line to the anchoring system, with the buoy end floating at the surface.
- Roll the Ark safely off the stern deck into the water or deploy the Ark into the water with a davit or A-frame. Attach the buoy end of the line to the positively buoyant Ark such that the structure floats above the anchoring system.
- Attachment of the Ark to the mooring system
NOTE: At this stage, the Ark structure is floating at the surface above the anchoring site with the integrated buoyancy elements (floats) providing flotation. The following tasks are completed underwater on SCUBA and require a team of at least three divers and two surface support personnel.- Attach the top block of a block and tackle pulley system to a secure attachment point on the base of the Ark, unspooling the pulley while descending toward the seafloor, and then attach the bottom block to the anchoring system (Supplemental File 1-Figure S19).
- Pull the line through the bottom block to engage the pulley, pulling the Ark to depth. The line should be locked into the cleat with each pull (Supplemental File 1-Figure S19).
NOTE: For Arks systems with high initial positive buoyancy, use a 6:1 block and tackle system for maximum purchase. Weights can also be temporarily attached to the Arks system to reduce the buoyant force necessary to sink the structure. - Continue to pull the Ark to depth until the downline and mooring attachment hardware can be connected to the anchor system. Use wire to mouse all the shackles.
- Inspect all the mooring components for integrity. Ensure the shackles are seated properly and the anchors are firmly in place.
- Slowly transfer the tension from the block and tackle to the mooring system. Remove the block and tackle, weights, and buoy line.
- Attachment of the ARMS to the Arks
- Retrieve the ARMS from the seeding location, and place into milk crates lined with 100 μm mesh to prevent the loss of small mobile invertebrates living within the ARMS. Transfer the ARMS to the Arks sites in tubs of shaded, cool seawater.
- Maneuver the ARMS through one of the larger triangular openings near the midline of the Ark such that the ARMS is inside the structure. Hold the ARMS firmly to one of the white baseplates mounted inside of the Ark framework.
- Secure a 1/2 in-13, 1.75 in long, stainless-steel hex head bolt through an open corner hole of the ARMS baseplate and the white, underlying HDPE baseplate, attach a stainless-steel locknut to the bolt protruding through the other side, and tighten down until snug. Repeat for the other three sides (Figure 2D).
- Push the ARMS back and forth to ensure firm attachment.
- Attachment of the corals to the Arks
- Fasten the coral plates containing corals epoxied to the limestone tile to the coral plate HDPE baseplates on the exterior of the Ark using 2 in long, 1/4 in-20, stainless steel hex head bolts, a washer, and a locknut at all four corners.
- Tighten the locknuts using a socket wrench to secure the coral plate in place.
- Assembly of the 2V geodesic frame
3. Coral Arks monitoring and maintenance
NOTE: Detailed fabrication instructions, including technical drawings for the manufacture of the components, are provided in Section 7 of Supplemental File 1.
- Measuring the in-water weight of the Arks
- Attach the submersible load cell to a block and tackle pulley system for use in temporarily transferring tension on the mooring line to the strain gauge system.
- Attach the base of the block and tackle to a secure location on the Ark mooring system, such as an intermediate shackle point or to the seafloor anchor. Attach the top of the load cell to a secure location on the Ark mounting framework (Supplemental File 1-Figure S33).
- Without removing or altering the mooring components on the Ark, pull the line through the block and tackle pulley system such that tension is transferred from the Ark mooring system to the pulley system, cleating the line with each pull (Supplemental File 1-Figure S33).
- Ensure the mooring line is completely slacked to allow the strain gauge to collect tension measurements (Supplemental File 1-Figure S33).
- Slowly transfer the tension from the block and tackle pulley system to the Ark mooring line, checking to ensure the shackles and other mooring components are properly seated and secure.
- For long-term data collection, integrate a load cell into the mooring system as an "in-line" component. Periodically switch out the dataloggers to retrieve the data.
- Long-term maintenance of the Arks
- Perform routine inspections of the Arks mooring system and conduct maintenance work as needed.
NOTE: See Supplemental File-Figure S18 for an example maintenance checklist. Biannual maintenance is recommended. - Ensure the anchors are continuing to provide maximum holding power (i.e., not backing out of the substrate).
- Clean the mooring lines of fouling organisms that can invade and compromise the integrity of the lines.
- Replace degrading components, such as the sacrificial anodes, shackles, and mooring lines, as needed (Supplemental File-Figure S18).
- Add supplemental buoyancy as needed by adding fixed buoyancy floats or air to the existing mooring buoys to compensate for biological mass accumulation.
- Perform routine inspections of the Arks mooring system and conduct maintenance work as needed.
Representative Results
The above methods provide assembly and installation instructions for two designs of Coral Arks systems. Prototypes for each design were assembled and field-tested in San Diego, USA, prior to long-term deployment to evaluate the drag characteristics and optimize the structural integrity based on modeled and empirical values of strength. The modeling efforts instrumental to the selection and refinement of both the Arks geometries presented here, including the results from wind tunnel testing, hydrodynamic simulations, and the in-water validation of the modeled values using prototype structures, are described in detail in Section 6 of Supplemental File 1. The results from the modeling and in-water testing of the "Shell" Arks design are shown here. Two structures of each design were then deployed at Caribbean field sites in Puerto Rico and Curaçao (four total Arks structures installed), and corals were translocated to the structures. Water quality, microbial community, and coral survival metrics associated with the "Shell" Arks design and two seafloor control sites were collected at several time points spanning 6 months to characterize and determine the changes in the environmental parameters and coral health associated with the Arks structures following natural recruitment and the addition of seeded ARMS.
Drag characteristics of Coral Arks
It is important to understand the drag characteristics of Coral Arks in order to design a structure and mooring that will survive the target environment. From a structural perspective, the hydrodynamic drag, in combination with the net buoyancy, imposes loadings within the structure, particularly on the mooring and its anchoring system. We conducted modeling and experimental measurements to estimate the drag characteristics of the Arks structures. The results of these tests for the "Shell" design of Arks structures are detailed below. Modeling was carried out by estimating the drag of the individual elements of the structure, summing these, and then combining the result into an effective drag coefficient as shown in equation (1) and equation (2):
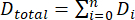 (1)
(1)
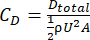 (2)
(2)
where Dtotal is the total drag of the structure estimated from the sum of the Di element drags, CD is the overall structure drag coefficient, is the fluid density, U is the flow speed of the object relative to the fluid, and A is the frontal area of the structure. In these calculations, the elements were all assumed to be cylinders, with their orientation to the flow dictated by the upright geometry of the Ark structure. The modeling was performed for the same prototype "Shell" system (a 2V geodesic sphere) that was used for tow testing (described below) prior to the construction of the final field systems. The prototype had a total frontal area of approximately 2.10 m2, and the modeling results indicated an effective drag coefficient for the entire structure of approximately 0.12. The model-predicted drag of the structure as a function of velocity is shown in Figure 4.
Experimental estimates of the drag force of the structure that would be experienced under different flow velocities were obtained by towing the Ark structure behind a vessel with a load cell spliced in-line with the towing line and a tilt sensor to record the changes in the Ark's orientation relative to the vertical axis at a range of tow speeds. Prior to towing, the in-water weight of the structure was determined, and sufficient additional weight was added to the structure to simulate a net buoyancy of approximately 200 kg (an initial target for the system). Based on the tension in the tow cable and the inclination angle of the Ark, the drag (Dtow) at each speed was determined using equation (3):
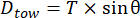 (3)
(3)
where T is the measured tension from the load cell, and is the tilt angle relative to the vertical axis. The resulting drag versus speed relationship is shown in Figure 4. A best fit drag curve (of the form Dtow α U2; see Figure 4), combined with estimates of the frontal area and the water density, was then used to determine the empirical drag coefficient of 0.13.
The Reynolds number during the tow testing (and the range used for the modeling) was in the range of 105-106, generally in the turbulent flow regimes. Typical values of the drag coefficient for a sphere in this Reynolds number range are between 0.2 and 0.4. For comparison purposes, a plot of the drag curve for a sphere with a drag coefficient of 0.3 is shown in Figure 4. Thus, the modeled and experimental estimates of the drag coefficient are in the order of two to three times smaller than for a sphere, which is consistent with the more open character of the structure.
To validate these modeled results, we also conducted field measurements of the response of two "Shell" Arks structures to flow. To achieve this, the same load cell was installed temporarily in line with the Ark main mooring line, a tilt sensor was installed on the Ark, and a current meter was installed at the site to simultaneously monitor the water speed. The buoyancy and drag components of the tension were then calculated from the tilt angle and the load cell measurements (Figure 5). The current speeds during the measurement period were relatively stable at about 20 cm/s, and the data set was relatively short; hence, the data were averaged over the period and used to compare the field drag and velocity response to the modeled and experimental towing estimates. These results show that under expected conditions at the deployment site (flow speeds up to 1.3 m/s during a typical storm event), the drag force on the system is expected to be less than 300 kg.
Both "Shell" structures in Vieques, Puerto Rico, survived a direct hit from the Category 1 Hurricane Fiona in September 2022 with no apparent damage to the structures, mooring, or anchoring system, providing an in situ test that supports the design. A nearby buoy (CARICOOS) recorded current speeds of 1.05 m/s at a 10 m depth at the deployment site, corresponding to a drag force of approximately 160 kg on the mooring systems. The systems were designed to withstand 1,600 kg of force (considering the anchor capacity and component breaking strength) and, therefore, are not expected to fail under ambient or typical storm conditions.
Net buoyancy monitoring for Coral Arks
The same approach described for validating the drag characteristics of the Ark structures was also used to develop a method for monitoring the net buoyancy of the Arks. As long as the physical structure of the Ark remains constant, the net buoyancy provides a rough proxy for monitoring the overall community calcification and, thus, the coral growth, as well as a maintenance metric to determine if the system has sufficient positive buoyancy to compensate for biological growth over time. The buoyancy component (B) of the mooring tension was calculated using the strain gauge and tilt sensor data in equation (4):
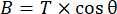 (4)
(4)
where T is the measured tension from the load cell, and is the tilt angle. The resulting time series of the net buoyancy is shown in Figure 5. Under the relatively stable current conditions present during the field monitoring events, we found the two "Shell" Arks structures deployed in Vieques, Puerto Rico, to have similar net buoyancies of 82.7 kg ± 1.0 kg (Ark 1) and 83.0 kg ± 0.9 kg (Ark 2) when averaged over the monitoring period (± one standard deviation) after all the corals and seeded ARMS units were translocated to the structures 6 months after the initial structure deployment. The results show that short-term monitoring during relatively stable periods of water flow can be used to determine the net buoyancy in the field to within ~1 kg, which should prove useful over the long term for monitoring changes in biomass.
Water quality and microbial community dynamics
Metrics associated with water quality and water column-associated microbial communities were measured on two midwater "Shell" Arks, which were anchored in 55 ft of water with the top of the Arks at a 25 ft depth, offshore of Isla Vieques, Puerto Rico (Figure 6C). The water quality metrics, microbial and viral abundances, and average microbe size from two Arks were compared to the same metrics from two nearby seafloor "control" sites, which were also at a 25 ft depth but much closer to shore (Figure 6D). The measurements shown were collected immediately after the installation of the Arks with an initial batch of translocated corals (November 2021) and 6 months later after a second batch of corals and seeded ARMS were translocated to the Arks (May 2022); they were then averaged across both sites (Arks and control sites) for comparison. As the seeded ARMS were transferred to the Arks at 6 months post-deployment, the accumulation of biological communities on the structures during the first 6 month period was associated with biofouling and natural recruitment.
The Arks environment exhibited higher average daytime light intensities (Figure 6A), higher average flow speeds (Figure 6C), lower dissolved organic carbon concentrations (Figure 6F), and lower diel fluctuations in dissolved oxygen concentrations (Figure 6G) than the benthic control sites. The Arks also displayed microbial communities with higher virus-to-microbe ratios than the control sites (Figure 7A), driven by a higher abundance of free viruses (Figure 7C) and a lower abundance of microbes (Figure 7B) in the midwater Arks environment. The microbial communities on the Arks were composed of, on average, physically smaller cells than the microbial communities at the seafloor sites (Figure 7D). Differences in temperature between the Arks and the control sites were not significant (Figure 6E). All of the above trends are consistent with better water quality and healthier microbial communities on the Arks than at the control sites. These conditions persisted through the initial 6 months of the deployment, during which a nascent biological community developed on the Arks through both the translocation of coral nubbins and natural recruitment from the water column and experienced successional changes, as well as through the addition of seeded ARMS onto the structures at month 6.
Coral survival
A cohort of corals comprising eight species and various morphologies were distributed to the Arks and benthic control sites both following the installation of the Arks (month 0) and following the addition of the seeded ARMS at month 6. The original parent colonies of each species of coral were fragmented into nubbins (2-8 cm in a given dimension) and attached to limestone coral plates (four to five nubbins per 20 cm2 plate) that were distributed equally at both the Arks and control sites, ensuring that the same species and genotypes were represented at both the midwater Arks sites and control sites. The survival of these translocated corals was assessed every 3 months at the Arks and control sites. Nine months after the translocation of the first cohort of corals, more corals were still alive on the Arks (80%, Figure 8) compared to the control sites (42%, Figure 8).
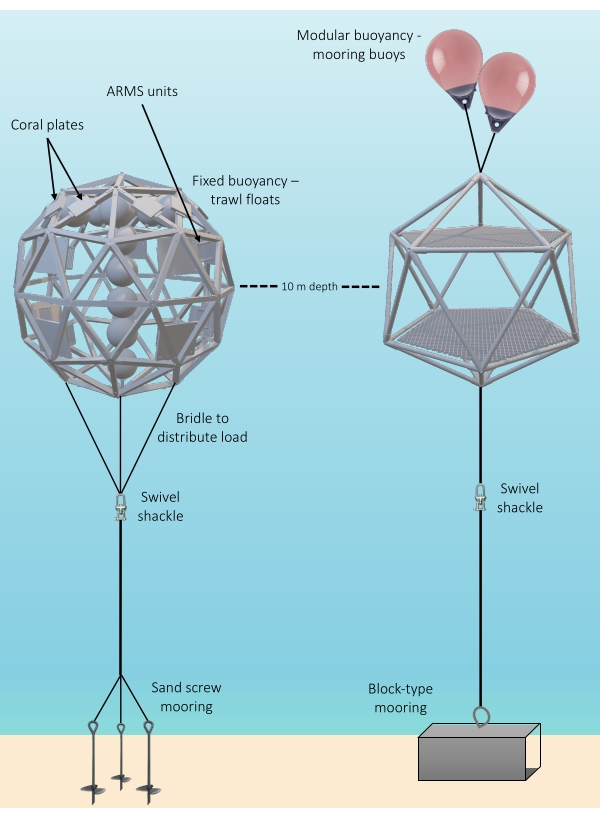
Figure 1: Diagram showing the structural components of two fully installed Coral Ark structures. Left, "Shell" and "Two-Platform" (right) Coral Arks structures are shown, together with two methods for providing positive buoyancy and two methods for anchoring. Abbreviation: ARMS = Autonomous Reef Monitoring Structures. Please click here to view a larger version of this figure.
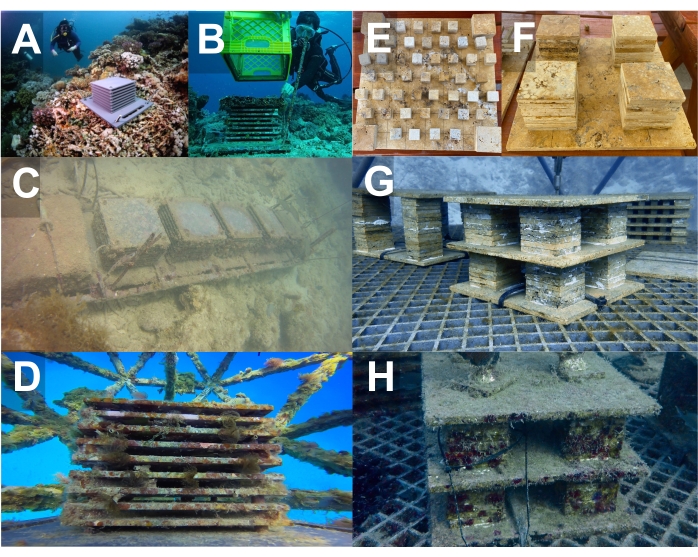
Figure 2: Design, deployment, and transfer of ARMS units. (A–D) PVC ARMS and (E–H) Limestone ARMS from seafloor seeding sites to Coral Arks. (A) Photo credit to Michael Berumen. (B) Photo credit to David Littschwager. Abbreviations: PVC = polyvinyl chloride; ARMS = Autonomous Reef Monitoring Structures. Please click here to view a larger version of this figure.
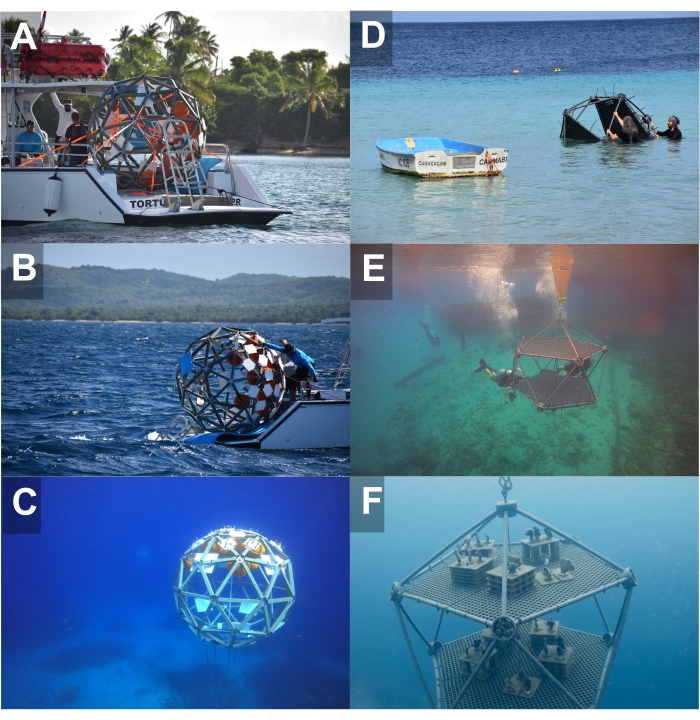
Figure 3: Images representing the deployment stages of Coral Arks, including transport to the site and full installation. (A–C) Shell type and (D–F) Two-Platform type systems. Please click here to view a larger version of this figure.
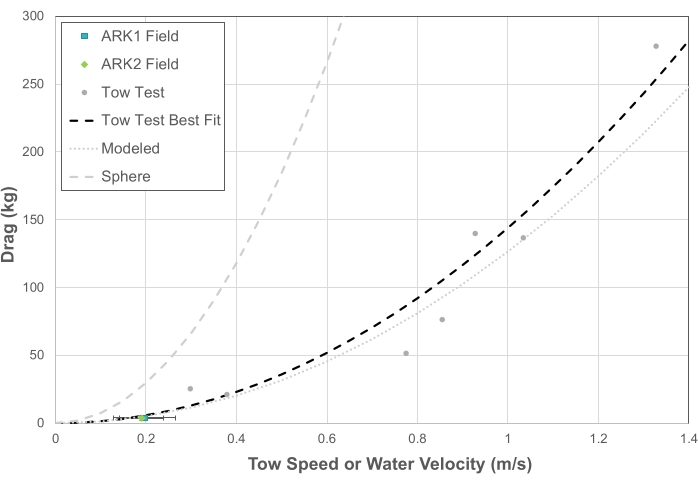
Figure 4: Drag characteristics of the "Shell" Ark structures based on modeling, experimental tow testing, and field validation relative to the drag of a sphere of the same approximate scale. "ARK1" and "ARK2" are identical "Shell" Ark structures installed at the same site in Vieques, Puerto Rico. Please click here to view a larger version of this figure.
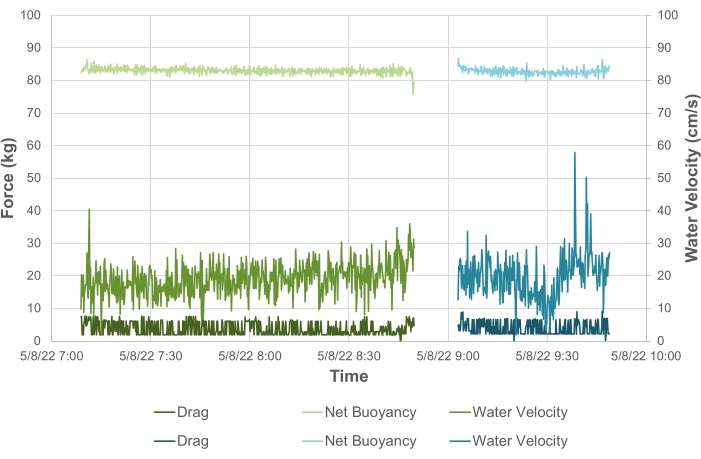
Figure 5: Measured net buoyancy values for two "Shell" Arks in Vieques, Puerto Rico. Shown are the water velocity (right axis, medium colors), net buoyancy (left axis, light colors), and calculated drag/tension on the mooring line (left axis, dark colors) for "Shell" Ark 1 (blue) and "Shell" Ark 2 (green). Please click here to view a larger version of this figure.
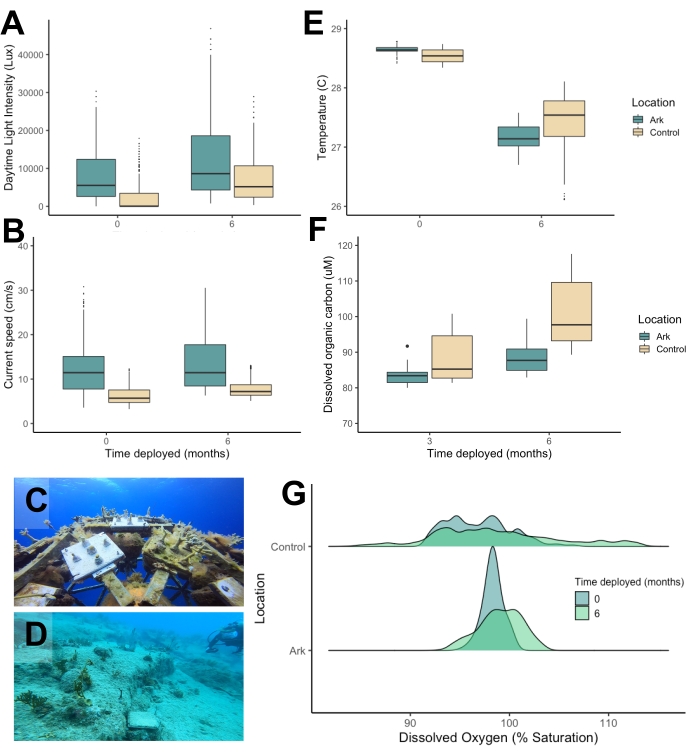
Figure 6: Water quality metrics associated with the "Shell" Arks and seafloor control sites in Vieques, Puerto Rico, immediately following the installation and 6 months afterward. (A) Daytime light intensity, (B) current speed, (C,D) photos taken 6 months post installation, (E) temperature, (F) dissolved organic carbon, (G) changes in dissolved oxygen levels in the Arks versus control sites over 6 months. Please click here to view a larger version of this figure.
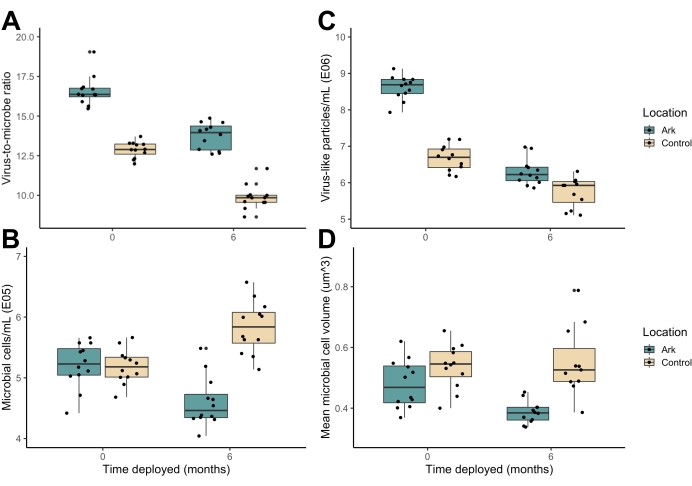
Figure 7: Metrics associated with the water column-associated microbial communities on the "Shell" Arks and seafloor control sites in Vieques, Puerto Rico immediately following installation and 6 months afterward. (A) Virus-to-microbe ratio, (B) bacterial cell abundance, (C) free virus abundance, and (D) average bacterial cell size. Please click here to view a larger version of this figure.
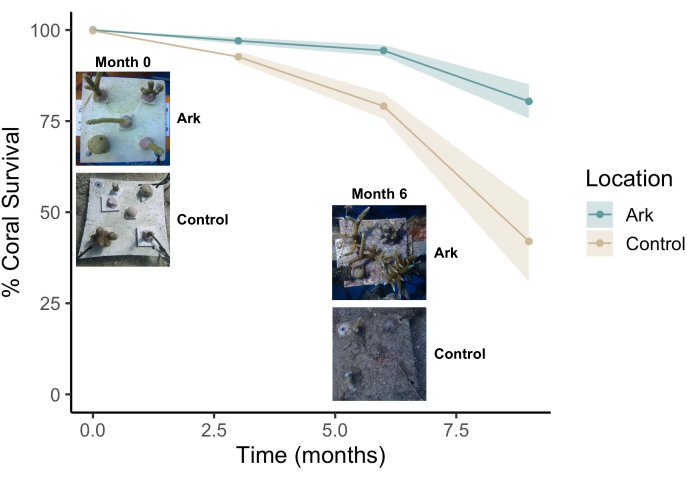
Figure 8: Proportion of surviving corals on the "Shell" Arks and seafloor control sites in Vieques, Puerto Rico during the first 9 months following translocation. The images represent the status of a single coral plate on the Arks (top) and on the benthic control sites (bottom) immediately following translocation (left) and 6 months after translocation (right). Please click here to view a larger version of this figure.
Table 1: ARMS construction and design considerations. Abbreviations: ARMS = Autonomous Reef Monitoring Structures; PVC = polyvinyl chloride. Please click here to download this Table.
Table 2: Coral Arks design considerations. Abbreviations: PVC = polyvinyl chloride; ARMS = Autonomous Reef Monitoring Structures; HDPE = high-density polyethylene. Please click here to download this Table.
Supplemental File. Please click here to download this file.
Discussion
The representative results presented above demonstrate that Coral Arks provide a habitat and improved water quality conditions for assembling reef communities on stable, in situ research platforms. Arks and seafloor control sites at the same depth displayed consistently different water quality profiles. Higher average current speeds and further distance from the coast reduced sedimentation and turbidity in the midwater environment at the Arks sites (Figure 6B), likely contributing to the lower measured dissolved organic carbon concentrations on the Arks (Figure 6F). Further, these improvements in water clarity resulted in elevated daytime light intensities on the Arks relative to the control sites (Figure 6A). Lower diel fluctuations in dissolved oxygen indicate improved oxygen availability for corals on the Arks compared to the benthos, especially at night (Figure 6G). These metrics have all been associated with improvements in coral survival42, growth43,44,45, and recovery from stress46,47 in past work and may be linked to enhanced survival outcomes of corals translocated to Arks as compared to benthic control sites (Figure 8). The fact that these conditions persist even after the accumulation of substantial biomass through biofouling indicates that natural recruitment processes do not diminish the improved water quality characteristics of the midwater environment. Arks were deployed 3 km offshore of the benthic control sites and likely benefitted from decreased inputs of terrestrially derived sediment, nutrients, and possibly fishing pressures that challenge nearshore sites. Siting Arks in areas with clean water and low human impact (such as offshore) may provide a better setting than heavily impacted coastal zones to propagate reef biodiversity for mesocosm-level experiments.
The preliminary findings also suggested that the midwater Arks experienced less microbialization, a central reef process associated with the degradation of benthic reef habitats4,48. High nutrient inputs and overfishing have been identified as drivers of reef-wide trophic feedback loops in which energetically destabilized microbial communities proliferate, resulting in the respiratory drawdown of metabolically available oxygen and the increased incidence of coral pathogens at the benthos6,49,50,51. The reduced abundance of free viruses on microbialized reefs, which serve as a primary lytic control on microbial community growth, indicate a breakdown in the trophic structure that favors further microbial expansion52. Water column-associated microbes on the Arks were both less abundant (Figure 7B) and physically smaller (Figure 7D) than at the seafloor sites. The Arks also displayed higher virus-to-microbe ratios (Figure 7A), abundance of free viruses (Figure 7C), and dissolved oxygen availability, particularly at night (Figure 6G). Taken together, these findings indicate that the midwater environment displayed less potential for microbialization relative to the seafloor sites. Arks, as mesocosms on which environmental conditions can be altered simply by vertical adjustment in the water column, offer an opportunity to mitigate and further explore the microbial and molecular mechanisms of reef degradation.
Geodesic spheres of two different frequencies were selected for the design of the Coral Arks presented here (Figure 1). Geodesic frequency (1V, 2V, 3V) indicates the number of repeating sub-elements in a geodesic sphere, with higher frequencies corresponding with a higher number of triangular sub-elements. From a structural perspective, geodesic polyhedra distribute mechanical stress throughout the structure, resulting in a high innate strength for their size53,54. These characteristics provide high durability and longevity but come at the cost of higher hydrodynamic drag, which can result in higher loadings on the mooring system. From a habitat perspective, the drag generated by an Ark system represents an indicator of the diffusion of momentum within the structure and, thus, the degree to which the internal ambient flow is reduced. The modeled and experimentally validated results indicate a 40%-70% reduction in the flow speed inside of the "Shell" Arks relative to the surrounding flow field due to the generation of turbulent flow inside the structures (see Section 6 of Supplemental File 1). While the optimal level of internal flow reduction is not clear (and differs with geodesic frequency), areas of reduced flow within the structure are important for creating niche habitats55,56, remineralizing nutrients57,58, and promoting the retention and settlement of larvae59,60. In general, larger and higher frequency geodesic structures, particularly at more exposed installation sites, require anchoring systems with higher holding power and more redundancy incorporated into the structural design.
The results from the field-based measurements of the drag component of tension on the "Shell" Ark mooring system closely matched those results generated from the modeled and experimental towing estimates (Figure 4) and were well within the expected design ranges. These results indicate that the assumptions of the hydrodynamic model are valid and that the model can predict drag forces over the background current ranges. However, while the deviations in the modeled and experimental data were small, the range of flows during the testing period, which were typical of ambient, non-storm flow speeds at the site, did not enable a rigorous validation over the full modeling spectrum. In predicting the design requirements of Coral Arks systems, modeling efforts should be combined with information on storm frequency and exposure at the planned deployment sites to design structures and mooring systems that can survive the anticipated hydrodynamic forces. The modeling work presented here can be used to design Ark systems at other sites with minimal inputs (desired Ark size, frequency, and average current speeds at the deployment site) by providing drag coefficients and maximum expected forces on the mooring and anchoring system.
Arks and ARMS systems are modular and may be built at different scales and with alternative materials than those described here. Although their ultimate longevity has not yet been determined, Coral Arks were designed to have an approximately 10 year life cycle. The material composition of the Arks and ARMS affects the longevity of the structures, the weight of the systems, and, therefore, the required buoyancy to offset the weight and may affect the response of early fouling communities (Supplemental File 1-Figure S7). For example, limestone provides a more natural substrate for biological colonization on the ARMS and is readily and inexpensively sourced on most carbonate reef islands, but it is more fragile and heavier than other materials such as PVC and fiberglass. These factors should be considered against site-specific characteristics to design ARMS, Arks, and mooring systems that best address the desired project outcomes.
The deployment sites for Coral Arks should also be selected based on the intended project goals (i.e., research, mitigation, or restoration). Factors to consider for site selection include the access to materials, reef state or condition, community investment/involvement, resource limitation, institutional support, and permit requirements. Coral Arks may provide opportunities to meet specific needs at sites that (1) contain living coral reefs that are in relatively poor condition and would benefit from restoration activities to enhance the coral recruitment, coral cover, coastal protection, or human food resources; (2) have a need for the translocation of corals to another location, which may occur, for example, when there are legal requirements to move living corals off of debris items slated for removal (at these sites, Coral Arks can be used in collaboration with, or in support of, existing restoration and outplanting efforts to improve translocation outcomes); (3) require research into novel conservation and restoration technologies using Coral Arks to improve the success of local efforts; or (4) have sufficiently distinct local conditions (i.e., different magnitude of anthropogenic impact), meaning standardized mesocosms could yield meaningful comparisons about reef processes and interventions. The specific approaches for monitoring aspects of the Coral Arks ecosystem such as biological growth, diversity, and water chemistry will vary between projects based on the project goals and site-specific variables.A representative outline for the scientific monitoring of Coral Arks conducted to date is provided in Section 5 of Supplemental File 1.
The design of Coral Arks structures can accommodate corals of nearly any species, size, and age and should provide improved conditions relative to those on a disturbed reef benthos. Depending on the growth and calcification rates observed on a given system, the addition of positive buoyancy to the Arks structures may be required to compensate for biological growth and to reduce the risk of sinking. Positively buoyant midwater structures can be weighed using a tension/compression load cell, or strain gauge, to determine if the in-water weight of the community is increasing (Figure 5). Periodic or long-term measurements using the load cell can complement other finer-resolution coral growth metrics to generate a metric of community-level growth/calcification and have been included as a regular maintenance task to determine if the system has sufficient positive buoyancy to compensate for this biological growth over time. In the case that an installed Ark can no longer be monitored or maintained, it could be relocated and/or the buoyancy could be removed to allow the Ark to be firmly attached to the benthos.
The methods described here provide researchers with a versatile toolkit for assembling midwater reef communities that can be sited at locations with improved water quality. By altering the depth or location of the Arks structures, changes in water quality parameters can be experimentally linked to changes in reef community structure and successional trajectories. This design feature allows researchers to exploit the abundant and underutilized space in the midwater environment to assemble and study coral reef mesocosms. The use of seeded ARMS to translocate cryptic biodiversity and deliver a "boost" to the natural recruitment of mobile grazing invertebrates provides a functional solution for reducing algal biofouling and, thus, benthic competition for corals. Using established and standardized sampling structures as components of this system provides added value by enabling the long-term monitoring of cryptic communities on Arks and comparison to datasets generated using ARMS as a global biodiversity census tool.
Coral Arks can serve as a more holistic, integrated, and self-regulating platform for propagating coral and invertebrate biomass that can then be outplanted to nearby degraded reefs and can provide a safe haven for corals to grow and reproduce in improved water quality conditions. As is currently being demonstrated in Puerto Rico, Arks can yield improved survival outcomes for mitigation projects involving the relocation of corals and reef biodiversity from debris items or degraded areas. Arks have relevance in long-term projects as a method to replace habitats for fish populations, test novel conservation strategies, and preserve native reef biodiversity. In the process, Arks provide versatile tools for conducting in situ studies of reef assemblies and ecological succession and may generate novel insights into reef connectivity.
Disclosures
The authors have nothing to disclose.
Acknowledgements
We thank Mark Vermeij, Kristen Marhaver, and the CARMABI Research Foundation in Curaçao for providing resources, support, and insight for this project. We thank the NAVFAC Atlantic Vieques Restoration Program and the Jacobs Engineering team for their substantial logistical and technical support in installing, maintaining, and monitoring the Coral Arks in Vieques. We are also grateful to Mike Anghera, Toni Luque, Cynthia Silveira, Natascha Varona, Andres Sanchez-Quinto, Lars ter Horst, and Ben Darby for their help and constructive input in the field. This research was funded by a Gordon and Betty Moore Foundation Aquatic Symbiosis Investigator Award to FLR <https://doi.org/10.37807/GBMF9207> and by the Department of Defense Environmental Security Technology Certification Program (RC20-5175).
Materials
| PVC ARMS | |||
| 316 Stainless Steel Hex Head Bolt, Partially Threaded, 8" length, 1/4"-20 Thread Size | McMaster Carr | 92186A569 | Bolts for PVC ARMS assembly Per unit: 4x |
| 316 Stainless Steel Hex Nut, Super-Corrosion-Resistant, 1/4"-20 Thread Size | McMaster Carr | 94805A029 | Nuts for PVC ARMS assembly Per unit: 8x |
| 316 Stainless Steel Nylon-Insert Locknut, Super-Corrosion-Resistant, 1/4"-20 Thread Size | McMaster Carr | 90715A125 | Locknuts for PVC ARMS assembly Per unit: 4x |
| 316 Stainless Steel Washer for 1/4" Screw Size, 0.281" ID, 0.625" OD | McMaster Carr | 90107A029 | Washers for PVC ARMS assembly Per unit: 8x |
| Nylon Unthreaded Spacers – 1/2" Long, 1/2" OD, Black | McMaster Carr | 90176A159 | Nylon spacers for PVC ARMS assembly Per unit: 20x |
| PVC Sheet Type 1, 0.25" Thick, Gray | McMaster Carr | 8747K215 | PVC for ARMS stacking plates. See Supplemental File 1-Figure SI 4. Per unit: 9x Refers to drawing: Yes |
| PVC Sheet Type 1, 0.5" Thick, Gray | McMaster Carr | 8747K217 | PVC for ARMS baseplates. See Supplemental File 1-Figure SI 1. Per unit: 1x Refers to drawing: Yes |
| PVC Sheet Type 1, 0.5" Thick, Gray | McMaster Carr | 8747K217 | PVC for ARMS long cross spacers. See Supplemental File 1-Figure SI 2. Per unit: 4x Refers to drawing: Yes |
| PVC Sheet Type 1, 0.5" Thick, Gray | McMaster Carr | 8747K217 | PVC for ARMS short cross spacers. See Supplemental File 1-Figure SI 3. Per unit: 8x Refers to drawing: Yes |
| Ratcheting Combination Wrench, 7/16" | McMaster Carr | 5163A15 | Wrenches to secure PVC ARMS hardware Per unit: 2x |
| Rebar, 3-ft Lengths, 1/2" Thick | McMaster Carr | 7480N115 | Rebar stakes to secure PVC ARMS to benthos. Mallet required. Per unit: 4x |
| Sequentially Numbered Metal Tags | McMaster Carr | 2208N349 | Numbered tags for ARMS ID Per unit: 1x |
| Limestone ARMS | |||
| DeWalt Wet Tile Saw | Home Depot | D24000S | Cut limestone tile into stackable pieces Per unit: 1x |
| Lift Bag, 50 lb Capacity | Amazon | B07GCNGRDR | Lift bag for transport of Limestone ARMS to benthos Per unit: 1x |
| Milk Crate, Heavy Duty, 13" x 19" x 11" | Amazon | B06XGBDJMD | Crate for transport of Limestone ARMS to benthos Per unit: 1x |
| Natural Limestone or Travertine Tile (Unfilled) – 12" x 12" | Bedrosians Tile & Stone | TRVSIENA1212T | Base material for Limestone ARMS layers and stacking pieces. See Supplemental File 1-Figure SI 7 and Figure SI 8. Per unit: 10x Refers to drawing: Yes |
| PC-11 Epoxy Adhesive Paste, Two-Part Marine Grade | Amazon | B008DZ1864 | Two-part epoxy for Limestone ARMS assembly |
| Shell Ark | |||
| Downline: 1" Nylon, 6' length thimble-to-thimble with stainless sailmaker thimble at top, heavy duty galvanized thimble at bottom | West Marine | Custom | Nylon mooring line for attaching Ark mooring bridle to anchor system. Per unit: 1 |
| Main structure: 105-B Epoxy | West Marine (made by West System) | 318352 | Epoxy to seal foam in struts. |
| Main structure: 205-B Hardener | West Marine (made by West System) | 318378 | Epoxy to seal foam in struts. |
| Mooring bridle: 3-1/8" X 2" small diamond base padeye with 7/8" bail | West Marine (Made by Harken) | 130560 | Padeyes for attaching mooring system to Ark base. Per unit: 5 |
| Main structure: 3/4" H-80 Divinycell Closed-Cell Foam, Plain Sheet 48" x 96" | Fiberglass Supply | L18-1110 | Buoyant foam for struts. Cut foam into 1.5" wide strips, 15.5" long for S1 struts and 19" long for S2 struts, add to struts. Per unit: 120 |
| Downline: 3/4" Stainless Masterlink | Lift-It (Made by Suncor) | S0652-0020 | Masterlink, connects top of swivel to lower portion of 5-point mooring bridle. Per unit: 1 |
| Mooring bridle: 3/8" Stainless Long D Shackles with Captive Self-Locking Pin | West Marine (Made by Wichard) | 116293 | High-strength shackles to connect pad eyes to mooring system. Per unit: 5 |
| Main structure: 316 SS, Pan Head Phillips Screw, 1/4-20, 3" Long | McMaster Carr | 91735A385 | Bolts to attach hull anodes to stainless struts Per unit: 2 |
| ARMS attachments: 316 Stainless Steel Nylon-Insert Locknut, Super-Corrosion-Resistant, 1/2"-13 Thread Size | McMaster | 90715A165 | Locknuts for attaching ARMS to ARMS mounting baseplates (8 per unit) Per unit: 80 |
| ARMS Baseplates: 316 Stainless Steel Nylon-Insert Locknut, Super-Corrosion-Resistant, 1/4"-20 Thread Size | McMaster | 90715A125 | Locknuts for ARMS mounting baseplates (struts and Stars) Per unit: 600 |
| Coral plate baseplates: 316 Stainless Steel Nylon-Insert Locknut, Super-Corrosion-Resistant, 1/4"-20 Thread Size | McMaster | 90715A125 | Locknuts for attaching coral plate baseplates to struts Per unit: 600 |
| Coral plate attach: 316 Stainless Steel Nylon-Insert Locknut, Super-Corrosion-Resistant, 1/4"-20 Thread Size | McMaster | 90715A125 | Locknuts to attach coral plates to baseplates Per unit: 80 |
| Mooring bridle: 316 Stainless Steel Nylon-Insert Locknut, Super-Corrosion-Resistant, 1/4"-20 Thread Size | McMaster | 90715A125 | Padeye locknuts for attaching pad eyes to struts. Per unit: 20 |
| Main structure: 316 Stainless Steel Nylon-Insert Locknut, Super-Corrosion-Resistant, 10-32 Thread Size | McMaster | 90715A115 | Locknuts for star-strut connections Per unit: 475 |
| Main structure: 316 Stainless Steel Pan Head Phillips Screw, 10-32 Thread, 2-1/2" Long | McMaster | 91735A368 | Bolts for star-strut connections Per unit: 475 |
| Mooring bridle: 316 Stainless Steel Phillips Flat Head Screws, 1/4"-20 Thread Size, 2-3/4" Long | McMaster | 91500A341 | Padeye bolts for attaching pad eyes to struts. Per unit: 15 |
| ARMS Baseplates: 316 Stainless Steel Phillips Flat Head Screws, 1/4"-20 Thread Size, 3" Long | McMaster | 91500A554 | Bolts for attaching ARMS mounting baseplates to Stars Per unit: 475 |
| Mooring bridle: 316 Stainless Steel Phillips Flat Head Screws, 1/4"-20 Thread Size, 3" Long | McMaster | 91500A554 | Padeye bolts for attaching pad eyes through struts & Stars. Per unit: 5 |
| Mooring bridle: 316 Stainless Steel Screw-Pin Shackle – for Lifting, 1/2" Thick | McMaster | 3583T15 | Shackles to connect lower bridle thimbles to small links on Masterlink. Per unit: 5 |
| ARMS attachments: 316 Stainless Steel Split Lock Washer for 1/2" Screw Size, 0.512" ID, 0.869" OD | McMaster | 92147A033 | Lock washers for attaching ARMS to ARMS mounting baseplates (4 per unit) Per unit: 40 |
| ARMS attachments: 316 Stainless Steel Washer for 1/2" Screw Size, 0.531" ID, 1.25" OD | McMaster | 90107A033 | Backing washers for attaching ARMS to ARMS mounting baseplates (4 per unit) Per unit: 40 |
| ARMS Baseplates: 316 Stainless Steel Washer for 1/4" Screw Size, 0.281" ID, 0.625" OD | McMaster | 90107A029 | Washers for attaching ARMS mounting baseplates to struts Per unit: 40 |
| Coral plate baseplates: 316 Stainless Steel Washer for 1/4" Screw Size, 0.281" ID, 0.625" OD | McMaster | 90107A029 | Washers for attaching coral plate baseplates to struts Per unit: 40 |
| Coral plate attach: 316 Stainless Steel Washer for 1/4" Screw Size, 0.281" ID, 0.625" OD | McMaster | 90107A029 | Washers to attach coral plates to baseplates Per unit: 160 |
| Main structure: 316 Stainless Steel Washer for Number 10 Screw Size, 0.203" ID, 0.438" OD | McMaster | 90107A011 | Washers for star-strut connections Per unit: 475 |
| Buoyancy: 316 Stainless Steel Washer, 1" Screw Size, 2" OD | McMaster | 90107A038 | Large washers for central rod (2 per float) Per unit: 22 |
| ARMS attachments: 316 Stainless Steel Washer, Oversized, 1/2" Screw, 1.5" OD, 0.052"- 0.072" Thickness | McMaster | 91525A145 | Oversized washers for attaching ARMS to ARMS mounting baseplates (4 per unit) Per unit: 40 |
| Coral plates: 3M Marine Adhesive Sealant – Fast Cure 5200 | McMaster | 67015A44 | Adhesive to glue limestone tiles to PVC coral baseplates. Drill out corners with masonry bit. |
| Buoyancy: 3M Marine Adhesive Sealant – Fast Cure 5200 | McMaster | 67015A44 | Adhesive for securing fiberglass threaded rods into trawl floats Per unit: 2 |
| Mooring bridle: 5/8" Dyneema with Stainless Sailmakers Thimbles at Top and Bottom | West Marine | Custom | 5-leg mooring bridle for attaching Ark to downline. Per unit: 5 |
| Downline: Clevis-to-Clevis Swivel – Not for Lifting, 316 Stainless Steel, 6-7/32" Long | McMaster | 37405T29 | Swivel, bottom connects to top of downline, top connects to large link in Masterlink. Per unit: 1 |
| Buoyancy: Fiberglass Hex Nut, 1"-8 Thread Size | McMaster | 91395A038 | Fiberglass hex nuts for securing fiberglass threaded rods into trawl floats Per unit: 30 |
| Buoyancy: Fiberglass Threaded Rod, 1"-8 Thread Size, 8 Feet Long | McMaster | 91315A238 | Fiberglass threaded rod to attach float to Ark. See Supplemental File 1-Figure SI 16. Per unit: 10 Refers to drawing: Yes |
| Anchor system: Galvanized Alloy Steel Shackle with Screw Pin – for Lifting, 1/2" Thick | McMaster | 3663T42 | Middle shackle from chain to pear link. Per unit: 3 |
| Anchor system: Galvanized Alloy Steel Shackle with Screw Pin – for Lifting, 3/4" Thick | McMaster | 3663T44 | Upper large shackle to connect pear link to lower downline thimble. Per unit: 1 |
| Anchor system: Galvanized Alloy Steel Shackle with Screw Pin – for Lifting, 3/4" Thick | McMaster | 3663T44 | Anchor shackle. Per unit: 3 |
| Anchor system: Galvanized Alloy Steel Shackle with Screw Pin – for Lifting, 3/8" Thick | McMaster | 3663T51 | Shackle to connect chain to upper middle shackle. Per unit: 3 |
| Anchor system: Galvanized Alloy Steel Shackle with Screw Pin – for Lifting, 3/8" Thick | McMaster | 3663T51 | Lower small shackle to connect chain and anchor shackle. Per unit: 3 |
| Install & Tools: HARKEN–57mm Carbo Air® Triple Block | West Marine | 200076 | Top of block and tackle Per unit: 1 |
| Install & Tools: HARKEN–57mm Carbo Air® Triple Block with Becket and Cam | West Marine | 1171644 | Base of block and tackle Per unit: 1 |
| ARMS Baseplates: Heat-Shrink Tubing, 0.50" ID Before Shrinking | McMaster | 7856K47 | Heatshrink for non-slip. Cut into 1.5" lengths, slide over a SS u-bolt bracket and use heat gun to tighten onto bracket. Per unit: 20 |
| Coral plate baseplates: Heat-Shrink Tubing, 0.50" ID Before Shrinking | McMaster | 7856K47 | Heatshrink for non-slip. Cut into 1.5" lengths, slide over a SS u-bolt bracket and use heat gun to tighten onto bracket. Per unit: 40 |
| Buoyancy: Heatshrink for covering threaded rods before mounting in floats, 14" sections | McMaster | 7856K66 | Heatshrink for non-slip. Cut into 14" lengths. Slide onto fiberglass rods with 1" exposed on one end and 2-1/4" exposed on the other. Use heat gun to shrink until snug. Per unit: 11 |
| Anchor system: High-Strength Grade 40/43 Chain-Not for Lifting, Galvanized Steel, 5/16 Trade Size | McMaster | 3588T23 | Chain to connect anchors and downline. Per unit: 3 |
| Install & Tools: LOW-STRETCH ROPE, 7/16" DIAMETER | McMaster | 3789T25 | Rope for block and tackle Per unit: 250 |
| ARMS Baseplates: Marine-Grade Moisture-Resistant HDPE, 48" x 48", 1/2" Thick | McMaster | 9785T82 | Sheeting for ARMS mounting baseplates. See Supplemental File 1-Figure SI 13. Per unit: 10 Refers to drawing: Yes |
| Coral plate baseplates: Marine-Grade Moisture-Resistant HDPE, 48" x 48", 1/2" Thick | McMaster | 9785T82 | Sheeting for coral plate baseplates. See Supplemental File 1-Figure SI 14. Per unit: 20 Refers to drawing: Yes |
| Mooring bridle: Martyr Collar Anode Zinc 3/4" x 2 1/8" x 2 1/8" | West Marine | 5538715 | Sacrificial anodes for Masterlinks on mooring lines Per unit: 2 |
| Main structure: Martyr Hull Anode Zinc 6 1/4" x 2 3/4" x 5/8" | West Marine | 484998 | Sacrificial anodes for stainless struts at Ark base Per unit: 3 |
| ARMS Baseplates: Mounting Plate for 1/4"-20 Thread Size, 2" ID 304 Stainless Steel U-Bolt | McMaster | 8896T156 | Bracket plate w/heatshrink, for attaching ARMS mounting baseplates to struts Per unit: 6 |
| Coral plate baseplates: Mounting Plate for 1/4"-20 Thread Size, 2" ID 304 Stainless Steel U-Bolt | McMaster | 8896T156 | Bracket plate w/heatshrink, for attaching coral plate baseplates to struts Per unit: 40 |
| Main structure: N1 Stars, 316 SS, 5mm Thick Connectors for DIY VikingDome F2 Sphere, modified | Viking Dome | ICO2-AISI | N1 Stars modified for central rod. Machine/weld connections to insert top and bottom of unthreaded fiberglass structural rod. See Supplemental File 1-Figure SI 10. Per unit: 2 |
| Main structure: N1 Stars, 316 SS, 5mm Thick Connectors for DIY VikingDome F2 Sphere, unmodified | Viking Dome | ICO2-AISI | Unmodified N1 Stars for Ark assembly. See Supplemental File 1-Figure SI 10 Per unit: 10 Refers to drawing: Yes |
| Main structure: N2 Stars, 316 SS, 5mm Thick Connectors for DIY VikingDome F2 Sphere, modified | Viking Dome | ICO2-AISI | N2 Stars modified for floats. Drill larger center hole to accommodate 1" threaded fiberglass rod. Per unit: 10 |
| Main structure: N2 Stars, 316 SS, 5mm Thick Connectors for DIY VikingDome F2 Sphere, modified | Viking Dome | ICO2-AISI | N2 Stars modified for pad eyes. Drill larger bolt hole (bit – 1/4") on outer hole of one arm for Padeye connector. Per unit: 5 |
| Main structure: N2 Stars, 316 SS, 5mm Thick Connectors for DIY VikingDome F2 Sphere, unmodified | Viking Dome | ICO2-AISI | Unmodified N2 Stars for Ark assembly Per unit: 15 |
| Anchor system: Pear-Shaped Link – Not for Lifting, Galvanized Steel, 3/4" Thick | McMaster | 3567T34 | Link to connect 3x 1/2" shackles to upper large shackle. Per unit: 1 |
| Install & Tools: Phillips Screwdriver, Size No. 2 | McMaster Carr | 5682A28 | Tighten down locknuts on star-strut bolts Per unit: 1 |
| Coral plates: PVC Sheet Type 1, Gray, 48" x 48", 1/4" Thick | McMaster | 8747K194 | PVC baseplates for coral plates. See Supplemental File 1-Figure SI 4. Per unit: 20 Refers to drawing: Yes |
| Install & Tools: Ratcheting Combination Wrench, 3/4" | McMaster Carr | 5163A21 | Attach ARMS to ARMS mounting baseplates Per unit: 2 |
| Install & Tools: Ratcheting Combination Wrench, 3/8" | McMaster Carr | 5163A14 | Tighten down locknuts on star-strut bolts Per unit: 2 |
| Install & Tools: Ratcheting Combination Wrench, 7/16" | McMaster Carr | 5163A15 | Attach coral plates to coral plate baseplates Per unit: 2 |
| Install & Tools: Round Bend-and-Stay Multipurpose Stainless Steel Wire, 0.012" diameter, 645 feet | McMaster | 9882K35 | Wire for mousing stainless shackles Per unit: 1 |
| Main structure: S1 Struts – Structural FRP Fiberglass Square Tube, 2" Wide x 2" High Outside, 1/4" Wall Thickness | McMaster | 8548K34 | Fiberglass S1 Struts. Cut to 20.905" long (531 mm), drill bolt holes (bit – 7/32"), fill w/ divinycell foam & epoxy. See Supplemental File 1-Figure SI 9 Per unit: 55 Refers to drawing: Yes |
| Main structure: S1 Struts (SS) – Corrosion-Resistant 316/316L Stainless Steel Rectangular Tube, 0.12" Wall Thickness, 2" x 2" Outside | McMaster | 2937K17 | Stainless S1 Struts. Cut to 20.905" long (531 mm), drill bolt holes (bit – 1/4"). See Supplemental File 1-Figure SI 9. Per unit: 5 Refers to drawing: Yes |
| Main structure: S2 Struts – Structural FRP Fiberglass Square Tube, 2" Wide x 2" High Outside, 1/4" Wall Thickness | McMaster | 8548K34 | Fiberglass S2 Struts. Cut to 24.331" long (618 mm), drill bolt holes (bit – 7/32"), fill w/ divinycell foam & epoxy. See Supplemental File 1-Figure SI 9. Per unit: 60 Refers to drawing: Yes |
| Anchor system: Skrew SK2500 | Spade Anchor USA | SK2500 | Two-plate sand screw anchors Per unit: 3 |
| Coral plates: Stainless Steel Washers for 1/4" Screw Size, 0.281" ID, 0.625" OD | McMaster | 90107A029 | Numbered tags for coral plates. Stamp SS washers with numbered stamps and glue to coral plate for later ID. Per unit: 100 |
| Main structure: Structural FRP Fiberglass Rod, 10 Feet Long, 1" Diameter | McMaster | 8543K26 | Central fiberglass rod, cut to Ark diameter Per unit: 1 |
| ARMS attachments: Super-Corrosion-Resistant 316 Stainless Steel Hex Head Screw, 1/2"-13 Thread Size, 1-3/4" Long | McMaster | 93190A718 | Bolts for attaching ARMS to ARMS mounting baseplates (4 per unit) Per unit: 40 |
| Coral plate attach: Super-Corrosion-Resistant 316 Stainless Steel Hex Head Screw, 1/4"-20 Thread Size, 2" Long, Fully Threaded | McMaster | 93190A550 | Bolts to attach coral plates to baseplates Per unit: 80 |
| ARMS Baseplates: Super-Corrosion-Resistant 316 Stainless Steel Hex Head Screw, 1/4"-20 Thread Size, 3-1/2" Long | McMaster | 92186A556 | Bolts for attaching ARMS mounting baseplates to struts Per unit: 40 |
| Coral plate baseplates: Super-Corrosion-Resistant 316 Stainless Steel Hex Head Screw, 1/4"-20 Thread Size, 3" Long, Partially Threaded | McMaster | 92186A554 | Bolts for attaching coral plate baseplates to struts Per unit: 160 |
| Buoyancy: TFLOAT 14" CENTERHOLE OR 437FM, modified | Seattle Marine | YUN12B-8 | 14" trawl floats for mounting to Stars. Slide fiberglass rod with heat shrink through trawl float. Add stainless washer and fiberglass hex nut on both sides. Seal washers with 3M 5200. Tighten nuts down. See Supplemental File 1-Figure SI 16. Per unit: 11 Refers to drawing: Yes |
| Buoyancy: TFLOAT 14" CENTERHOLE OR 437FM, unmodified | Seattle Marine | YUN12B-8 | 14" trawl float Per unit: 2 |
| ARMS Baseplates: Thick-Wall Dark Gray PVC Pipe for Water, Unthreaded, 1/4 Pipe Size, 5 Feet Long | McMaster | 48855K41 | Star standoffs for attaching ARMS mounting baseplates to Stars. Cut to 1.75" long sections. Per unit: 40 |
| Coral plates: Unfilled, Natural Travertine Flooring Tile, 16" x 16" | Home Depot | 304540080 | Limestone tiles for coral plates. Cut to 9" x 9" tiles using wet tile saw. Per unit: 20 |
| Buoyancy: Vibration-Damping Routing Clamp, Weld mount, Polypropylene with Stainless Steel Plates, 1" ID | McMaster | 3015T47 | Attachment for central rod and float Per unit: 1 |
| Buoyancy: Water- and Steam-Resistant Fiberglass Washer for 1" Screw Size, 1.015" ID, 1.755" OD | McMaster | 93493A110 | Fiberglass washers for securing fiberglass threaded rods into trawl floats Per unit: 20 |
| Install & Tools: Zinc-Galvanized Steel Wire, 0.014" diameter, 475 feet long | McMaster | 8872K19 | Wire for mousing galvanized shackles Per unit: 1 |
| Two Platform Ark | |||
| Downline: 1" Nylon, 15' length thimble-to-thimble with SS Sailmaker Thimble spliced at top, galvanized thimble spliced at bottom | West Marine | Custom | Runs from bottom of swivel shackle (SS) to top of anchor system (galvanized) Per unit: 1x |
| Downline: 1/2" Spectra Rope with SS316 Sailmakers Thimbles Spliced at Top and Bottom | West Marine | Custom | Runs from bottom of Ark to top of swivel shackle. Per unit: 2x |
| Buoyancy: 1/2" Spectra Rope with SS316 Sailmakers Thimbles Spliced at Top and Bottom | West Marine | Custom | Connects mooring buoy to top eye on Ark Per unit: 2x |
| Main structure: 3/8 x 36 Inch SS Thimble Eye Swages and 5/8 Jaw-Jaw Turnbuckle Cable Assembly | Pacific Rigging & Loft | Custom | Custom rigging system with turnbuckle, 3/8" SS wire rope swaged into PVC end caps Per unit: 1x |
| Main structure: 304 SS U-Bolt with Mounting Plate, 1/4"-20, 2" ID | McMaster Carr | 8896T123 | For joining fiberglass platforms using I-beams Per unit: 10x |
| Main structure: 316 SS Hex Nut, 1/4"-20 | McMaster Carr | 94804A029 | For locking struts in hubs Per unit: 120x |
| Main structure: 316 SS Nylon-Insert Locknut, 1/4"-20 | McMaster Carr | 90715A125 | For locking struts in hubs Per unit: 240x |
| Main structure: 316 SS Pan Head Phillips Screw, 1/4"-20 Thread, 2.5" Long | McMaster Carr | 91735A384 | For locking struts in hubs Per unit: 120x |
| Downline: 316 SS Safety-Pin Shackle, 1/2" Thick | McMaster Carr | 3860T25 | Connect Ark bottom eye to 1/2" Spectra rope. Per unit: 1x |
| Buoyancy: 316 SS Safety-Pin Shackle, 1/2" Thick | McMaster Carr | 3860T25 | Connects bottom of 1/2" rope to top Ark eye Per unit: 2x |
| Buoyancy: 316 SS Safety-Pin Shackle, 7/16" Thick | McMaster Carr | 3860T24 | Connects mooring buoy to 1/2" rope Per unit: 2x |
| Install & Tools: Arbor with 7/16" Hex for 1-1/2" Diameter Hole Saw | McMaster Carr | 4066A63 | Drill holes in 6" PVC (Hubs) Per unit: 1x |
| Main structure: Clamping U-bolt, 304 SS, 1/4"-20 Thread Size, 9/16" ID | McMaster Carr | 3042T149 | For clamping SS wire rope at Ark vertices Per unit: 15x |
| Downline: Clevis-to-Clevis Swivel, 316 SS, 5-7/16" Long | McMaster Carr | 37405T28 | Swivel shackle between 1/2" spectra rope and 1" nylon downline Per unit: 1x |
| Main structure: Corrosion-Resistant Wire Rope, 316 SS, 1/8" Thick | McMaster Carr | 8908T44 | String through assembled Ark and clamp at vertices Per unit: 250ft |
| Main structure: Fiberglass Molded Grating, Square Grid, 1" Grid Height, 1-1/2" x 1-1/2" Square Grid, Grit Surface, 70% Open Area | McNichols | MS-S-100 | Cut to half pentagon shape, mirror images. See Figure S23. Per unit: 2x Refers to drawing: Yes |
| Anchor system: Galvanized Alloy Steel Screw-Pin Shackle, 1/2" Thick | McMaster Carr | 3663T42 | Connects base of 1" nylon downline to anchor chain Per unit: 1x |
| Anchor system: Galvanized Alloy Steel Screw-Pin Shackle, 3/8" Thick | McMaster Carr | 3663T51 | Connects anchor chain together Per unit: 1x |
| Anchor system: Grade 30 Chain, Galvanized Steel, 1/4 Trade Size | McMaster Carr | 3592T45 | Anchor chain |
| Install & Tools: HARKEN–57 mm Carbo Air Triple Block | West Marine | 200076 | Top of block and tackle Per unit: 1x |
| Install & Tools: HARKEN–57 mm Carbo Air Triple Block with Becket and Cam | West Marine | 1171644 | Base of block and tackle Per unit: 1x |
| Install & Tools: Hole Saw, 1-15/16" Cutting Depth, 1-1/2" Diameter | McMaster Carr | 4066A27 | Drill holes in 6" PVC (Hubs) Per unit: 1x |
| Install & Tools: Low Pressure Inflator Nozzle | Amazon (Made by Trident) | B00KAI940E | Inflate mooring buoys underwater Per unit: 1x |
| Install & Tools: LOW-STRETCH ROPE, 7/16" DIAMETER | McMaster | 3789T25 | Rope for block and tackle Per unit: 100ft |
| Main structure: Nylon Cable Ties, UV Resistant Heavy Duty, 19" long, 250 lb strength | CableTiesAndMore | CT19BK | Use to secure platforms to Ark framework Per unit: 30x |
| Install & Tools: Phillips Screwdriver, Size No. 3 | McMaster Carr | 5682A29 | For locking struts in hubs Per unit: 1x |
| Buoyancy: Polyform Buoy, A-5 Series All-Purpose Buoy, 27" | West Marine (Made by PolyformUS) | 11630142 | Mooring buoy for buoyancy. Per unit: 2x |
| Main structure: PVC Pipe, Schedule 80, 1" diameter | McMaster Carr | 48855K13 | Struts. Cut to 1.2 m (4 ft) lengths, drill to accommodate bolts Per unit: 30x |
| Main structure: PVC Pipe, Schedule 80, 6" diameter | McMaster Carr | 48855K42 | Hubs. Cut into 4" lengths, drill 5 holes symmetrically around midline using 1-1/2" hole saw. See Supplemental File 1-Figure S22. Per unit: 12x Refers to drawing: Yes |
| Main structure: PVC Thick Wall Pipe Fitting, End Cap, Schedule 80, 6 " diameter, Female | PRMFiltration (Made by ERA) | PVC80CAP600X | End caps for top and bottom of Ark. Cut off bottom 2 inches. Per unit: 2x |
| Install & Tools: Ratcheting Combination Wrench, 7/16" | McMaster Carr | 5163A15 | For locking struts in hubs Per unit: 1x |
| Install & Tools: Ratcheting PVC Cutter, 1-1/4" | McMaster Carr | 8336A11 | Cut 1" PVC into struts Per unit: 1x |
| Main structure: Ring, 18-8 SS, for 5/32 Chain Trade Size, 3/4" Inside Length | McMaster Carr | 3769T71 | Substitute for 1/2" SS wire rope clamps. Per unit: 12x |
| Install & Tools: Round Bend-and-Stay Multipurpose Stainless Steel Wire, 0.012" diameter, 645 feet | McMaster | 9882K35 | Wire for mousing stainless shackles Per unit: 1 |
| Main structure: Structural FRP Fiberglass I-Beam, 1/4" Wall Thickness, 1-1/2" Wide x 3" High, 5 ft long | McMaster Carr | 9468T41 | Cut to 5 1-ft long sections. Per unit: 1x |
| Install & Tools: Underwater Lift Bag, 220 lbs Lift Capacity | Subsalve Commercial | C-200 | Transport Ark to deployment site Per unit: 1x |
| Install & Tools: Zinc-Galvanized Steel Wire, 0.014" diameter, 475 feet long | McMaster | 8872K19 | Wire for mousing galvanized shackles Per unit: 1x |
| Strain Gauge | |||
| 316 Stainless Steel Eyebolt, for Lifting, M16 x 2 Thread Size, 27 mm Thread Length | McMaster Carr | 3130T14 | For strain gauge eyebolts Per unit: 2x |
| Bridge101A Data Logger, 30 mV | MadgeTech | Bridge101A-30 | Collect voltage data from load cell. Per unit: 1x |
| Chemical-Resistant PVC Rod, 2" Diameter | McMaster Carr | 8745K26 | For datalogger housing endcap. See Supplemental File 1-Figure S32. Per unit: 1x Refers to drawing: Yes |
| Clamping U-Bolt, 304 SS, 5/16"-18 Thread Size, 1-3/8" ID | McMaster Carr | 3042T154 | For attachment of datalogger housing to strain gauge. Per unit: 1x |
| Dow Corning Molykote 44 Medium Grease Lubricant | Amazon (Made by Dow Corning) | B001VY1EL8 | For mating male and female underwater connectors. Per unit: 1x |
| STA-8 Stainless Steel S Type Tension and Compression Load Cell | LCM Systems | STA-8-1T-SUB | Load cell instrument for assessment of in-water weight. Per unit: 1x |
| Standard-Wall Clear Blue Rigid PVC Pipe for Water, Unthreaded, 1-1/2 Pipe Size, 2 ft | McMaster Carr | 49035K47 | For datalogger housing. See Supplemental File 1-Figure S31. Per unit: 1x Refers to drawing: Yes |
| Standard-Wall PVC Pipe Fitting for Water, Cap, White, 1-1/2 Pipe Size Socket Female | McMaster Carr | 4880K55 | For datalogger housing. Per unit: 2x |
| Structural FRP Fiberglass Sheet, 12" Wide x 12" Long, 3/16" Thick | McMaster Carr | 8537K24 | For attachment of datalogger housing to strain gauge. Per unit: 1x |
| SubConn Micro Circular Connector, Female, 4-port | McCartney (Made by SubConn) | MCBH4F | Install into machined housing endcap. Per unit: 1x |
| SubConn Micro Circular Connector, Male, 4-contact | McCartney (Made by SubConn) | MCIL4M | Splice to load cell wiring and waterproof connection. Per unit: 1x |
| Threadlocker, Loctite 262, 0.34 FL. oz Bottle | McMaster Carr | 91458A170 | For strain gauge eyebolts Per unit: 1x |
| Vibration-Damping Routing Clamp, Weld-Mount, Polypropylene with Zinc-Plated Steel Top Plate, 1-7/8" ID | McMaster Carr | 3015T39 | For attachment of datalogger housing to strain gauge. Per unit: 1x |
References
- Pandolfi, J. M., et al. Global trajectories of the long-term decline of coral reef ecosystems. Science. 301 (5635), 955-958 (2003).
- Hughes, T. P., et al. Phase shifts, herbivory, and the resilience of coral reefs to climate change. Current Biology. 17 (4), 360-365 (2007).
- McManus, J. W., Polsenberg, J. F. Coral-algal phase shifts on coral reefs: Ecological and environmental aspects. Progress in Oceanography. 60 (2-4), 263-279 (2004).
- Haas, A. F., et al. Global microbialization of coral reefs. Nature Microbiology. 1, 16042 (2016).
- Dinsdale, E. A., et al. Microbial ecology of four coral atolls in the Northern Line Islands. PLoS One. 3 (2), 1584 (2008).
- Zaneveld, J. R., et al. Overfishing and nutrient pollution interact with temperature to disrupt coral reefs down to microbial scales. Nature Communications. 7, 11833 (2016).
- Estes, J. A., et al. Trophic downgrading of planet earth. Science. 333 (6040), 301-306 (2011).
- Houk, P., Musburger, C. Trophic interactions and ecological stability across coral reefs in the Marshall Islands. Marine Ecology Progress Series. 488, 23-34 (2013).
- Pearman, J. K., Anlauf, H., Irigoien, X., Carvalho, S. Please mind the gap – Visual census and cryptic biodiversity assessment at central Red Sea coral reefs. Marine Environmental Research. 118, 20-30 (2016).
- Stella, J. S., Pratchett, M. S., Hutchings, P. A., Jones, G. P., Gibson, R. N., Atkinson, R. J. A., Gordon, J. D. M. Coral-associated invertebrates: Diversity, ecological importance and vulnerability to disturbance. Oceanography and Marine Biology: An Annual Review, edited by. , (2011).
- Stewart, H. L., Holbrook, S. J., Schmitt, R. J., Brooks, A. J. Symbiotic crabs maintain coral health by clearing sediments. Coral Reefs. 25 (4), 609-615 (2006).
- Williams, S. M. The reduction of harmful algae on Caribbean coral reefs through the reintroduction of a keystone herbivore, the long-spined sea urchin Diadema antillarum. Restoration Ecology. 30 (1), 13475 (2022).
- Francis, F. T., Filbee-Dexter, K., Yan, H. F., Côté, I. M. Invertebrate herbivores: Overlooked allies in the recovery of degraded coral reefs. Global Ecology and Conservation. 17, 00593 (2019).
- De Goeij, J. M., et al. Surviving in a marine desert: The sponge loop retains resources within coral reefs. Science. 342 (6154), 108-110 (2013).
- Rix, L., et al. Differential recycling of coral and algal dissolved organic matter via the sponge loop. Functional Ecology. 31 (3), 778-789 (2017).
- Plaisance, L., Caley, M. J., Brainard, R. E., Knowlton, N. The diversity of coral reefs: What are we missing. PLoS One. 6 (10), 25026 (2011).
- Leray, M., Knowlton, N. DNA barcoding and metabarcoding of standardized samples reveal patterns of marine benthic diversity. Proceedings of the National Academy of Sciences of the United States of America. 112 (7), 2076-2081 (2015).
- Pearman, J. K., et al. Disentangling the complex microbial community of coral reefs using standardized Autonomous Reef Monitoring Structures (ARMS). Molecular Ecology. 28 (15), 3496-3507 (2019).
- Pearman, J. K., et al. Cross-shelf investigation of coral reef cryptic benthic organisms reveals diversity patterns of the hidden majority. Scientific Reports. 8, 8090 (2018).
- Carvalho, S., et al. Beyond the visual: Using metabarcoding to characterize the hidden reef cryptobiome. Proceedings of the Royal Society B: Biological Sciences. 286 (1896), 20182697 (2019).
- Hartmann, A. C., et al. Meta-mass shift chemical profiling of metabolomes from coral reefs. Proceedings of the National Academy of Sciences of the United States of America. 114 (44), 11685-11690 (2017).
- Ransome, E., et al. The importance of standardization for biodiversity comparisons: A case study using autonomous reef monitoring structures (ARMS) and metabarcoding to measure cryptic diversity on Mo’orea coral reefs, French Polynesia. PLoS One. 12 (4), 0175066 (2017).
- Pennesi, C., Danovaro, R. Assessing marine environmental status through microphytobenthos assemblages colonizing the Autonomous Reef Monitoring Structures (ARMS) and their potential in coastal marine restoration. Marine Pollution Bulletin. 125 (1-2), 56-65 (2017).
- Bartley, R., et al. Relating sediment impacts on coral reefs to watershed sources, processes and management: A review. Science of the Total Environment. 468-469, 1138-1153 (2014).
- Häder, D. P., et al. Anthropogenic pollution of aquatic ecosystems: Emerging problems with global implications. Science of the Total Environment. 713, 136586 (2020).
- Bianchi, D., Carozza, D. A., Galbraith, E. D., Guiet, J., DeVries, T. Estimating global biomass and biogeochemical cycling of marine fish with and without fishing. Science Advances. 7 (41), (2021).
- Rogers, C. S. Responses of coral reefs and reef organisms to sedimentation. Marine Ecology Progress Series. 62, 185-202 (1990).
- Fabricius, K. E. Effects of terrestrial runoff on the ecology of corals and coral reefs: Review and synthesis. Marine Pollution Bulletin. 50 (2), 125-146 (2005).
- Littler, M. M., Littler, D. S., Brooks, B. L. Harmful algae on tropical coral reefs: Bottom-up eutrophication and top-down herbivory. Harmful Algae. 5 (5), 565-585 (2006).
- Scofield, V., Jacques, S. M. S., Guimarães, J. R. D., Farjalla, V. F. Potential changes in bacterial metabolism associated with increased water temperature and nutrient inputs in tropical humic lagoons. Frontiers in Microbiology. 6, 310 (2015).
- Cárdenas, A., et al. Excess labile carbon promotes the expression of virulence factors in coral reef bacterioplankton. ISME Journal. 12, 59-76 (2018).
- Johnson, M. D., et al. Rapid ecosystem-scale consequences of acute deoxygenation on a Caribbean coral reef. Nature Communications. 12, 4522 (2021).
- Altieri, A. H., et al. Tropical dead zones and mass mortalities on coral reefs. Proceedings of the National Academy of Sciences of the United States of America. 114 (14), 3660-3665 (2017).
- Timmers, M. A., et al. Biodiversity of coral reef cryptobiota shuffles but does not decline under the combined stressors of ocean warming and acidification. Proceedings of the National Academy of Sciences of the United States of America. 118 (39), 2103275118 (2021).
- Enochs, I. C., et al. Shift from coral to macroalgae dominance on a volcanically acidified reef. Nature Climate Change. 5 (12), 1083-1088 (2015).
- Nelson, H. R., Altieri, A. H. Oxygen: The universal currency on coral reefs. Coral Reefs. 38, 177-198 (2019).
- Wallace, R. B., Baumann, H., Grear, J. S., Aller, R. C., Gobler, C. J. Coastal ocean acidification: The other eutrophication problem. Estuarine, Coastal and Shelf Science. 148, 1-13 (2014).
- Haas, A. F., et al. Effects of coral reef benthic primary producers on dissolved organic carbon and microbial activity. PLoS One. 6 (11), 27973 (2011).
- Shafir, S., Van Rijn, J., Rinkevich, B. A mid-water coral nursery. Proceedings of the 10th International Coral Reef Symposium. , 1674-1679 (2006).
- Rinkevich, B. The active reef restoration toolbox is a vehicle for coral resilience and adaptation in a changing world. Journal of Marine Science and Engineering. 7 (7), 201 (2019).
- Nakamura, T., Van Woesik, R. Water-flow rates and passive diffusion partially explain differential survival of corals during the 1998 bleaching event. Marine Ecology Progress Series. 212, 301-304 (2001).
- Dennison, W. C., Barnes, D. J. Effect of water motion on coral photosynthesis and calcification. Journal of Experimental Marine Biology and Ecology. 115 (1), 67-77 (1988).
- Mass, T., Genin, A., Shavit, U., Grinstein, M., Tchernov, D. Flow enhances photosynthesis in marine benthic autotrophs by increasing the efflux of oxygen from the organism to the water. Proceedings of the National Academy of Sciences of the United States of America. 107 (6), 2527-2531 (2010).
- Finelli, C. M., Helmuth, B. S., Pentcheff, N. D., Wethey, D. S. Intracolony variability in photosynthesis by corals is affected by water flow: Role of oxygen flux. Marine Ecology Progress Series. 349, 103-110 (2007).
- Nakamura, T., Yamasaki, H., Van Woesik, R. Water flow facilitates recovery from bleaching in the coral Stylophora pistillata. Marine Ecology Progress Series. 256, 287-291 (2003).
- Nakamura, T., Yamasaki, H. Requirement of water-flow for sustainable growth of Pocilloporid corals during high temperature periods. Marine Pollution Bulletin. 50 (10), 1115-1120 (2005).
- McDole, T., et al. Assessing coral reefs on a Pacific-wide scale using the microbialization score. PLoS One. 7 (9), 43233 (2012).
- Haas, A. F., Jantzen, C., Naumann, M. S., Iglesias-Prieto, R., Wild, C. Organic matter release by the dominant primary producers in a Caribbean reef lagoon: Implication for in situ O2 availability. Marine Ecology Progress Series. 409, 27-39 (2010).
- Haas, A. F., et al. Influence of coral and algal exudates on microbially mediated reef metabolism. PeerJ. 1, 108 (2013).
- Silveira, C. B., et al. Microbial processes driving coral reef organic carbon flow. FEMS Microbiology Reviews. 41 (4), 575-595 (2017).
- Knowles, B., et al. Lytic to temperate switching of viral communities. Nature. 531 (7595), 466-470 (2016).
- Szmit, R. Geometry design and structural analysis of steel single-layer geodesic domes. 2017 Baltic Geodetic Congress (BGC Geomatics). , 205-209 (2017).
- Laila, T., Arruda, A., Barbosa, J., Moura, E. The constructive advantages of Buckminster Fuller’s geodesic domes and their relationship to the built environment ergonomics. Advances in Ergonomics in Design. Proceedings of the AHFE 2017 International Conference on Ergonomics in Design, July 17-21, 2017. , (2018).
- Graham, N. A. J., Nash, K. L. The importance of structural complexity in coral reef ecosystems. Coral Reefs. 32, 315-326 (2013).
- Alldredge, A. L., King, J. M. Distribution, abundance, and substrate preferences of demersal reef zooplankton at Lizard Island Lagoon, Great Barrier Reef. Marine Biology. 41, 317-333 (1977).
- Scheffers, S. R., Nieuwland, G., Bak, R. P. M., Van Duyl, F. C. Removal of bacteria and nutrient dynamics within the coral reef framework of Curaçao (Netherlands Antilles). Coral Reefs. 23 (3), 413-422 (2004).
- Van Duyl, F. C., Scheffers, S. R., Thomas, F. I. M., Driscoll, M. The effect of water exchange on bacterioplankton depletion and inorganic nutrient dynamics in coral reef cavities. Coral Reefs. 25, 23-36 (2006).
- Reidenbach, M. A., Stocking, J. B., Szczyrba, L., Wendelken, C. Hydrodynamic interactions with coral topography and its impact on larval settlement. Coral Reefs. 40 (2), 505-519 (2021).
- Reidenbach, M. A., Koseff, J. R., Koehl, M. A. R. Hydrodynamic forces on larvae affect their settlement on coral reefs in turbulent, wavedriven flow. Limnology and Oceanography. 54 (1), 318-330 (2009).

