Creating a Box-Cavity Defect Model in the Cortical Bone of Rat Femora
Summary
Here, we present a protocol for creating a box-cavity defect in rat femoral diaphysis tissue. This model can assess biomaterial performance under biomechanical stress and explore mechanisms of bone regeneration related to intramembranous osteogenesis.
Abstract
Severe bone defects or complex fractures can result in serious complications such as nonunion or insufficient bone healing. Tissue engineering, which involves the application of cells, scaffolds, and cytokines, is considered a promising solution for bone regeneration. Consequently, various animal models that simulate bone defects play a crucial role in exploring the therapeutic potential of tissue engineering for bone healing. In this study, we established a box-shaped cortical bone defect model in the mid-femur of rats, which could serve as an ideal model for assessing the function of biomaterials in promoting bone healing. This box-shaped cortical bone defect was drilled using an oral low-speed handpiece and shaped by a lathe needle. Post-operative micro-CT analysis was immediately conducted to confirm the successful establishment of the box-cavity cortical bone defect. The femurs on the operated side of the rats were then harvested at multiple time points post-surgery (0 days, 2 weeks, 4 weeks, and 6 weeks). The healing process of each sample's defect area was evaluated using micro-CT, hematoxylin and eosin (H&E) staining, and Masson trichrome staining. These results demonstrated a healing pattern consistent with intramembranous ossification, with healing essentially complete by 6 weeks. The categorization of this animal model's healing process provides an effective in vivo method for investigating novel biomaterials and drugs that target intramembranous ossification during bone tissue defect healing.
Introduction
Fractured and defective bone often results from trauma, tumors, inflammation, and congenital malformations1,2. Although bone tissue in young healthy individuals typically possesses robust regenerative abilities3, defects exceeding a critical size or healing impediments due to systemic diseases (e.g., diabetes, osteoporosis, and infections) may still lead to complications such as bone discontinuity or impaired healing4. To address this clinical challenge, bone grafting or biomaterials are commonly used to replace severely defective bone or to reconstruct large bone segments. However, these treatments have limitations. For instance, although considered the gold standard, autologous bone grafting suffers from restricted donor supply and potential donor site complications5,6. Allografts also present certain risks, such as immune-mediated rejection, potential transmission of diseases, and negative impacts on the biomechanical and biological properties of the graft7.
Recent years have witnessed a surge in research focusing on bone defect healing mechanisms. The use of alternative biomaterials and advancements in tissue engineering have emerged as prominent topics within the domain of bone regeneration8. Before these biomaterials can be applied to human therapy, they must be tested in vitro and in vivo to ensure their efficacy and safety. However, the reduced complexity of in vitro environments and the absence of immune and inflammatory responses limit the evaluation of various biomaterials in vitro. Consequently, the establishment of animal models for various types of bone tissue defects is needed9. Animal models allow the evaluation of biomaterials under different loading conditions, facilitate understanding of species-specific bone characteristics, and provide insight into the similarity between animal models and human clinical situations. These advantages are essential for studying bone-scaffold interactions and translating research findings into clinical practice9,10.
Currently, mechanical bone defect animal models are widely used to validate the performance of biomaterials, with cranial bone defect models and segmental bone defect models being the most commonly applied methods11. Segmental bone defect models, often utilized to mimic severe long bone or tibial trauma ending in bone nonunion, are advantageous due to their uniform dimensions and defined anatomical positions, simplifying radiological or histological evaluations of new bone formation and revascularization. However, these models require metal implants to stabilize bilateral fracture segments and necessitate a complex healing process involving both endochondral and intramembranous ossification12. On the other hand, calvarial bone defect models have become a primary screening tool for evaluating biomaterials due to their standardized defect diameters, convenient surgical access, and the supportive function of dura mater and soft tissue13. Although they are widely used for modeling intramembranous bone formation in clinically relevant scenarios, they are unsuitable for evaluating bone healing under biomechanical loading conditions due to their non-load-bearing nature during the healing process14.
To address these limitations, we established a box-cavity cortical bone defect model in the femoral diaphysis tissue of rats. Utilizing micro-computed tomography (CT) three-dimensional (3D) reconstruction, and histopathological staining (Hematoxylin and eosin [HE] and Masson), we analyzed the healing process of this model under hemostasis conditions. We aim to offer fresh insights for evaluating biomaterial performance under biomechanical loading conditions and for studying the bioengineering and mechanism of bone regeneration vis-à-vis intramembranous ossification.
Protocol
All animal procedures in this study were reviewed and approved by the Ethical Committee of the West China School of Stomatology, Sichuan University (WCHSIRB-D-2021-597). Sprague-Dawley rats (male, body weight 300 g) were used for the present study.
1. Presurgical preparation
- Instrument preparation
- Refer to Figure 1A for the surgical instruments used in this study: electric shaver, tissue scissors, ophthalmic scissors, ophthalmic forceps, disposable scalpel, periosteal separator, oral low-speed handpiece, oral probe, disposable irrigation vac, needle holder, 3.0 suture.
- Prepare an oral probe and mark the large curved end of the probe with a marker pen according to the diameter of the defect (Figure 1B). Use this to determine the size of the defect during the surgery.
- Sterilize all surgical materials and instruments used to perform the procedure before use. Pack the desired materials in folded cloth or wrapping paper and seal them with autoclave tape for steam sterilization (125-135°C for 20-25 min).
- Surgical area preparation: Disinfect the operating table and the environment around the table by spraying with 75% alcohol. Create an approximately 60 cm x 90 cm sterile area with autoclaved drapes on the operating table.
- Anesthesia preparation
- Anesthetize the rats intraperitoneally with 10% ketamine hydrochloride (50-100 mg/kg) and 2% xylazine 2 mg/kg). Use subcutaneous injection of carprofen (5 mg/kg) for preoperative and intraoperative analgesia. Examine the depth of anesthesia by toe pinch test. After anesthesia, apply sterile eye ointment to the eyes to prevent dry eyes and corneal injury.
2. Surgical procedure
- Place the rat in a lateral recumbency position on the sterile surgical table and remove the lower limb hairs with an electric shaver.
- Use 5% iodophor solution and 75% alcohol to disinfect the skin tissue in the surgical area.
- Locate the proximal and distal femur and make a 2.5 cm incision along the long axis of the femur to cut through the skin tissue of the rat.
- Separate the skin layer from the fascia with ophthalmic forceps and tissue scissors, and expose the lateral approach to the femur through the biceps femoris and lateral femoral muscles.
- Locate the intersection of the two muscle septa (a white tissue line) and carefully separate with a disposable surgical blade along the muscle border until the femoral surface is reached.
NOTE: When using a disposable blade to separate the muscle, it is important to separate along the muscle septum and be careful of causing vascular injury within the soft tissue. Beginners and those unfamiliar with the anatomy must use ophthalmic forceps and periosteal separators in blunt dissection between the two muscle bulks.
- Apply a periosteal separator to bluntly separate the femoral surface muscles and expose the middle of the femoral diaphysis.
- Use a sterile marker pen to mark the area of the defect site on the mid-surface of the femoral diaphysis, located at the top of the lateral 1/3 oblique crest of the femoral head.
- Use the oral low-speed handpiece with a 1.2 mm diameter slow-motion ball drill to drill a small hole perpendicular to the bone surface at the marked site, destroying the periosteum and the bone cortex with a depth deep enough to reach the bone marrow cavity. At this drilling depth level, expand the size of the hole parallel to this depth in all directions, trimming to achieve a box-cavity shape.
- Use a labeled oral probe parallel to the edge of the defect to determine the defect diameter and morphology during and after preparation.
- Close the muscle and skin layers with 3-0 monofilament absorbable sutures, respectively, and disinfect the surgical area with 5% iodophor from the inside out.
3. Post-operative care
- After surgery, administer carprofen (5 mg/kg) subcutaneously and put the rat on a constant temperature heating pad until recovery from anesthesia. When the rat regains consciousness, gently move it to a cage that contains dry, autoclaved bedding.
- Continue analgesia for 24 h and post-operative monitoring for 1 week after surgery.
4. Sample collection and analysis
- Humanly euthanize the rat after surgery by injecting pentobarbital sodium 100-200 mg/kg intraperitoneally. Carefully separate the muscle and fascial tissue on the surface of the femur and remove the femur on the operated side completely. Collect specimens at 0 days (Figure 2A, B), 2 weeks, 4 weeks, and 6 weeks postoperatively.
- Fix the femoral specimens in 4% paraformaldehyde for 24 h. Analyze the structure of the femora by using micro-computed tomography. Set the scanning parameters as follows: X-ray tube potential, 70 kVp; filter, AL 0.5 mm; X-ray intensity, 0.2 mA; voxel size, 17 µm; and integration time, 1 × 300 ms. Reconstruct 3D model images using bitmap data.
- Decalcify the specimens in 10% EDTA for 8 weeks before dehydrating the femoral specimens in a graded series of ethanol dilutions. Then, embed the samples in paraffin wax15.
- Cut the embedded samples into 5 µm paraffin sections from the sagittal plane.
- Stain sections with both a hematoxylin and eosin (H&E) staining kit and a Masson staining kit. Observe the healing of the defect area by histopathology.
Representative Results
In this protocol, we successfully establish a rat femoral box-cavity defect model with dimensions of 4.5 mm x 1.5 mm by drilling. In order to analyze the healing process, we collected the femoral tissue on the operated side at 0 days, 2 weeks, 4 weeks, and 6 weeks after surgery, which are the key time points of endochondral ossification, intramembranous ossification, and bone remodeling during the healing process of femoral trauma in rats2. On post-operative day 0, reconstruction of the 3D model from micro-CT bitmap data showed that we successfully modeled a box-cavity defect in the femur with a size of 4.5 mm x 1.5 mm deep to the marrow cavity (Figure 3A). Results of micro-CT showed mineralized trabecular bone formation in the interstitial space of the cortical bone defect 2 weeks after model creation (Figure 3B). The surface of the new bone tissue in the defect area showed mature, dense cortical bone, and trabecular bone tissue was still visible on the medullary side 4 weeks postoperatively (Figure 3C). After 6 weeks, the defect area was almost composed of mature, dense cortical bone, with only a small amount of trabecular bone tissue remaining near the medullary side, indicating that the defect area had basically healed (Figure 3D).
We also analyze the collected specimens by H&E staining and Masson trichrome staining (Figure 4). The results showed that naïve trabecular-like bone tissue was formed in the defect area 2 weeks postoperatively, and the periosteum around the defect area thickened and connected with the newborn trabecular bone tissue in the defect area (Figure 4A,A',B,B'). At 4 weeks after creating the defect, dense cortical bone tissue was formed on the surface of the defect area and connected with the cortical bone on both sides of the defect; trabecular-like bone tissue was resorbed heavily on the medullary side (Figure 4C,C',D,D'). Mature cortical bone tissue was formed in the defect area, and the trabecular bone tissue on the medullary side was almost completely resorbed 6 weeks postoperatively (Figure 4E,E',F,F'), indicating that the defect's healing process was almost complete.
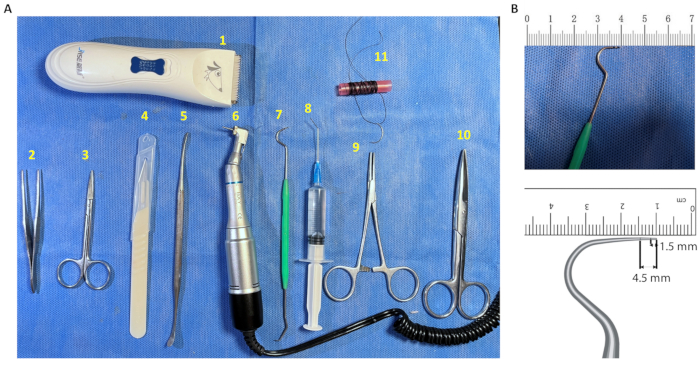
Figure 1: Instruments required for defect creation. (A) Surgical instruments. (1) Electric shaver; (2) Ophthalmic forceps; (3) Ophthalmic scissors; (4) Disposable scalpel; (5) Periosteal separator; (6) Oral low-speed handpiece; (7) Modified oral probe; (8) Disposable irrigation vac; (9) Needle holder; (10) Tissue scissors; (11) 3.0 suture. (B) Oral probes labeled with the dimensions of the defect diameter. The large curved end of the sterile disposable oral probe was marked with a 1.5 mm and 4.5 mm scale for intraoperative measurement of the diameter of the defect area. Please click here to view a larger version of this figure.
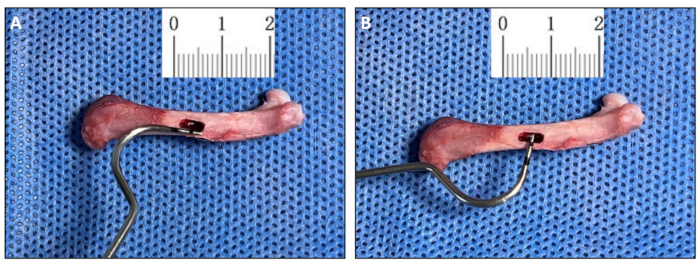
Figure 2: Morphology of the box cavity defect (Day 0). (A,B) Measurement of Day 0 post-surgery samples using a modified oral probe that was used to create the box cavity defect (4.5 mm x 1.2 mm). Please click here to view a larger version of this figure.
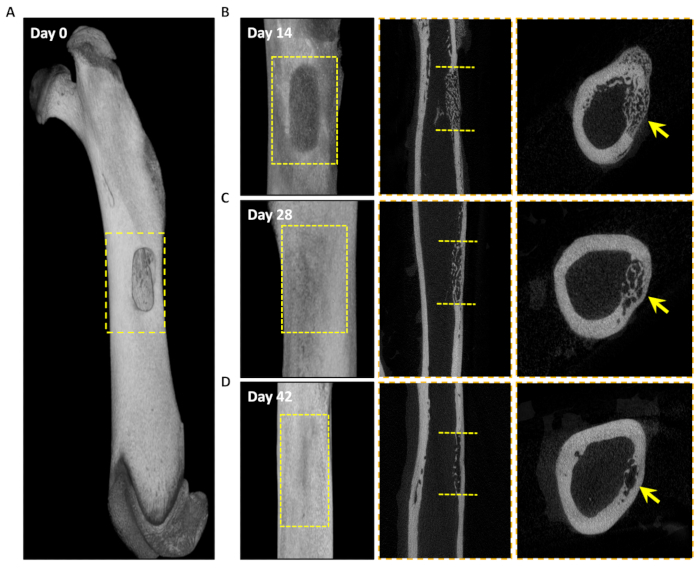
Figure 3: Micro-CT analysis of the healing process of the defect area. 3D reconstructed images of the defect area at 0 days, 14 days, 28 days, and 42 days after surgery. (A) Longitudinal plane . (B–D) Longitudinal (left panel), sagittal (middle) and cross-sectional (right panel) micro-CT images of healing of the defect area at 14 days, 28 days, and 42 days. The yellow dashed box in A, B, C, and D represents the defect area. The yellow dashed line represents the edges of the defect, and the yellow arrow represents the new bone tissue in the defect area. Please click here to view a larger version of this figure.
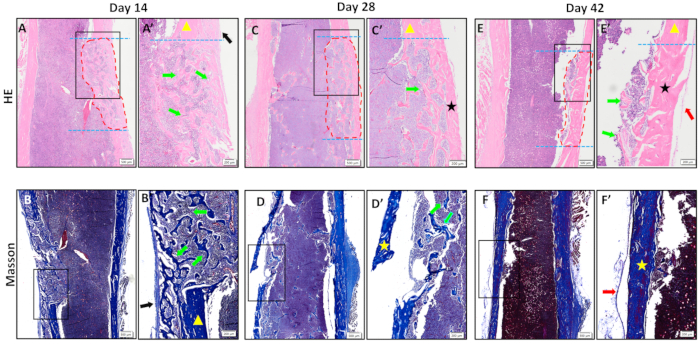
Figure 4. Histopathological results. (A-F) H&E and Masson staining of the defect area at 14 days, 28 days, and 42 days.(A'-F') The zoomed-in images of the black rectangular boxes shown in A-F.Trabecular-like mineralized bone tissue was formed in the defect area at 14 days after surgery; cortical bone tissue was formed on the surface of the defect area and connected to both ends of the defect area at 28 days after surgery; mature cortical bone tissue was formed in the defect area at 42 days after surgery, and healing was basically completed. The blue dotted line indicates the edge of the defect; the area circled by the red dotted line is the new bone tissue in the defect area; the black arrow is the thickened periosteum; the yellow triangle is the old cortical bone; the green arrow is the new trabecular-like bone tissue; the black and the yellow stars are the new cortical bone tissue; and the red arrow is the new periosteum. Scale bars: 500 µm (A-F), 200 µm (A'-F'). Please click here to view a larger version of this figure.
Discussion
Preclinical animal models are vital for examining bone healing and the influence of biomaterials on bone regeneration. This protocol illustrates a femoral box-cavity defect model replicating the intramembranous bone formation process associated with clinical bone regeneration. The defect area was intraoperatively standardized using a pre-marked oral probe. Micro-CT and histopathological staining results showed progressive healing over 6 weeks, with thickened periosteum and new trabecular bone formation, followed by dense cortical bone formation. No cartilage tissue was observed during the healing process, indicating intramembranous osteogenesis. This finding presents fresh perspectives for studying the role of biomaterials or drugs in clinical bone regeneration.
This protocol requires careful attention to several factors: (i) Deep anesthesia is a prerequisite to prevent animal distress and ensure accurate defect localization. Anesthesia depth and vital signs should be vigilantly monitored. (ii) During muscle separation, meticulous attention should be paid to prevent vascular injury, which could hinder recovery. (iii) In preparing the defect hole, keep the right-hand fulcrum stable and always perpendicular to the bone surface to ensure the box defect is at the same depth. The drill depth should be controlled to remove cortical bone tissue without going excessively deep. This avoids delaying bone healing due to excessive mechanical destruction of the bone marrow, resulting in the removal of mesenchymal stem cells (MSCs) and disruption of the inflammatory response16. (iv) The cavity preparation necessitates an assistant to continuously irrigate the area with saline to mitigate potential tissue damage caused by heat during bone ablation while maintaining the surgical field's visibility.
Bone fracture, segmental bone defect, and cranial bone defect models are the commonly used animal models in investigating bone regeneration. The bone fracture model reflects the actual process of trauma, but the results may vary due to differences in fracture cross-sections among experimental animals17. The segmental bone defect model effectively simulates fracture complications such as nonunion or delayed healing. Due to its anatomical positioning, this model facilitates the evaluation of new bone formation and revascularization through radiographic or histological methods18. However, this method presents considerable variation in defect size among subjects; the requirement for splint fixation or internal fixation with metal implants also limits its application11. The calvarial defect model is characterized by no internal fixation, high reproducibility in size and anatomical position, and a stable mechanical environment14, which also restricts the application in evaluating the biological response to physiological, biomechanical loading of implanted material13. Moreover, in the absence of a graft bone, the invasion of the defect by the dura mater and overlying soft tissue can hinder bone regeneration, making these models sensitive to the performance of the scaffold material18. Therefore, this protocol proposes the establishment of a box-cavity defect in the rat femoral diaphysis, which allows for intraoperative measurement of the defect diameter using a simple tool18. This approach offers better control over defect standardization than the fracture or segmental defect models and eliminates the need for internal or external fixation. Furthermore, this model is robust against impairment of bone formation due to soft tissue erosion. This protocol, however, presents certain limitations: (i) Improper execution or excessive defect diameters can lead to severe hemorrhage, shock, or even fatality in rats. Thus, to ensure the safety and welfare of the animals, it is imperative to practice rat cadavers before performing the procedure on living subjects. (ii) Moreover, the absence of the Haversian system in rodent cortex19 makes it hard to completely reproduce the natural clinical healing process of human bone fracture/defect. Rats, as rodents, are cost-effective, require minimal maintenance, and are safe and easy to handle, providing higher reproducibility than larger animals such as rabbits, dogs, and goats20.
In summary, we developed a rat femoral diaphysis box-cavity defect model and tracked the healing process utilizing micro-CT and histopathologic staining techniques. This protocol furnishes a comprehensive and generalizable method for scrutinizing intramembranous bone formation within clinically significant bone regeneration models. Furthermore, it proposes innovative perspectives for the biological performance evaluation of substitute biomaterials, bone regeneration-associated drugs, scaffolds, etc. It also facilitates the preclinical validation of emergent therapeutic strategies for bone tissue engineering.
Disclosures
The authors have nothing to disclose.
Acknowledgements
This study was funded by grants from the National Natural Science Foundation of China 82101000 (H. W.), U21A20368 (L. Y.), and 82100982 (F. L.), and supported by Sichuan Science and Technology Program 2023NSFSC1499 (H. W.).
Materials
| 1.2 mm slow speed ball drill | Dreybird Medical Equipment Co., Ltd. | RA3-012 | For preparation of box cavity defects |
| 3.0 suture | Chengdu Shifeng Co., Ltd. | None | For suturing wounds |
| 4% paraformaldehyde | Biosharp | BL539A | For fix the femoral specimens |
| Cotton balls | Haishi Hainuo Group Co., Ltd. | 20120047 | For skin sterilization and cleaning of surgical field |
| Cotton sticks | Lakong Medical Devices Co., Ltd. | M6500R | For skin disinfection |
| Dental technician grinding machine | Marathon | N3-140232 | For preparation of box cavity defects |
| Disposable scalpel | Hangzhou Huawei Medical Supplies Co., Ltd. | 20100227 | For creating skin incisions as well as to sharply separate muscle tissue |
| Electric shaver | JASE | BM320210 | Removal of hair tissue from the surgical area |
| Hematoxylin and Eosin Stain kit | Biosharp | C1005 | For the histological analysis of the specimens |
| Masson’s Trichrome Stain Kit | Solarbio | G1340 | For the histological analysis of the specimens |
| Micro CT | Scanco medical ag | µCT 45 | For analyzing the healing of defects in femoral samples |
| Needle holder | Chengdu Shifeng Co., Ltd. | None | For suture-holding needles |
| Olympus Research Grade Whole Slide Scanning System VS200 | Chengdu Knowledge Technology Co. | VS200 | For analyzing the results of HE staining and Masson staining |
| Ophthalmic forceps | Chengdu Shifeng Co., Ltd. | None | For clamping skin, muscle tissue |
| Ophthalmic scissors | Chengdu Shifeng Co., Ltd. | None | For forming a skin incision approach |
| Oral low-speed handpiece | Marathon | Y221101003 | For preparation of box cavity defects |
| Oral probe | Shanghai Sangda Medical Insurance Co., Ltd. | 20000143 | For measuring the diameter of defects |
| Periosteal separator | Chengdu Shifeng Co., Ltd. | None | For blunt separation of muscle tissue |
| Sprague–Dawley rats | Byrness Weil Biotech Ltd | None | For the establishment of femoral bone boxy cavitary defect |
| Tissue scissors | Chengdu Shifeng Co., Ltd. | None | For forming a skin incision approach |
References
- Einhorn, T. A., Gerstenfeld, L. C. Fracture healing: mechanisms and interventions. Nat Rev Rheumatol. 11 (1), 45-54 (2015).
- Claes, L., Recknagel, S., Ignatius, A. Fracture healing under healthy and inflammatory conditions. Nat Rev Rheumatol. 8 (3), 133-143 (2012).
- Holmes, D. Nonunion bone fracture: a quicker fix. Nature. 550 (7677), S193 (2017).
- Dimitriou, R., Jones, E., McGonagle, D., Giannoudis, P. V. Bone regeneration: current concepts and future directions. BMC Med. 9, 66 (2011).
- Schmidt, A. H. Autologous bone graft: Is it still the gold standard? Injury. 52 (Suppl 2). , S18-S22 (2021).
- Baldwin, P., et al. Autograft, allograft, and bone graft substitutes: Clinical evidence and indications for use in the setting of orthopaedic trauma surgery. J Orthop Trauma. 33 (4), 203-213 (2019).
- Muscolo, D. L., Ayerza, M. A., Aponte-Tinao, L. A. Massive allograft use in orthopedic oncology. Orthop Clin North Am. 37 (1), 65-74 (2006).
- Yu, Y., et al. Biomimetic periosteum-bone substitute composed of preosteoblast-derived matrix and hydrogel for large segmental bone defect repair. Acta Biomater. 113, 317-327 (2020).
- Pearce, A. I., Richards, R. G., Milz, S., Schneider, E., Pearce, S. G. Animal models for implant biomaterial research in bone: a review. Eur Cell Mater. 13, 1-10 (2007).
- McGovern, J. A., Griffin, M., Hutmacher, D. W. Animal models for bone tissue engineering and modelling disease. Dis Model Mech. 11 (4), dmm033084 (2018).
- Bigham-Sadegh, A., Oryan, A. Selection of animal models for preclinical strategies in evaluating the fracture healing, bone graft substitutes and bone tissue regeneration and engineering. Connect Tissue Res. 56 (3), 175-194 (2015).
- Horner, E. A., et al. Long bone defect models for tissue engineering applications: criteria for choice. Tissue Eng Part B Rev. 16 (2), 263-271 (2010).
- Vajgel, A., et al. A systematic review on the critical size defect model. Clin Oral Implants Res. 25 (8), 879-893 (2014).
- Samsonraj, R. M., et al. A versatile protocol for studying calvarial bone defect healing in a mouse model. Tissue Eng Part C Methods. 23 (11), 686-693 (2017).
- Yu, F., Li, F., Zheng, L., Ye, L. Epigenetic controls of Sonic hedgehog guarantee fidelity of epithelial adult stem cells trajectory in regeneration. Sci Adv. 8 (29), eabn4977 (2022).
- Colnot, C. Skeletal cell fate decisions within periosteum and bone marrow during bone regeneration. J Bone Miner Res. 24 (2), 274-282 (2009).
- Bhandari, M., Shaughnessy, S. A minimally invasive percutaneous technique of intramedullary nail insertion in an animal model of fracture healing. Arch Orthop Trauma Surg. 121 (10), 591-593 (2001).
- Muschler, G. F., Raut, V. P., Patterson, T. E., Wenke, J. C., Hollinger, J. O. The design and use of animal models for translational research in bone tissue engineering and regenerative medicine. Tissue Eng Part B Rev. 16 (1), 123-145 (2010).
- Clifford, J., Rosen, M. D. . Primer on the Metabolic Bone Diseases and Disorders of Mineral Metabolism. , (2019).
- Wong, R. M. Y., et al. A systematic review of current osteoporotic metaphyseal fracture animal models. Bone Joint Res. 7 (1), 6-11 (2018).

