Repeated Transcranial Magnetic Stimulation Combined with Action Observation Training in Children with Spastic Cerebral Palsy
Summary
Children with spastic cerebral palsy (SCP) have limb spasticity, movement disorders, and abnormal posture due to injury to the cerebral cortex motor area, resulting in inability to stand and walk normally. Therefore, alleviating limb spasticity and enhancing gross motor function in children with SCP have become important therapeutic goals.
Abstract
This study presents the results of a randomized controlled trial utilizing a 2 x 2 factorial design, comparing the effects of repeated transcranial magnetic stimulation (rTMS) and action observation training (AOT) intervention methods on spasticity, balance function, and motor function in children with spastic cerebral palsy (SCP). The study aimed to investigate whether the combination of the two interventions produces greater improvement than either treatment alone or conventional treatment.
Subject children in this study, in accordance with the random number table, were randomly divided into four groups: conventional group, rTMS group, AOT group, and combined intervention group. All the children in the four groups received conventional rehabilitation treatment, on the basis of which they were given different therapeutic programs of rehabilitation measures. The conventional group had no other treatment while the rTMS group received rTMS, the AOT group received AOT and the combined intervention group was given a combined intervention of rTMS and AOT. They were trained five days per week for 12 weeks. Changes in scores of spasticity, balance function, walking ability, and gross motor function were assessed at the onset of the training program and upon completion of 12 weeks of treatment.
A total of 64 Children with SCP completed the study, and their results were analyzed. The total gross motor function efficiency of 87.50% in the experimental group was significantly higher than that of 25.00% in the conventional group, 62.50% in the rTMS group, and 68.75% in the AOT group. The preliminary results showed that combined intervention of rTMS and AOT could effectively improve the balance function and motor function of children, and the therapeutic effect of the combined intervention was better than that of conventional treatment, rTMS or AOT alone. Finally, clinical efficacy and optimal treatment parameters of the combined intervention were clarified to provide a clinical basis for therapists to conduct lower limb function rehabilitation for children with SCP.
Introduction
Cerebral palsy1 (CP) is one of the most common disabling disorders in children and is a group of persistent syndromes caused by non-progressive brain damage in developing fetuses and infants, including central dyskinesia, abnormal posture, and limited mobility. Currently, there are approximately 17 million children worldwide affected by cerebral palsy2, with over 5 million cases in China. Among various forms of cerebral palsy, spastic cerebral palsy (SCP) accounts for roughly 80%3. Children with SCP suffer from brain damage resulting in muscle spasms, diminished sensory function, impaired muscle coordination, and decreased balance capabilities, all of which hinder independent walking and daily activities. Current rehabilitation measures for enhancing gross motor function in children with SCP encompass activities such as core stability training4, task-oriented training5, constraint-induced movement therapy6, and mirror visual feedback therapy7. These measures can improve clinical symptoms of children by regulating and reshaping the central nervous system from bottom to top by improving the functions of peripheral organs.
Transcranial magnetic stimulation (TMS)8,9 is a painless, non-invasive, and safe therapeutic method. rTMS refers to the continuous release of stimuli at equal intervals after a single command, which is one of the regular repetitive stimulation patterns. Based on the principle of electromagnetic induction and electromagnetic conversion, it applies transient currents through stimulation coils to form a pulsed magnetic field to penetrate the skull, generate induction current to stimulate neurons and trigger a series of physiological and biochemical responses. rTMS has been applied to the monitoring, evaluation, and treatment of neurological diseases, providing a novel approach to exploring the structure and function of the brain10. Existing studies11,12 have shown that rTMS has varying therapeutic effects on limb spasms and motor function in children with CP. Moreover, the effect of rTMS treatment combined with other rehabilitation techniques is more noticeable.
AOT is a rehabilitation method that utilizes the Mirror Neuron System (MNS) to establish motor learning and memory13,14. The essence of AOT is to make the observer carefully observe actions in the video and subsequently try their best to imitate what they observed. The observing process might trigger activation in the neural network known as MNS, akin to the activation that occurs when one is personally engaged in executing those actions15, which provides a neurophysiological basis for AOT. AOT has shown success in improving motor skills in patients with movement disorders, yielding positive outcomes in stroke recovery and rehabilitation of upper limb motor function in cerebral palsy patients16,17.
Our previous studies have found that AOT based on the mirror neuron system could effectively improve the balance and walking ability of children with cerebral palsy18. In addition, studies19 have shown that rTMS can improve muscle spasticity, movement, and gait in children with SCP, but treatment standards of rTMS applied to children with cerebral palsy have not been unified, and it is an urgent problem to explore the influence of different parameters of rTMS on children with CP, to provide a personalized and precise treatment standard. We believe that combining rTMS with AOT has the potential to develop into a valuable physical therapy strategy for the neurological rehabilitation of cerebral palsy. Both rTMS and AOT stimulated the cerebral cortex in children with SCP20, thereby aiding gross motor function development. This study aimed to find out whether the combination of rTMS and AOT could achieve a greater synergistic effect than conventional treatment or rTMS or AOT alone.
Protocol
This study was carried out in strict accordance with the national standards for human experimentation, and the clinical study was approved by the Ethics Association of Xiangya Boai Rehabilitation Hospital (ethics approval number: 20211223). The children's guardians agreed to participate in this training program and signed informed consent forms. Children with SCP were recruited in the Xiangya Boai Rehabilitation Hospital and Xiangya Hospital of Central South University from February 2022 to December 2022.
1. Preparation before the experiment
- To ensure the accuracy of study results, provide relevant training to rehabilitation therapists and assess their skills in advance to make sure that they can guide the children in the training.
NOTE: The rTMS technicians should be trained and qualified to ensure the safety of children. - Conduct rTMS safety screening with parents before the experiment, including questioning them about evidence of seizures and medications taken.
NOTE: Medications can lower the seizure threshold.
2. Recruitment
- Recruit children with SCP whose clinical diagnosis and classification meet the criteria of China Cerebral Palsy Rehabilitation Treatment Guidelines (2022)21.
- Ensure that the children are between 3 and 6 years old.
- Ensure that the children have Gesell Developmental Diagnostic Scale (GDDS) adaptive behavioral area development quotient (DQ) > 55 so that they can actively participate in training.
- Ensure that the children have Gross motor function classification (GMFCS) I-III.
- Ensure that the children can walk more than 10 m independently or with a walker.
- Exclude the following: children with other types of CP; children scheduled for medical rehabilitation or surgery during the study period; children with epilepsy, severe intellectual impairment, visual impairment, or attention deficit; children with cochlear implants or intracranial implants.
- Before the training, obtain informed consent forms from children's guardians for voluntary participation in this training program 1x a day, 5 days per week for 12 weeks.
3. Pretreatment assessment
- Analyze the baseline data collected from recruited children with SCP, including name, sex, age, cerebral palsy spasticity type, GMFCS, cognitive level, ankle foot orthosis wear, previous or current medical treatment, and surgical history.
- Rehabilitation assessment
NOTE: Studies have shown that the Comprehensive Spasticity Scale (CSS) used to assess the degree of lower limb spasm in children with SCP has good reliability and validity22.- Determine the degree of spasm by examining the lower limb Achilles tendon reflex, the muscle tension of the ankle plantar flexor group, and the ankle clonus. Use the following evaluation criteria: no spasm, <7; mild spasm, 7-9; moderate spasm, 10-12; and severe spasm, 13-16.
- Assess balance function according to the Pediatric Balance Scale (PBS)23, which includes 14 tested items. Each item is divided into five levels with 0-4 points for each item and 56 points in total. The higher the score, the better the balance function.
NOTE: PBS is based on the BBS Children's Revision, mainly adjusting the order of the tested items, shortening the time for maintaining posture, and clarifying test instructions. - Assess the children's walking ability based on the 10 m walking speed (10MWS)24.
- Ask children to walk as fast as possible on a runway with specifications of width x length =20 cm x 15 m, independently or wearing an ankle-foot orthotic or using a walking aid for children with GMFCS class III.
- Ask children to start walking at the 15 m marker; start timing at 12 m, and stop at 2 m to measure the time taken for the middle 10 m. Ask the children to perform three consecutive 10MWT tests and calculate the walking speed (m/s).
NOTE: The functional level of the children was graded I-III according to GMFCS, meaning they have certain standing and walking ability. - Evaluate the gross motor function level of the children by selecting a standing position in area D (13 items) and walking, running, and jumping in area E (24 items) in the Gross Motor Function Measure (GMFM). Calculate the score according to the completion degree of each item (0-3 points) and get the total score of Zones D and E. The higher the score, the better the gross motor function.
- Calculate the efficacy rate using equations (1) and (2) and assess the clinical effectiveness of gross motor function interventions by measuring the difference in the scores from the GMFM-88E section before and after treatment.
Efficacy rate (%) =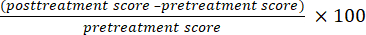 (1)
(1)
Efficacy rate = significant effective rate + effective rate (2)- Consider the outcome to be significantly effective if the efficacy rate is >50%.
- Consider the outcome to be effective if the efficacy rate is between 20% and 50%.
- Consider the outcome to be ineffective if the efficacy rate is <20%.
4. Therapeutic method
NOTE: Explain the principles and objectives of the training program, as well as possible adverse effects and safety issues during the training process, to the guardians of the children.
- Randomly divide the children with SCP into four different groups: a conventional group, a rTMS group, an AOT group, and a combination intervention group according to the numerical table method (16 cases in each group in this study).
- Ensure that all the children receive conventional rehabilitation treatment, based on which they will be given different rehabilitation treatment measures. The conventional group receives no other rehabilitation treatment; the rTMS group receives rTMS; the AOT group receives AOT; and the study group receives rTMS combined with AOT.
- Explain in detail to the families of the subject children the possibilities of their participation in training programs of conventional rehabilitation, rTMS, AOT, and combined interventions. Ensure that the researchers are blinded to the allocation of the subjects and are not involved in the assessment.
- According to the admission order of these children, generate a numerical list as a spreadsheet. Randomize into groups as follows: numbers 1, 5, 9… for the regular group; numbers 2, 6, 10… for the rTMS group; numbers 3, 7, 11… for the AOT group; and numbers 4, 8, 12… for the combined intervention group. To ensure privacy, use a curtain to provide each child with a separate space during each treatment session.
- Conventional rehabilitation
- Exercise therapy
- Explain the principle and purpose of exercise therapy to the guardians of the children and notify them that the duration of treatment is 30 min.
- According to their muscle strength rating, instruct the children to perform muscle strength exercises for the training of hip abductor muscles, hip posterior extensor muscles, and ankle dorsal extensor muscles, 10x per set and three sets per day.
- According to their balance function level, instruct the children to conduct balance training such as standing without support, standing with feet together, and standing on one leg, with a duration of 10 min each time.
- According to their walking ability, instruct the children to carry out walking training such as handholding, independent walking, and crossing obstacles, with a duration of 10 min each time.
- Hydrotherapy
- Explain the principle, process, and purpose of hydrotherapy to guardians of the children and notify them that the duration of treatment is 20 min.
- When using the special hydrotherapy machine for children, adjust the water temperature to an appropriate temperature of 37-38 °C.
- Ask the parents to help their children take off their clothes, put on waterproof swimming trunks and hydrotherapy collars, and then get them into the hydrotherapy machine.
- Click on the bubble bath button to start treatment and instruct the children to stand, turn around, and take steps alternately with both feet in the water.
- Massage treatment
- Have the children diagnosed first.
- Explain the effects of massage treatment to the guardians of the children and notify them that the duration of treatment is 30 min.
- Place the children in the prone position and apply alternating pinching and pushing techniques 5-10x along both sides of the coronary artery and the bladder meridian.
- Place the children in the prone position and press, using the end of the thumb, the acupuncture points of Futu, Liangqiu, Zusanli, Jiexi, Huantiao, Chengfu, and Wuizhong from the top down.
- Place the children in the supine or prone position and apply rhythmic lifting and pinching 10-20x with the thumb and other four fingers alternately on the quadriceps, hamstrings, and calf triceps muscle at the same time.
- Exercise therapy
- rTMS
NOTE: The physical therapist engaged in performing rTMS first participated in the training arranged by the manufacturer. For the safety of the participants, the therapist or other trained medical professionals must supervise the entire treatment process and inform the guardians in advance that the pulse generated by rTMS may cause temporary and mild pain and slight twitching of the face and limbs when it hits the scalp.- rTMS patient preparation
- At the first visit, let the physical therapist know about the basic information and medical history of the subject children.
- Explain the process of rTMS treatment and possible responses to the guardians of the children and notify them that the duration of treatment is approximately 30 min.
- Place the children in the supine or seated position, degrease their hands with medical alcohol, and choose a suitable positioning cap for each of them according to their head circumference. Place the naso-occipital line on the positioning cap on the median line of the participating children, and the crossing point of the naso-occipital line and the temporal-parietal line at the midpoint of the line between the eyebrow and the occipital posterior carina.
NOTE: The appropriate size should be selected according to their head circumferences. - Affix the recording electrode to the abdominal muscle of the abductor pollicis brevis of the participating children, the reference electrode to the tendon of the abductor pollicis brevis, and the bottom electrode to the wrist.
- rTMS operation preparation
- Start the computer application on the rTMS instrument, input the basic information and diagnoses of the participating children, and determine the treatment plan.
- The stimulation coil is a circular air-cooled coil, the center of which coincides with the contralateral M1 area corresponding to the identified abductor pollicis brevis. Place the coil at 45° to the scalp.
NOTE: The positioning cap was designed according to the electroencephalography (EEG) 10-20 electrode arrangement system, which could quickly locate the M1 region. - Select a stimulation intensity of 30% and stimulate the M1 region corresponding to the contralateral abductor pollicis brevis muscle by manual monopulse.
NOTE: During the treatment, the participating children should hold their position to avoid deviation of the stimulation target. - Observe the motor evoked potentials interface graph and gradually reduce the stimulation intensity. Define the RMT as the minimum stimulus intensity that will elicit this response if at least 5 out of 10 consecutive stimulations of the child's primary motor cortex (M1) induce a motor potential strength of more than 50 mV in the contralateral abductor hallucis brevis muscle (Figure 1A).
- Click on the confirm button, save the RMT record, and then enter the treatment parameter setting page.
- rTMS treatment
- Use the following settings to perform low-frequency stimulation on the M1 region of the undamaged cerebral cortex: stimulation frequency 1 Hz, stimulation intensity 80% RMT, stimulation number 10, stimulation time 10 s, the number of repetitions 80, and the total number of pulses 80025,26 (see Figure 1B).
- Use the following settings to perform high-frequency stimulation of the M1 region of the damaged cerebral cortex: stimulation frequency 5 Hz, stimulation intensity 100% RMT, stimulation number 15, stimulation time 3 s, repetition times 80, and the total number of pulses 1,20010(see Figure 1C).
- Observe the responses of the participating children. For example, if the children start crying due to scalp beating, have excessive facial twitching, or show signs of a seizure and other adverse reactions, stop the treatment immediately.
NOTE: For children with spastic diplegia/quadriplegia, high-frequency stimulation of the M1 region of the damaged cerebral cortex was performed alternately on both sides of the damaged brain. For those with spastic hemiplegia, the M1 region of the undamaged cerebral cortex was stimulated at a low frequency, while the damaged cerebral cortex of M1 area was stimulated at a high frequency.
- rTMS patient preparation
- AOT
NOTE: AOT requires physical therapists to video subjects' body movements in advance and supervise the safety and training accuracy of the whole treatment process.- Explain the principle, process, and precautions of AOT to the guardians of the children, and notify them that the duration is 30 min.
- Have two professional physiotherapists shoot videos of body movements from three different angles: front, side, and back, and design six body movements for the purpose of improving lower limb balance and walking function.
- The first movement goes from sitting to standing: move the body forward from the upright sitting position by lifting the pelvis and extending the knee joint, gradually converting to the standing position, and then sit down. Perform this 3x consecutively (Figure 2A).
- The second movement is a standing exercise: move the body weight to the left or right as far as possible and then back to the neutral position. Perform it 3x in turn (Figure 2B).
- The third movement is also a standing exercise: move the body weight forward or backward as far as possible and then return to the neutral position. Perform it 3x in turn (Figure 2C).
- The fourth movement is also a standing exercise: rotate the body to the left or right as far as possible and then return to the neutral position. Perform it 3x in turn (Figure 2D).
- The fifth movement is a standing exercise: walk sideways to the left or right and then return to the neutral position. Perform it 3x in turn (Figure 2E).
- The sixth movement is a standing exercise: alternately step up or down a 10 cm high step with the left and right feet. Perform it 3x in turn (Figure 2F).
- Get the children gathered in a quiet room and sitting 5 m away from an 86 inch TV in comfortable positions.
- Have the physiotherapist explain the body movements first so that the children can then focus on watching the video.
- After watching the video, ask the children to imitate these movements.
5. Post treatment evaluation
NOTE: The pretreatment and 12 week post treatment evaluation for each patient would be completed by the same pediatric rehabilitation doctor.
- After 12 weeks of different rehabilitation measures for the four groups of children, have the same pediatric rehabilitation doctor re-evaluate the clinical efficacy of CSS, PBS, 10 MWS, GMFM, and gross motor function.
- Record the guardians' satisfaction and feedback on the treatment plan, including the degree of enjoyment of the treatment and improvement in self-awareness, as well as their wishes and suggestions for continuing the treatment.
6. Statistical analysis
- Enter the demographics, CSS, PBS, 10 m walking speed (10MWS), GMFM, and effective rate (%) assessment data into the software for statistical analysis.
- Analyze the counting data using the χ2 Test or Fisher Probabilistic Precision method.
- Determine whether the data conform to a normal distribution and express normally distributed data as mean ± SD.
- Use repetitive measurement deviation analysis for comparison among the four groups and perform repeated measures analysis of variance under the mixed model to verify whether the results and variation scores of each group are consistent with the approximately normal distribution.
- Consider time as an intra-group factor and intervention an inter-group factor. If there is any interaction between these two factors, test the inter-group difference at each time point at a= 0.05. If there is no interaction effect, test the main effect. Consider differences to be statistically significant at P < 0.05.
Representative Results
This paper presents the results of 64 children with SCP (Supplementary Table S1 and Supplementary File 1), who were randomly divided into four groups according to the numerical table method and given different rehabilitation measures for 12 weeks. During the entire process, the participating children had no adverse reactions such as headaches, dizziness, and seizures.
The demographic data of the four groups of children are shown in Table 1. Before treatment, there were no significant differences in the sex ratio, age, spastic category of cerebral palsy, GMFCS, GDDS developmental quotient (GDDS DQ), and usage of assistive devices (ankle foot orthosis or walker) (all P > 0.05).
The CSS scores of the four groups of children before and after the 12-week treatment have been compared in Table 2. After 12 weeks of training, the CCS scores of all four groups significantly decreased, with the combination intervention group showing significantly greater changes than the other three groups. The time effect and the interaction effect between groups and time were significant (P < 0.05), while the effect between groups was not significant (P > 0.05).
The scores of PBS, 10MWS, and GMFM before and after the 12-week training of the four groups are shown in Table 3, Table 4, and Table 5. Compared to the pretraining scores, the scores of PBS, 10MWS, and GMFM of all four groups significantly increased after the treatment, with the scores of the combination intervention group improving the most. The time effect and the interaction effect between groups and time were significant (P < 0.05), while the effect between groups was not significant (P > 0.05).
The clinical effects on gross motor function in all four groups of children are represented in Table 6. The total efficacy rate of gross motor function in the combination intervention group was 87.50%, which was significantly higher than those of the remaining three groups (rTMS group 62.50%, AOT group 68.75%, conventional group 25.00%) (χ2 = 13.850, P = 0.003).
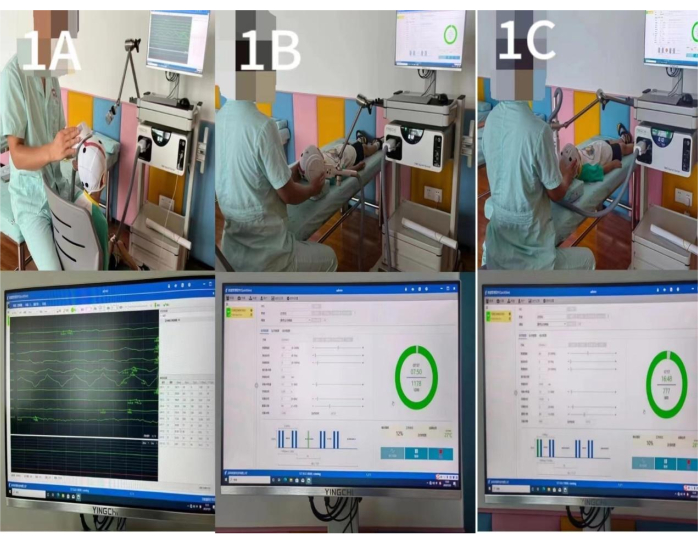
Figure 1: Stimulation of the M1 area. (A) Threshold determination: The value of RMT was measured when the contralateral M1 region corresponding to the abductor pollicis breve muscle, which was stimulated with a single pulse to generate a motion-evoked potential. (B) Low-frequency stimulation on the M1 region of the undamaged cerebral cortex: stimulation frequency was 1Hz, stimulation intensity was 80% RMT, stimulation number was 10, stimulation time lasted for 10 s, the number of repetitions was 80 times, and the total number of pulses was 800. (C) High-frequency stimulation of the M1 region of the damaged cerebral cortex: stimulation frequency was 5 Hz, stimulation intensity was 100% RMT, stimulation number was 15, stimulation time lasted for 3 s, repetition times was 80 times, and the total number of pulses was 1,200. Please click here to view a larger version of this figure.
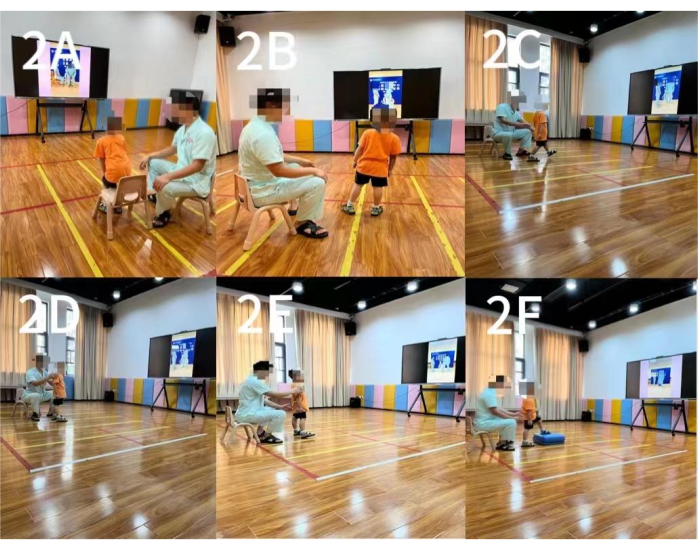
Figure 2: Action observation training. (A) The first movement: Sit-to-Stop Conversion. (B) The second movement: left/right bodyweight shifting. (C) The third movement: back/forth bodyweight shifting. (D) The fourth movement: left/right body rotation. (E) The fifth movement: left/right walking. (F) The sixth movement: stepping up/down alternatively. Please click here to view a larger version of this figure.
| (n=64, x±s) | |||||||||
| Item | Conventional Group | rTMS Group | AOT Group | combination intervention group | χ2 /F | P | |||
| Number (n) | 16 | 16 | 16 | 16 | |||||
| Sex Ratio (Male/Female) | 10/6 | 10/6 | 13/3 | 9/7 | 2.494 | 0.476 | |||
| Age (Year) | 4.44±0.80 | 4.74±0.68 | 4.71±0.54 | 4.63±0.68 | 0.654 | 0.683 | |||
| Classification of spastic cerebral palsy (spastic hemip legia/spastic dip legia/spastic quadriplegia) | 02-07-2007 | 5/10/1 | 6/9/1 | 5/10/1 | 2.105 | 0.945 | |||
| GMFCS (n) (Level I/II/III) | 7/3/6 | 6/5/5 | 8/5/3 | 8/5/3 | 2.750 | 0.868 | |||
| GDDS DQ | 70.06±10.25 | 70.13±9.44 | 71.56±12.58 | 69.25±6.89 | 0.148 | 0.931 | |||
| Usage of assistive devices (n) Yes/No |
5/11 |
3/13 | 3/13 | 4/12 | 1.780 | 0.699 | |||
Table 1: Comparison of the basic information of the four groups of children (n = 64, x ± s). *One-way analysis of variance. Before the training, there were no significant differences in sex ratio, age, spastic category of cerebral palsy, GMFCS, GDDS developmental quotient (GDDS DQ), and usage of assistive devices (ankle foot orthosis or walker), indicating comparability. Abbreviations: GMFCS = Gross motor function classification; GDDS DQ = Gesell developmental diagnosis schedule developmental quotient.
| Group | Number (n) | BEFORE | AFTER | F | P | ||
| Conventional Group | 16 | 10.63±1.67 | 10.19±1.76 | ||||
| rTMS Group | 16 | 10.88±1.41 | 9.75±1.13 | ||||
| AOT Group | 16 | 10.75±1.13 | 9.75±1.00 | ||||
| combination intervention group | 16 | 10.69±1.01 | 8.88±1.02 | ||||
| Group Factor | 0.774 | 0.513 | |||||
| Time Factor | 228.261 | <0.001 | |||||
| Group Factor * Time Factor | 15.217 | <0.001 | |||||
Table 2: Comparison of CSS scores in the four groups of children (n = 64, x ± s). After treatment, repeated-measures ANOVA results showed that the time effect and the interaction effect between groups and time were significant (P < 0.05), while the effect between groups was not significant (P > 0.05). Abbreviations: CSS = Comprehensive Spasticity Scale; rTMS = repeated transcranial magnetic stimulation; AOT = action observation training.
| Group | Number (n) | BEFORE | AFTER | F | P | ||
| Conventional Group | 16 | 28.25±9.38 | 31.13±9.22 | ||||
| rTMS Group | 16 | 29.44±10.05 | 35.56±9.82 | ||||
| AOT Group | 16 | 29.25±9.84 | 35.94±8.62 | ||||
| combination intervention group | 16 | 29.81±11.59 | 41.88±8.03 | ||||
| Group Factor | 1.12 | 0.348 | |||||
| Time Factor | 371.208 | <0.001 | |||||
| Group Factor * Time Factor | 27.954 | <0.001 | |||||
Table 3: Comparison of PBS scores in the four groups of children (n = 64, x ± s). After treatment, repeated-measures ANOVA results showed that the time effect and the interaction effect between groups and time were significant (P < 0.05), while the effect between groups was not significant (P > 0.05). Abbreviations: PBS = Pediatric Balance Scale; rTMS = repeated transcranial magnetic stimulation; AOT = action observation training.
| Group | Number (n) | BEFORE | AFTER | F | P | ||
| Conventiona Group | 16 | 1.02±0.14 | 1.10±0.16 | ||||
| rTMS Group | 16 | 0.98±0.18 | 1.15±0.16 | ||||
| AOT Group | 16 | 0.99±0.12 | 1.15±0.09 | ||||
| combination intervention group | 16 | 1.02±0.15 | 1.24±0.11 | ||||
| Group Factor | 0.946 | 0.424 | |||||
| Time Factor | 501.551 | <0.001 | |||||
| Group Factor * Time Factor | 19.275 | <0.001 | |||||
Table 4: Comparison of 10MWS scores in the four groups of children (n = 64, x ± s). After treatment, repeated-measures ANOVA results showed that the time effect and the interaction effect between groups and time were significant (P < 0.05), while the effect between groups was not significant (P > 0.05). Abbreviations: 10MWS = 10 m walking speed; rTMS = repeated transcranial magnetic stimulation; AOT = action observation training.
| Group | Number (n) | BEFORE | AFTER | F | P | ||
| Conventiona Group | 16 | 46.63±20.05 | 54.00±22.19 | ||||
| rTMS Group | 16 | 48.94±19.96 | 61.94±20.61 | ||||
| AOT Group | 16 | 50.25±15.25 | 63.63±16.40 | ||||
| combination intervention group | 16 | 50.94±18.43 | 75.69±17.86 | ||||
| Group Factor | 1.300 | 0.283 | |||||
| Time Factor | 502.502 | <0.001 | |||||
| Group Factor * Time Factor | 31.184 | <0.001 | |||||
Table 5: Comparison of GMFM scores in the four groups of children (n = 64, x ± s). After treatment, repeated-measures ANOVA results showed that the repeated-measures ANOVA results showed that the time effect and the interaction effect between groups and time were significant (P < 0.05), while the effect between groups was not significant (P > 0.05). Abbreviations: GMFM = gross motor function measure; rTMS = repeated transcranial magnetic stimulation; AOT = action observation training.
| Group | Number | obvious effective | Effective | Ineffective | Effective rate | ||
| Conventional Group | 16 | 1 | 3 | 12 | 25.00% | ||
| rTMS Group | 16 | 3 | 7 | 6 | 62.50% | ||
| AOT Group | 16 | 2 | 9 | 5 | 68.75% | ||
| combination intervention group | 16 | 7 | 7 | 2 | 87.50% | ||
| χ2 | 13.850 | ||||||
| P | 0.003 | ||||||
| Abbreviations: rTMS = repeated transcranial magnetic stimulation; AOT = action observation training. | |||||||
Table 6: Comparison of gross motor function of all four groups of children (n [%]). Abbreviations: rTMS = repeated transcranial magnetic stimulation; AOT = action observation training.
Supplementary File 1: Case information of a patient. The patient was treated with rTMS combined with AOT. After 12 weeks of treatment, the data of various evaluation indicators before and after treatment were compared to determine the clinical effect of the combined intervention on the patient's gross motor function. Please click here to download this File.
Supplementary Table S1: Patient data. Please click here to download this File.
Discussion
For children with SCP, increased activity of γ and α neurons leads toinhibition of corticospinal tract input, which results in increased muscle tension known as spasm. As limb spasms significantly affect the development of lower limb motor function of children with SCP, one of the crucial training goals is to reduce spasticity. Currently, stepwise treatment strategies are employed to alleviate spasticity, including rehabilitation nursing, physical therapy, orthopedic brace application, botulinum toxin injections27, drug treatments, and surgical interventions28. In this study, rehabilitation techniques such as exercise therapy, hydrotherapy, massage therapy, and rTMS were used to relieve spasms in children with SCP. Table 2 shows that the CSS scores in the combination intervention group exhibited greater improvement than the other three groups, suggesting that combined intervention of rTMS and AOT is more effective in relieving the spasticity of children with SCP. Rajak et al.12 suggested that increasing the frequency of rTMS treatment can help reduce muscle spasms in CP children and facilitate the development of motor function. Gupta et al.29 treated 20 cases of cerebral palsy patients with rTMS at 5 Hz and 10 Hz for 20 days, stimulating the motor cortex of the brain, and results showed that the muscle tension of rTMS treatment was significantly lower than that of the conventional group. This finding was consistent with the result of the present study in which the CSS scores of the experimental group were significantly better than those of the other three groups of children.
Before accepting rTMS treatment, it was necessary to measure the motor threshold of the children first to determine their treatment intensity. For children with spastic hemiplegia, the healthy side was evaluated first, then the affected side. The motor threshold of the healthy side was used to determine the treatment intensity. During rTMS treatment, positioning caps should be worn correctly, the coil stimulation center should coincide with the fixed point determined by the coil, and the coil should be placed at a 45°with the scalp. In this study, the M1 region was selected as a stimulation site to improve lower limb movement disorders. Low-frequency stimulation with 1 Hz frequency can reduce cerebral blood flow and excitability of the stimulation side and increase the excitability of the contralateral side. High-frequency stimulation with 5 Hz frequency could directly improve cortical excitability of the stimulated side. The aim is to achieve better inter-hemispheric inhibition, and alternating low- and high-frequency stimulation is conducive to the recovery of motor function.
AOT represents a promising approach for cerebral palsy intervention, restoring damaged brain networks to re-establish motor function as an adjunct to physiotherapy15,30. According to this mechanism, scholars have explored AOT's synergy with constraint-induced therapy6, whole-body vibration training31, and brain-computer interface systems32 in cerebral palsy rehabilitation. AOT must present meaningful actions to observers and requires children to have a certain cognitive ability to imitate the actions in the video. The key point of AOT was observation, not execution of actions. As balance disorders could affect independent walking and daily activities of children with SCP, enhancing their balance control ability was particularly important. This study incorporated AOT programs involving functional movements to enhance lower limb balance and walking abilities. The children attentively watched the video, and physical therapists informed them of more details of the actions presented in the video and guided and supervised the children to perform the actions correctly. During training, physiotherapists need to elucidate movement details to children and adopt incentives to ensure spontaneous participation. AOT has achieved good therapeutic effects in remote rehabilitation environments for children with CP due to its simple operation33. Therefore, parents are encouraged to implement intensive home-based training to enhance training effectiveness. Meanwhile, the training effectiveness is closely related to the training program that patients adhere to during the observation and/or execution of movements. Different learning and performance outcomes will be produced based on varying attention levels paid to observe the training resources34. Kim et al.35 found no substantial difference when comparing the results of short and long-term AOT interventions.
In this study, on the basis of conventional rehabilitation, the effects of four different ways of intervention, namely, conventional rehabilitation measures alone, rTMS, AOT, and combined intervention of rTMS and AOT, on the gross motor function of Children with SCP were respectively administered to explore whether the combined intervention has a synergistic effect. Table 3, Table 4, and Table 5 illustrate that after 12 weeks of treatment, scores of PBS, 10MWS, and GMFM improved for all groups. However, the combined intervention group exhibited the most substantial enhancement in PBS, 10MWS, and GMFM scores compared to the conventional, rTMS, and AOT groups. Moreover, its gross motor function effective rate was also significantly higher. Table 6 shows that the gross motor function effective rate in the combined intervention group was 87.50%, surpassing the conventional group (25.00%), rTMS group(62.50%), and AOT group (68.75%) (χ2=13.850, P=0.003), suggesting that the combined intervention of rTMS and AOT is more effective in improving the motor function of children with SCP. The regulation mechanisms might be: first, the rTMS site is the M1 region of the lower limb motor region of the cerebral cortex, which is close to the region where AOT activates MNS. Movement observation and execution strengthen the connection between the motor cortex and the M1 region through MNS36. Second, Sun et al.37confirmed that rTMS regulates the excitability of the target cerebral cortex by applying a magnetic field to the target cerebral region to generate an inductive current, which can propagate along the corticospinal tracts and peripheral motor nerves, leading to muscle responses that promote the recovery of limb motor function.AOT can provide children with the expected experience and preparation of the action by first observing the video, and promote the task-dependent neuronal network activity when performing the same task38. Third, rTMS stimulation of lateral motor cortical areas induces trans-synaptic activation of subcortical circuits via induced currents, producing reduced motor and muscle spastic activity to establish correct movement patterns39. Action videos of the AOT program can be observed repeatedly and simultaneously enhance motor memory and promote cerebral cortex remodeling through repeated execution of the program40. The combined intervention could synergistically regulate cortical activity, increase the excitability of the motor cortex and the recruitment of motor units, reduce spasticity and muscle hyperreflexia, and enhance the effect of mutual interaction. For these reasons, rTMS combined with AOT may have a synergistic effect to optimize neuroplastic changes and improve motor function recovery.
The combination of rTMS and AOT emerges as an effective strategy for individualized rehabilitation for lower limb dysfunction in children with SCP, improving balance and motor function. There are also shortcomings in this study: placebo effects may be present for rTMS since a sham rTMS condition was not used. Most of the assessment methods used in the study were rehabilitation assessment scales, and no objective assessment with imaging such as neurophysiology, electroencephalography, or functional MRI was applied. Besides, large-scale, long-term clinical studies and randomized controlled trials with objective neurophysiological or imaging evaluation are required to obtain standardized treatment parameters for rTMS therapy and AOT or to determine the therapeutic effect.
Disclosures
The authors have nothing to disclose.
Acknowledgements
This study was supported by the funding of the Xiangya Boai Rehabilitation Hospital.
Materials
| K6 multimedia scene interactive training system | Hunan Le Jiekang technology Co., LTD | Program implementation | |
| KLW-SLL type spa machine for children | Nanjing Kanglongwei Technology Industrial Co., LTD | Conventional therapy | |
| Pulse magnetic field stimulator model YRD CCY-II | Shenzhen Yingzhi technology Co., LTD | Program implementation | |
| SPSS26 software | IBM | Statistic analysis |
References
- Sadowska, M., Sarecka-Hujar, B., Kopyta, I. Cerebral palsy: current opinions on definition, epidemiology, risk factors, classification and treatment options. Neuropsychiatric Dis Treat. 16, 1505-1518 (2020).
- Alpay, S. Z., Kim, S. K., Oh, K. J., Graham, S. F. Advances in cerebral palsy biomarkers. Adv Clin Chem. 100, 139-169 (2016).
- Paul, S., Nahar, A., Bhagawati, M., Kunwar, A. J. A review on recent advances of cerebral palsy. Oxid Med Cell Longev. 2022, 2622310 (2022).
- Huang, C., et al. Efficacy and safety of core stability training on gait of children with cerebral palsy: A protocol for a systematic review and meta-analysis. 의학. 99 (2), e18609 (2020).
- Kwon, H. Y., Ahn, S. Y. Effect of task-oriented training and high-variability practice on gross motor performance and activities of daily living in children with spastic diplegia. J Phys Ther Sci. 28 (10), 2843-2848 (2016).
- Jamali, A. R., Amini, M. The effects of constraint-induced movement therapy on functions of cerebral palsy children. Iran J Child Neurol. 12 (4), 16-27 (2018).
- Park, E. J., Baek, S. H., Park, S. Systematic review of the effects of mirror therapy in children with cerebral palsy. J Phys Ther Sci. 28 (11), 3227-3231 (2016).
- Fan, J., et al. The effectiveness and safety of repetitive transcranial magnetic stimulation on spasticity after upper motor neuron injury: A systematic review and meta-analysis. Front Neural Circuits. 16, 973561 (2022).
- Zewdie, E., et al. Safety and tolerability of transcranial magnetic and direct current stimulation in children: Prospective single center evidence from 3.5 million stimulations. Brain Stimul. 13 (3), 565-575 (2020).
- Dadashi, F., et al. The effects of repetitive transcranial magnetic stimulation (rTMS) on balance control in children with cerebral palsy. Annu Int Conf IEEE Eng Med Biol Soc. 2019, 5241-5244 (2019).
- Feng, J. Y., et al. Effect of infra-low-frequency transcranial magnetic stimulation on motor function in children with spastic cerebral palsy. Chinese Journal of Contemporary Pediatrics. 15 (3), 187-191 (2013).
- Rajak, B. L., Gupta, M., Bhatia, D., Mukherjee, A. Increasing number of therapy sessions of repetitive transcranial magnetic stimulation improves motor development by reducing muscle spasticity in cerebral palsy children. Ann Indian Acad Neurol. 22 (3), 302-307 (2019).
- Novak, I., et al. State of the evidence traffic lights 2019: systematic review of interventions for preventing and treating children with cerebral palsy. Curr Neurol Neurosci Rep. 20 (2), 3 (2020).
- Buchignani, B., et al. Action observation training for rehabilitation in brain injuries: a systematic review and meta-analysis. BMC Neurol. 19 (1), 344 (2019).
- Buccino, G. Action observation treatment: a novel tool in neurorehabilitation. Philos Trans R Soc Lond B Biol Sci. 369 (1644), 20130185 (2014).
- Lee, H. J., Kim, Y. M., Lee, D. K. The effects of action observation training and mirror therapy on gait and balance in stroke patients. J Phys Ther Sci. 29 (3), 523-526 (2017).
- Peng, T. H., et al. Action observation therapy for improving arm function, walking ability, and daily activity performance after stroke: a systematic review and meta-analysis. Clin Rehabil. 33 (8), 1277-1285 (2019).
- Fan, T. L., et al. Effects of action observation therapy based on mirror neuron theoryon the balance and walking ability of children with cerebral palsy. Chin J Child Health Care. 31 (2), 220-224 (2023).
- Noh, J. S., Lim, J. H., Choi, T. W., Jang, S. G., Pyun, S. B. Effects and safety of combined rTMS and action observation for recovery of function in the upper extremities in stroke patients: A randomized controlled trial. Restor Neurol Neurosci. 37 (3), 219-230 (2019).
- Parvin, S., et al. Therapeutic effects of repetitive transcranial magnetic stimulation on corticospinal tract activities and neuromuscular properties in children with cerebral palsy. Annu Int Conf IEEE Eng Med Biol Soc. 2018, 2218-2221 (2018).
- Children Rehabilitation Committee of China Rehabilitation Medical Association, China Rehabilitation Association for Disabled Children Cerebral Palsy Rehabilitation Committee, Chinese medical doctor association pediatric rehabilitation committee. & Chinese rehabilitation Guidelines for Cerebral Palsy (2022) Editorial board. Chinese Rehabilitation Guidelines for Cerebral Palsy (2022) Chapter 1: Generality. Chin J Appl Clin Pediatr. 37 (12), 887-892 (2022).
- Subspecialty Group of Rehabilitation, t. S. o. P.C. M. A. Rehabilitation strategy and recommendation for motor dysfunction in children with cerebral palsy. Zhonghua Er Ke Za Zhi. 58 (2), 91-95 (2020).
- Alimi, L., Kalantari, M., Nazeri, A. R., Akbarzade, B. A. Test-retest & inter-rater reliability of Persian version of Pediatric Balance Scale in children with spastic cerebral palsy. Iran J Child Neurol. 13 (4), 163-171 (2019).
- Thompson, P., et al. Test-retest reliability of the 10-metre fast walk test and 6-minute walk test in ambulatory school-aged children with cerebral palsy. Dev Med Child Neurol. 50 (5), 370-376 (2008).
- Yang, Z., Qiao, L., He, J., Zhao, X., Zhang, M. Effects of repetitive transcranial magnetic stimulation combined with functional electrical stimulation on hand function of stroke: A randomized controlled trial. NeuroRehabilitation. 51 (2), 283-289 (2022).
- Li, X., et al. Effect of virtual reality combined with repetitive transcranial magnetic stimulation on musculoskeletal pain and motor development in children with spastic cerebral palsy: a protocol for a randomized controlled clinical trial. BMC Neurology. 23 (1), 339 (2023).
- Multani, I., Manji, J., Hastings-Ison, T., Khot, A., Graham, K. Botulinum toxin in the management of children with cerebral palsy. Paediatr Drugs. 21 (4), 261-281 (2019).
- Volpon Santos, M., Carneiro, V. M., Oliveira, P., Caldas, C. A. T., Machado, H. R. Surgical results of selective dorsal rhizotomy for the treatment of spastic cerebral palsy. J Pediatr Neurosci. 16 (1), 24-29 (2021).
- Gupta, M., Rajak, B. L., Bhatia, D., Mukherjee, A. Neuromodulatory effect of repetitive transcranial magnetic stimulation pulses on functional motor performances of spastic cerebral palsy children. J Med Eng Technol. 42 (5), 352-358 (2018).
- Abdelhaleem, N., et al. Effect of action observation therapy on motor function in children with cerebral palsy: a systematic review of randomized controlled trials with meta-analysis. Clin Rehabil. 35 (1), 51-63 (2021).
- Jung, Y., Chung, E. J., Chun, H. L., Lee, B. H. Effects of whole-body vibration combined with action observation on gross motor function, balance, and gait in children with spastic cerebral palsy: a preliminary study. J Exerc Rehabil. 16 (3), 249-257 (2020).
- Rossi, F., et al. Combining action observation treatment with a brain-computer interface system: perspectives on neurorehabilitation. Sensors (Basel). 21 (24), 8504 (2021).
- Beani, E., Menici, V., Ferrari, A., Cioni, G., Sgandurra, G. Feasibility of a home-based action observation training for children with unilateral cerebral palsy: an explorative atudy. Front Neurol. 11, 16 (2020).
- Carson, H. J., Collins, D. J. Commentary: Motor imagery during action observation: a brief review of evidence, theory and future research opportunities. Front Hum Neurosci. 11, 25 (2017).
- Kim, D. H., An, D. H., Yoo, W. G. Effects of live and video form action observation training on upper limb function in children with hemiparetic cerebral palsy. Technol Health Care. 26 (3), 437-443 (2018).
- Qi, F., Nitsche, M. A., Ren, X., Wang, D., Wang, L. Top-down and bottom-up stimulation techniques combined with action observation treatment in stroke rehabilitation: a perspective. Front Neurol. 14, 1156987 (2023).
- Sun, Y. Y., et al. Effects of repetitive transcranial magnetic stimulation on motor function and language ability in cerebral palsy: A systematic review and meta-analysis. Front Pediatr. 11, 835472 (2023).
- Jeong, Y. A., Lee, B. H. Effect of action observation training on spasticity, gross motor function, and balance in children with diplegia cerebral palsy. Children (Basel). 7 (6), 64 (2020).
- Gupta, M., Lal Rajak, B., Bhatia, D., Mukherjee, A. Effect of r-TMS over standard therapy in decreasing muscle tone of spastic cerebral palsy patients. J Med Eng Technol. 40 (4), 210-216 (2016).
- Ryan, D., et al. Effect of action observation therapy in the rehabilitation of neurologic and musculoskeletal conditions: a systematic review. Arch Rehabil Res Clin Transl. 3 (1), 100106 (2021).

