Construction of Out-of-Equilibrium Metabolic Networks in Nano- and Micrometer-Sized Vesicles
Summary
We present a protocol for reconstituting membrane proteins and encapsulating enzymes and other water-soluble components in lipid vesicles of sub-micrometer and micrometer size.
Abstract
We present a method to incorporate into vesicles complex protein networks, involving integral membrane proteins, enzymes, and fluorescence-based sensors, using purified components. This method is relevant for the design and construction of bioreactors and the study of complex out-of-equilibrium metabolic reaction networks. We start by reconstituting (multiple) membrane proteins into large unilamellar vesicles (LUVs) according to a previously developed protocol. We then encapsulate a mixture of purified enzymes, metabolites, and fluorescence-based sensors (fluorescent proteins or dyes) via freeze-thaw-extrusion and remove non-incorporated components by centrifugation and/or size-exclusion chromatography. The performance of the metabolic networks is measured in real time by monitoring the ATP/ADP ratio, metabolite concentration, internal pH, or other parameters by fluorescence readout. Our membrane protein-containing vesicles of 100-400 nm diameter can be converted into giant-unilamellar vesicles (GUVs), using existing but optimized procedures. The approach enables the inclusion of soluble components (enzymes, metabolites, sensors) into micrometer-size vesicles, thus upscaling the volume of the bioreactors by orders of magnitude. The metabolic network containing GUVs are trapped in microfluidic devices for analysis by optical microscopy.
Introduction
The field of bottom-up synthetic biology focuses on constructing (minimal) cells1,2 and metabolic bioreactors for biotechnological3,4 or biomedical purposes5,6,7,8. The construction of synthetic cells provides a unique platform that allows researchers to study (membrane) proteins in well-defined conditions mimicking those of native environments, enabling the discovery of emergent properties and concealed biochemical functions of proteins and reaction networks9. As an intermediate step towards an autonomously functioning synthetic cell, modules are developed that capture essential features of living cells such as metabolic energy conservation, protein and lipid synthesis, and homeostasis. Such modules not only enhance our understanding of life but also have potential applications in the fields of medicine8 and biotechnology10.
Transmembrane proteins are at the heart of virtually any metabolic network as they transport molecules in or out of the cell, signal, and respond to the quality of the environment, and play numerous biosynthetic roles. Thus, the engineering of metabolic modules in synthetic cells requires in most cases the reconstitution of integral and/or peripheral membrane proteins into a membrane bilayer composed of specific lipids and high integrity (low permeability). The handling of these membrane proteins is challenging and requires specific knowledge and experimental skills.
Several methods have been developed to reconstitute membrane proteins within phospholipid vesicles, most often with the purpose of studying the function11,12, regulation13, kinetic properties14,15, lipid dependence15,16, and/or stability17 of a specific protein. These methods involve the rapid dilution of detergent-solubilized protein into aqueous media in the presence of lipids18, the removal of detergents by incubating detergent-solubilized protein with detergent-destabilized lipid vesicles and absorption of the detergent(s) onto polystyrene beads19, or the removal of detergents by dialysis or size-exclusion chromatography20. Organic solvents have been used to form lipid vesicles, for example, via the formation of oil-water interphases21, but the majority of integral membrane proteins are inactivated when exposed to such solvents.
In our laboratory, we mostly reconstitute membrane proteins by the detergent-absorption method to form large-unilamellar vesicles (LUVs)19. This method allows the co-reconstitution of multiple membrane proteins and the encapsulation in the vesicle lumen of enzymes, metabolites, and probes22,23. The membrane protein-containing LUVs can be converted into giant-unilamellar vesicles (GUVs) with/without encapsulation of water-soluble components, using either electroformation24 or gel-assisted swelling25 and specific conditions to preserve the integrity of the membrane proteins26.
This paper presents a protocol for the reconstitution in LUVs of an out-of-equilibrium metabolic network that regenerates ATP through the breakdown of L-arginine into L-ornithine27. The formation of ATP is coupled to the production of glycerol-3-phosphate (G3P), an important building block for phospholipid synthesis22,28. The metabolic pathway consists of two integral membrane proteins, an arginine/ornithine (ArcD) and a G3P/Pi antiporter (GlpT). In addition, three soluble enzymes (ArcA, ArcB, ArcC) are required for the recycling of ATP, and GlpK is used to convert glycerol into glycerol 3-phosphate, using the ATP from the breakdown of L-arginine, see Figure 1 for a schematic overview of the pathway. This protocol represents a good starting point for the future construction of even more complex reaction networks-for the synthesis of lipids or proteins or the division of cells. The lipid composition of the vesicles supports the activity of a wide variety of integral membrane proteins and has been optimized for the transport of diverse molecules into or out of the vesicles27,29,30.
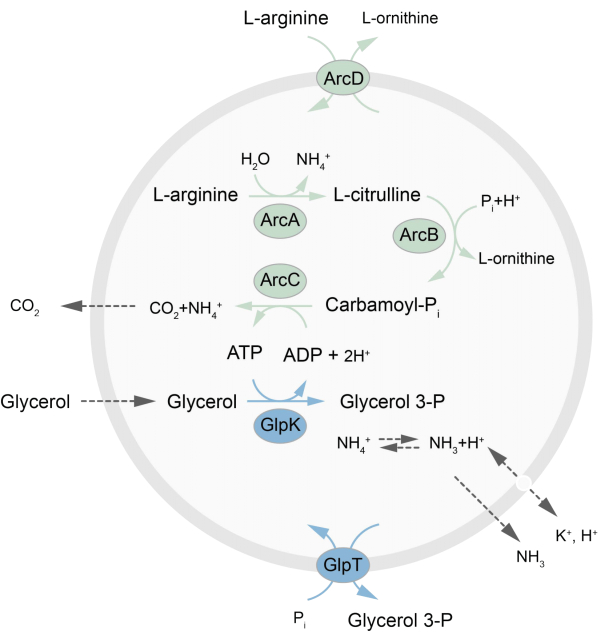
Figure 1: Overview of the pathway for ATP production and glycerol 3-phosphate synthesis and excretion. Please click here to view a larger version of this figure.
In short, purified membrane proteins (solubilized in dodecyl-β-D-maltoside, DDM) are added to preformed lipid vesicles that have been destabilized with Triton X-100, which allows the insertion of the proteins into the membrane. The detergent molecules are subsequently (slowly) removed by the addition of activated polystyrene beads, resulting in the formation of well-sealed proteoliposomes. Soluble components can then be added to the vesicles and encapsulated via freeze-thaw cycles, which traps the molecules in the process of membrane fusion. The obtained vesicles are highly heterogeneous and many are multilamellar. They are then extruded through a polycarbonate filter with a pore size of 400, 200, or 100 nm, which yields more uniformly sized vesicles; the smaller the pore size, the more homogeneous and unilamellar the vesicles but at the price of a smaller internal volume. Non-incorporated proteins and small molecules are removed from the external solution by size-exclusion chromatography. The proteoLUVs can be converted into micrometer size vesicles by gel-assisted swelling, and these proteoGUVs are then collected and trapped in a microfluidic chip for microscopic characterization and manipulation. Figure 2 shows a schematic overview of the full protocol.
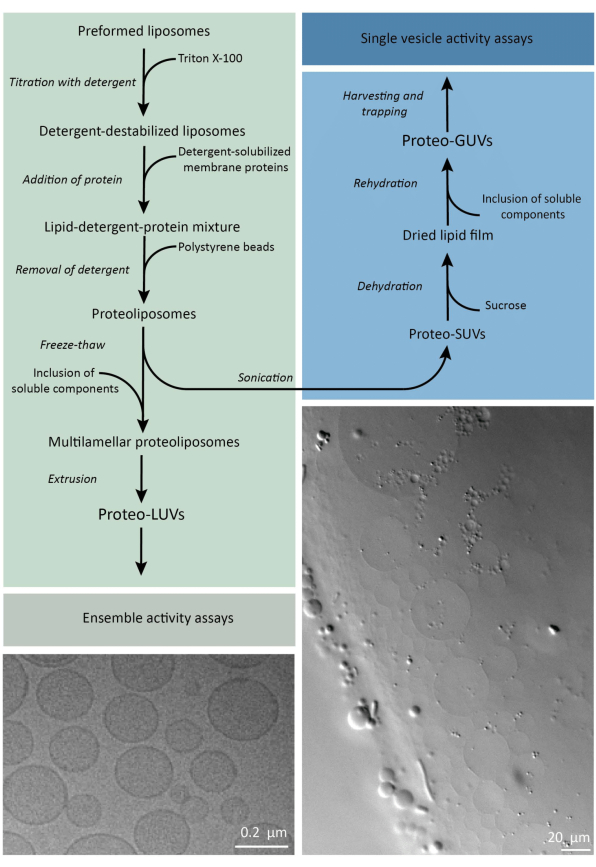
Figure 2: Overview of the protocol for reconstituting membrane proteins and encapsulating enzymes and water-soluble components in lipid vesicles of sub-micrometer (LUVs) and micrometer size (GUVs). Please click here to view a larger version of this figure.
The reconstitution and encapsulation protocols work well and the functionality of the proteins is retained, but the proteoLUVs and proteoGUVs are heterogeneous in size. Microfluidic approaches31,32 allow the formation of micrometer-sized vesicles that are more homogeneous in size, but functional reconstitution of membrane proteins is generally not possible because residual solvent in the bilayer inactivates the proteins. The proteoLUVs range in size from 100 to 400 nm, and at low concentrations of enzymes, the encapsulation may lead to vesicles with incomplete metabolic pathways (stochastic effects; see Figure 3). LUVs are ideal for constructing specific metabolic modules, as shown here for the production of ATP and building blocks like G3P. Such proteoLUVs can potentially be encapsulated in GUVs and serve as organelle-like compartments for the host vesicles.
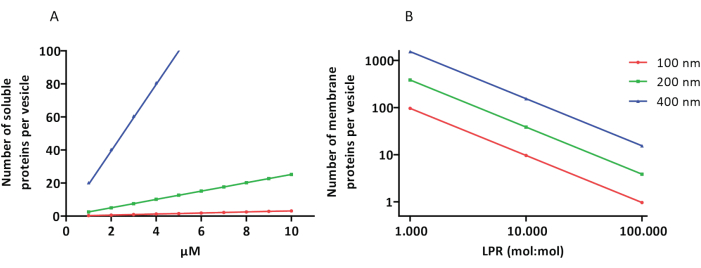
Figure 3: Number of molecules per vesicle with a diameter of 100, 200, or 400 nm. (A) When the encapsulated proteins (enzymes, probes) are in the range of 1-10 µM. (B) The reconstitution is done at 1 to 1,000, 1 to 10,000, and 1 to 100,000 membrane proteins per lipid (mol/mol). We make the assumption that molecules are encapsulated at the indicated concentrations and incorporated in the membrane at these protein-to-lipid ratios. For some enzymes, we have seen that they bind to membranes, which can increase their apparent concentration in the vesicles. Abbreviation: LPR = Lipid-Protein-Ratio Please click here to view a larger version of this figure.
Protocol
Representative Results
Discussion
We present a protocol for the synthesis of (membrane) protein containing sub-micrometer size lipid vesicles (proteoLUVs), and the conversion of proteoLUVs into giant-unilamellar vesicles (proteoGUVs). The protocol should be applicable for the reconstitution of other membrane proteins13,19,30,40 and the encapsulation of metabolic networks other than the L-arginine breakdown and glycerol 3-phosph…
Disclosures
The authors have nothing to disclose.
Acknowledgements
The authors thank Aditya Iyer for the cloning of the pBAD-PercevalHR gene and Gea Schuurman-Wolters for aiding with protein production and purification. The research was funded by the NWO Gravitation program "Building a Synthetic Cell" (BaSyC).
Materials
| Agarose | Sigma Aldrich | A9414-25g | |
| Amicon cut-off filter | Sigma Aldrich | Milipore centrifugal filter units Amicon Ultra | |
| BioBeads | BioRad | 152-3920 | |
| CHCl3 | Macron Fine Chemicals | MFCD00000826 | |
| D(+)-Glucose | Formedium | – | |
| D(+)-Sucrose | Formedium | – | |
| DDM | Glycon | D97002 -C | |
| Diethyl Ether | Biosolve | 52805 | |
| DMSO | Sigma-Aldrich | 276855-100ml | |
| DOPC | Avanti | 850375P-1g | |
| DOPE | Avanti | 850725P-1g | |
| DOPG | Avanti | 840475P-1g | |
| DTT | Formedium | DTT005 | |
| EtOH | J.T.Baker Avantor | MFCD00003568 | |
| Extruder | Avestin Inc | LF-1 | |
| Fluorimeter | Jasco | Spectrofluorometer FP-8300 | |
| Glycerol | BOOM | 51171608 | |
| Gravity flow column | Bio-Rad | 732-1010 | |
| Hamilton syringe 100 µL | Hamilton | 7656-01 | |
| Hamilton syringe 1000 µL | Hamilton | 81320 | |
| Handheld LCP dispenser | Art Robbins Instruments | 620-411-00 | |
| Handheld Sonicator | Hielscher Ultrasound Technology | UP50H | |
| HCl | BOOM | x76021889.1000 | |
| Imidazole | Roth | X998.4-250g | |
| K2HPO4 | Supelco | 1.05099.1000 | |
| KCl | BOOM | 76028270.1 | |
| KH2PO4 | Supelco | 1.04873.1000 | |
| Kimwipe | Kimtech Science | 7552 | |
| Large Falcon tube centrifuge | Eppendorf | Centrifuge 5810 R | |
| L-Arginine | Sigma-Aldrich | A5006-100G | |
| Light microscope | Leica | DM LS2 | |
| L-Ornithine | Roth | T204.1 | |
| LSM Laser Scanning Confocal Microscope | Zeiss | LSM 710 ConfoCor 3 | |
| MgCl2 | Sigma-Aldrich | M2670-1KG | |
| Microfluidic chip | Homemade | PDMS based | DOI: https://doi.org/10.1039/C8LC01275J |
| Na-ADP | Sigma-Aldrich | A2754-1G | |
| NaCl | Supelco | 1.06404.1000 | |
| Nanodrop Spectrometer | Isogen Life Science | ND-1000 spectrophotometer NanoDrop | |
| NaOH | Supelco | 1.06498.1000 | |
| Needles for GUVs | Henke-Ject | 14-14575 | 27 G x 3/4'' 0.4 x 20 mm |
| Needles for microfluidics | Henke-Ject | 14-15538 | 18 G x 1 1/2'' 1.2 x 40 mm |
| Ni2+ Sepharose | Cytiva | 17526802 | |
| Nigericin | Sigma-Aldrich | N7143-5MG | |
| Nutator | VWR | 83007-210 | |
| Osmolality meter | Gonotec Salmenkipp | Osmomat 3000 basic freezing point osmometer | |
| Plasmacleaner | Plasma Etch | PE-Avenger | |
| Polycarbonate filter | Cytiva Whatman | Nuclepor Track-Etch Membrane Product: 10417104 | 0.4 µm |
| Polycarbonate ultracentrifuge tube | Beckman Coulter | 355647 | |
| Pyranine | Acros Organics | H1529-1G | |
| Quartz cuvette (black) | Hellma Analytics | 108B-10-40 | |
| Sephadex G-75 resin | GE Healthcare | 17-0050-01 | |
| Sonicator | Sonics Sonics & Materials INC | Sonics vibra cell | |
| Syringe filter | Sarstedt | Filtropur S plus 0.2 | 0.2 µm |
| Syringe pump | Harvard Apparatus | A-42467 | |
| Tabletop centrifuge | Eppendorf | centrifuge 5418 | |
| Teflon spacer | Homemade | Teflon based | 45 x 26 x 1.5 or 45 x 26 x 3 or 20 x 20 x 3 mm |
| Tris | PanReac AppliChem | A1086.1000 | |
| Triton X-100 | Sigma Aldrich | T8787-100 ml | |
| Ultracentrifuge | Beckman Coulter | Optima Max-E | |
| UV lamp | Spectroline | ENB-280C/FE | |
| UV/VIS Spectrometer | Jasco | V730 spectrophotometer | |
| Valinomycin | Sigma-Aldrich | V0627-10MG | |
| Widefield fluorescence microscope | Zeiss | AxioObserver | |
| β-Casein | Sigma Aldrich | C5890-500g |
References
- Hirschi, S., Ward, T. R., Meier, W. P., Müller, D. J., Fotiadis, D. Synthetic biology: bottom-up assembly of molecular systems. Chem Rev. 122 (21), 16294-16328 (2022).
- Ivanov, I., et al. Bottom-up synthesis of artificial cells: recent highlights and future challenges. Annu Rev Chem Biomol. Eng. 12 (1), 287-308 (2021).
- Clomburg, J. M., Crumbley, A. M., Gonzalez, R. Industrial biomanufacturing: The future of chemical production. Science. 355 (6320), (2017).
- Shi, T., Han, P., You, C., Zhang, Y. -. H. P. J. An in vitro synthetic biology platform for emerging industrial biomanufacturing: Bottom-up pathway design. Synth Syst Biotechnol. 3 (3), 186-195 (2018).
- Wang, A., et al. Liver-target and glucose-responsive polymersomes toward mimicking endogenous insulin secretion with improved hepatic glucose utilization. Adv Funct Mater. 30 (13), 1910168 (2020).
- Kanter, G., et al. Cell-free production of scFv fusion proteins: an efficient approach for personalized lymphoma vaccines. Blood. 109 (8), 3393-3399 (2007).
- Zeltins, A. Construction and characterization of virus-like particles: a review. Mol Biotechnol. 53 (1), 92-107 (2013).
- Jain, K. K. Synthetic biology and personalized medicine. Med Princ Pract. 22 (3), 209-219 (2013).
- Schwille, P., Frohn, B. P. Hidden protein functions and what they may teach us. Trends Cell Biol. 32 (2), 102-109 (2022).
- Sachsenmeier, P. Industry 5.0-The relevance and implications of bionics and synthetic biology. 공학. 2 (2), 225-229 (2016).
- Schmidt, D., Jiang, Q. -. X., MacKinnon, R. Phospholipids and the origin of cationic gating charges in voltage sensors. Nature. 444 (7120), 775-779 (2006).
- Godoy-Hernandez, A., et al. Rapid and highly stable membrane reconstitution by LAiR enables the study of physiological integral membrane protein functions. ACS Cent Sci. 9 (3), 494-507 (2023).
- Sikkema, H. R., et al. Gating by ionic strength and safety check by cyclic-di-AMP in the ABC transporter OpuA. Sci Adv. 6 (47), 7697 (2020).
- Foucaud, C., Poolman, B. Lactose transport system of Streptococcus thermophilus. Functional reconstitution of the protein and characterization of the kinetic mechanism of transport. J Biol Chem. 267 (31), 22087-22094 (1992).
- Yoneda, J. S., Sebinelli, H. G., Itri, R., Ciancaglini, P. Overview on solubilization and lipid reconstitution of Na,K-ATPase: enzyme kinetic and biophysical characterization. Biophys Rev. 12 (1), 49-64 (2020).
- Simidjiev, I., et al. Self-assembly of large, ordered lamellae from non-bilayer lipids and integral membrane proteins in vitro. Proc Natl Acad Sci. 97 (4), 1473-1476 (2000).
- Harris, N. J., Booth, P. J. Folding and stability of membrane transport proteins in vitro. Biochim Biophys Acta BBA – Biomembr. 1818 (4), 1055-1066 (2012).
- Jackson, M. L., Litman, B. J. Rhodopsin-egg phosphatidylcholine reconstitution by an octyl glucoside dilution procedure. Biochim Biophys Acta BBA – Biomembr. 812 (2), 369-376 (1985).
- Geertsma, E. R., Nik Mahmood, N. A. B., Schuurman-Wolters, G. K., Poolman, B. Membrane reconstitution of ABC transporters and assays of translocator function. Nat Protoc. 3 (2), 256-266 (2008).
- Rigaud, J. -. L., Pitard, B., Levy, D. Reconstitution of membrane proteins into liposomes: application to energy-transducing membrane proteins. Biochim Biophys Acta BBA – Bioenerg. 1231 (3), 223-246 (1995).
- Szoka, F., Papahadjopoulos, D. Procedure for preparation of liposomes with large internal aqueous space and high capture by reverse-phase evaporation. Proc Natl Acad Sci. 75 (9), 4194-4198 (1978).
- . Synthetic Organelles for Energy Conservation and Delivery of Building Blocks for Lipid Biosynthesis Available from: https://www.researchsquare.com/article/rs-3385355/v1 (2023)
- Lee, K. Y., et al. Photosynthetic artificial organelles sustain and control ATP-dependent reactions in a protocellular system. Nat Biotechnol. 36 (6), 530-535 (2018).
- Méléard, P., Bagatolli, L. A., Pott, T. Giant unilamellar vesicle electroformation. Methods in Enzymology. , 161-176 (2009).
- Garten, M., Aimon, S., Bassereau, P., Toombes, G. E. S. Reconstitution of a transmembrane protein, the voltage-gated ion channel, KvAP, into giant unilamellar vesicles for microscopy and patch clamp studies. J. Vis. Exp. (95), e52281 (2015).
- Doeven, M. K., et al. lateral mobility and function of membrane proteins incorporated into giant unilamellar vesicles. Biophys J. 88 (2), 1134-1142 (2005).
- Pols, T., et al. A synthetic metabolic network for physicochemical homeostasis. Nat Commun. 10 (1), 4239 (2019).
- Bailoni, E., Poolman, B. ATP recycling fuels sustainable glycerol 3-phosphate formation in synthetic cells fed by dynamic dialysis. ACS Synth Biol. 11 (7), 2348-2360 (2022).
- Van Der Heide, T. On the osmotic signal and osmosensing mechanism of an ABC transport system for glycine betaine. EMBO J. 20 (24), 7022-7032 (2001).
- Van’T Klooster, J. S., et al. Membrane lipid requirements of the lysine transporter Lyp1 from Saccharomyces cerevisiae. J Mol Biol. 432 (14), 4023-4031 (2020).
- Lou, G., Anderluzzi, G., Woods, S., Roberts, C. W., Perrie, Y. A novel microfluidic-based approach to formulate size-tuneable large unilamellar cationic liposomes: Formulation, cellular uptake and biodistribution investigations. Eur J Pharm Biopharm. 143, 51-60 (2019).
- Weiss, M., et al. Sequential bottom-up assembly of mechanically stabilized synthetic cells by microfluidics. Nat Mater. 17 (1), 89-96 (2018).
- Pols, T., Singh, S., Deelman-Driessen, C., Gaastra, B. F., Poolman, B. Enzymology of the pathway for ATP production by arginine breakdown. FEBS J. 288 (1), 293-309 (2021).
- Yandrapalli, N., Robinson, T. Ultra-high capacity microfluidic trapping of giant vesicles for high-throughput membrane studies. Lab Chip. 19 (4), 626-633 (2019).
- Elias, M., et al. Microfluidic characterization of biomimetic membrane mechanics with an on-chip micropipette. Micro Nano Eng. 8, 100064 (2020).
- Robinson, T., Kuhn, P., Eyer, K., Dittrich, P. S. Microfluidic trapping of giant unilamellar vesicles to study transport through a membrane pore. Biomicrofluidics. 7 (4), 044105 (2013).
- Cooper, A., Girish, V., Subramaniam, A. B. Osmotic Pressure Enables High-Yield Assembly of Giant Vesicles in Solutions of Physiological Ionic Strengths. Langmuir. 39 (15), 5579-5590 (2023).
- Tantama, M., Martínez-François, J. R., Mongeon, R., Yellen, G. Imaging energy status in live cells with a fluorescent biosensor of the intracellular ATP-to-ADP ratio. Nat Commun. 4 (1), 2550 (2013).
- Setyawati, I., et al. In vitro reconstitution of dynamically interacting integral membrane subunits of energy-coupling factor transporters. eLife. 9, e64389 (2020).
- Oropeza-Guzman, E., Ríos-Ramírez, M., Ruiz-Suárez, J. C. Leveraging the coffee ring effect for a defect-free electroformation of giant unilamellar vesicles. Langmuir. 35 (50), 16528-16535 (2019).
- Estes, D. J., Mayer, M. Electroformation of giant liposomes from spin-coated films of lipids. Colloids Surf B Biointerfaces. 42 (2), 115-123 (2005).

