복부대동맥류의 정량적 변형 매핑
English
소셜에 공유하기
개요
출처: 한나 엘 세불1,아르빈 에이치 소프리야트나1,존 J. 보일2, 크레이그 J. 괴르겐1
1 웰던 생물 의학 공학 대학, 퍼듀 대학, 웨스트 라파예트, 인디애나
2 기계 공학 및 재료 과학, 세인트 루이스워싱턴 대학, 세인트 루이스, 미주리
혈관, 피부, 힘줄 및 기타 장기와 같은 연조직의 기계적 행동은 탄성과 강도를 제공하는 엘라스틴과 콜라겐의 구성에 의해 강하게 영향을 받습니다. 이 단백질의 섬유 방향은 연조직의 유형에 따라 다르며 병들게 된 조직에서 변경될 수 있는 단일 바람직한 방향에서 복잡한 메쉬 네트워크에 이르기까지 다양합니다. 따라서 연조직은 세포 및 장기 수준에서 비상적으로 행동하여 3차원 특성화의 필요성을 만듭니다. 복잡한 생물학적 조직 또는 구조 내에서 변형 필드를 안정적으로 추정하는 방법을 개발하는 것은 질병을 기계적으로 특성화하고 이해하는 데 중요합니다. 스트레인은 연조직이 시간이 지남에 따라 상대적으로 변형되는 방법을 나타내며 다양한 추정을 통해 수학적으로 설명될 수 있습니다.
시간이 지남에 따라 이미지 데이터를 수집하면 변형과 변형을 추정할 수 있습니다. 그러나, 모든 의료 영상 양식은 생체 내 변형에서 정확하게 추정하는 어려움을 증가 소음의 일부 양을 포함. 여기에 설명된 기술은 직접 변형 추정(DDE) 방법을 사용하여 볼륨 이미지 데이터에서 공간적으로 다양한 3D 스트레인 필드를 계산하여 이러한 문제를 성공적으로 극복합니다.
현재 변형 추정 방법에는 디지털 이미지 상관관계(DIC) 및 디지털 볼륨 상관관계가 포함됩니다. 안타깝게도 DIC는 2D 평면의 변형을 정확하게 추정할 수 있으며 이 방법의 적용을 심각하게 제한할 수 있습니다. 유용하지만 DIC와 같은 2D 메서드는 3D 변형을 겪는 영역에서 균주를 정량화하는 데 어려움을 겪습니다. 이는 평면 외 이동으로 변형 오류가 발생하기 때문입니다. 디지털 볼륨 상관 관계는 초기 볼륨 데이터를 영역으로 나누고 변형된 볼륨의 가장 유사한 영역을 찾아 평면 내 오류를 줄이는 보다 적용 가능한 방법입니다. 그러나 이 방법은 노이즈에 민감하다는 것을 증명하며 재료의 기계적 특성에 대한 가정이 필요합니다.
여기에서 입증된 기술은 DDE 방법을 사용하여 이러한 문제를 제거하므로 의료 이미징 데이터의 분석에 매우 유용합니다. 또한, 높은 또는 국소화 된 변형에 강력합니다. 여기서는 게이트, 체적 4D 초음파 데이터의 수집, 분석 가능한 형식으로의 변환, 3D 변형 및 해당 Green-Lagrange 균주를 추정하기 위한 사용자 지정 Matlab 코드의 사용에 대해 설명합니다. 녹색-Lagrange 스트레인 텐서는 변위의 최소 제곱 핏(LSF)에서 F를 계산할 수 있기 때문에 많은 3D 스트레인 추정 방법으로 구현됩니다. 아래 방정식은 녹색-Lagrange 스트레인 텐서, E를나타내며, 여기서 F와 나는 변형 그라데이션과 두 번째 차순 아이덴티티 텐서를 각각 나타낸다.
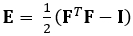 (1)
(1)
Principles
Procedure
Results
Using the procedure described above, 4D ultrasound of an angiotensin II-induced suprarenal dissecting abdominal aortic aneurysm (AAA) of a mouse was acquired. Multiple short-axis EKV video loops were acquired along the aorta and combined to create 4D data, as shown in Figure 1. This data was then converted into a MAT file using a custom code, which was then analyzed in a 3D strain calculation code using a warping function. After optimizing the parameters of the code for a specific data set, a representative, long-axis view with corresponding strain values was produced as well as a 3D slice visualization plot with an overlaid strain color map (Figure 2). This DDE technique and strain data highlight the heterogeneous spatial variations in strain, particularly when a thrombus is present. These results can then be correlated with vessel structure to determine the relationship between in vivo deformation and aneurysm composition.
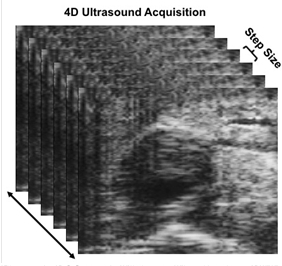
Figure 1: ECG-gated kilohertz visualization (EKV) loops of the aorta are acquired from manually inputted starting and ending locations, following a step size of 0.2 mm.
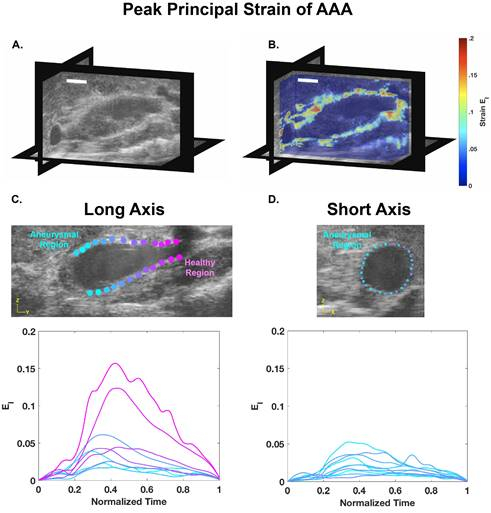
Figure 2: 4D high frequency ultrasound data of a murine dissecting abdominal aortic aneurysm represented at systole (A) with principal strain fields estimated and overlaid (B) (Scalebar = 5 mm). Long- and short-axis views representing both aneurysmal and healthy regions corresponding principal strain over one cardiac cycle (systole: t= 0.4) (C, D). These data show relatively high strain levels in healthy regions and reduced strain values within the dissecting aneurysm.
Applications and Summary
Localized in vivo mechanical characterization is an important part of understanding the growth and remodeling of biological tissues. Compared to existing approaches, the strain quantification procedure described here uses an improved method of accurately calculating 3D strain through optimally warping the undeformed image before cross-correlation. This method does not use any material assumptions in determining strains within tissue volumes. Unfortunately, the strain estimation is reliable only down to a kernel size of 15x15x15 voxels when using ultrasound data, suggesting that this DDE approach may not detect subtle features within a strain field. Despite this limitation, it remains an important tool for investigating mechanical responses, diagnosing pathology, and improving disease models.
Many areas of research beyond aortic aneurysms can benefit from this strain measurement tool. Cardiac strain can also be easily quantified using this method. Because the myocardium undergoes 3D deformation during the cardiac cycle, quantifying strain in three dimensions is integral to reliably characterizing the dynamics of this tissue. Reliable strain data is especially important when tracking disease progression in animal models.
3D strain analysis can also be applied to intestinal ultrasound imaging. Mechanical characterization of intestinal tissue is most commonly conducted in vitro. However, this is not always a true representation of the actual behavior of the intestines in vivo because of effects from surrounding structures. As an example of clinically translating this approach, calculating the strain from images of intestinal fibrosis due to abnormal luminal pressure could provide early detection of problematic areas that require surgical intervention.
Beyond the larger scale applications, this method can also be applied to the cellular level by using higher resolution imaging techniques, such as confocal microscopy. Characterizing the extracellular matrix is important for understanding how cells communicate. Much research has been conducted on the biochemical characterization, but understanding how communication can be conducted through mechanical responses requires an understanding of deformation and strain. Bulk strain is not beneficial because there is no way to determine the origin of the deformation change. Applying a high-resolution DDE approach could directly reveal how the extracellular matrix responds to mechanical changes.
ACKNOWLEDGEMENTS
We would like to acknowledge John Boyle, Guy Genin, and Stavros Thomopoulos for the contribution of the DDE custom Matlab code capable of directly estimating Lagrange-Green strain.
내레이션 대본
Three-dimensional strain imaging is used to estimate deformation of soft tissues over time and understand disease. The mechanical behavior of soft tissues, such as skin, blood vessels, tendons, and other organs, is strongly influenced by their extracellular composition, which can become altered from aging and disease. Within complex biological tissues, it is important to characterize these changes, which can significantly affect the mechanical and functional properties of an organ.
Quantitative strain mapping uses volumetric image data and a direct deformation estimation method to calculate the spatially varying three-dimensional strain fields. This video will illustrate the principles of strain mapping, demonstrate how quantitative strain mapping is used to estimate strain fields within complex biological tissues, and discuss other applications.
Biological tissues are strongly influenced by the composition and orientation of elastin and collagen. The protein elastin is a highly elastic component of tissues that continually stretch and contract, such as blood vessels and the lungs. Collagen is the most abundant protein in the body, and is assembled from individual triple-helical polymers that are bundled into larger fibers that provide structural integrity to tissues ranging from skin to bones.
The orientation of these proteins ranges from aligned fibers to fibrous mesh networks, which affects the mechanical properties of the tissue. Strain is a measure of the relative deformation of soft tissues over time, and can be used to visualize injury and disease. It is described and mapped using mathematical estimations.
To map strain in complex organs, such as the heart, four-dimensional ultrasound data, which provides high resolution, spatial, and temporal information, can be used. Then the direct deformation estimation method, or DDE, is applied to the data. A code is used to estimate the 3D deformation and corresponding Green-Lagrange strains using the following equation.
The Green-Lagrange strain tensor depends on the deformation gradient tensor and the second order identity tensor. Deformation gradient tensors are traditionally estimated from displacement fields. In the DDE method, a warping function is optimized to be directly analogous to the deformation tensor. The warping function depends on both spatial position and the warping parameter. The calculation of deformation is directly incorporated into the warping function. The first nine elements represent the deformation gradient tensor.
This method is used to estimate both large and localized deformations in soft tissues. Now that we understand the principles of strain mapping, let’s now see how strain mapping is performed to detect aortic aneurysms in mice.
To begin setup, open the Vivo 2100 software and connect the laptop to the ultrasound system. Make sure the physiological monitoring unit is on to measure heart rate and temperature. Then initialize the 3D motor stage.
Install the ultrasound transducer and ensure that all proper connections are made. Next, anesthetize the animal that will be imaged using 3% isoflurane in a knock-down chamber. Once the mouse is anesthetized, move it to the heated stage and secure a nose cone to deliver 1-2% isoflurane. Apply ophthalmic ointment to the eyes and secure the paws to the stage electrodes to monitor the animal’s respiration and heart rate. Then insert a rectal temperature probe. Apply depilatory cream to remove hair from the area of interest, and then apply a generous amount of warm ultrasound gel to the depilated area.
To start the image acquisition, first, open the imaging window and select B mode. Then lower the transducer onto the animal and use the x and y-axis knobs on the stage to locate the area of interest. Monitor the respiratory rate to make sure it does not decrease substantially. Position the transducer in the middle of the region of interest. Then approximate the distance required to cover the entire region of interest.
Enter these dimensions in the MATLAB code and choose a step size of 0.08 millimeters. Make sure the animal’s heart and respiratory rates are stable, then run the MATLAB code.
After image acquisition, export the data as raw XML files and convert them into MAT files. Make sure to input the number of frames, step size, and output resolution. Then re-sample the matrix in through-plane.
Import the new MAT file into the 3D strain analysis code. It may be necessary to rescale the file to reduce the computation time. Then, input the region to be analyzed. Approximate the number of pixels in a two-dimensional slice of the tracked feature and select the mesh template either as a simple box or manually chosen polygons. Choose the optimal pixel number for the mesh size. Compute the Jacobians and the gradients. Repeat for each region. Then apply the warping function.
Next, using Cartesian deformations calculated from DDE, determine the eigenvalues and eigenvectors of the deformation. Then, select the slices that you want to plot strain values for by scrolling through the long axis, sort axis, and coronal axis views.
Press Select Manifold for Analysis. Then use the cursor to place markers along the aortic wall, including the thrombus, aneurysm, and healthy parts of the aorta. Repeat for all views. Finally, use color mapping to plot the results of the strain field over the region of interest.
Let us have a close look at the example of an angiotensin II-induced suprarenal dissecting abdominal aortic aneurysm acquired from a mouse. First, multiple short axis ECG-gated kilohertz visualization loops are obtained at a given step size along the aorta and combined to create 4D data.
After performing 3D strain calculation using an optimized warping function, the 3D slice visualization plot of the infrarenal aorta is obtained. The color map of principal green strain is overlaid to highlight regions of heterogeneous aortic wall strain. In addition, long axis and short axis views reveal heterogeneous spatial variations in strain, particularly when a thrombus is present.
Corresponding strain plots show higher strain values in healthy regions of the aorta in the long axis, while the aneurysmal region shows decreased strain in the short axis.
Accurate quantitative strain visualization using direct deformation estimation is a useful tool used in various biomedical applications.
For instance, cardiac strain can be quantified. During the cardiac cycle, the myocardium undergoes 3D deformation. Quantifying strain in three dimensions is integral to reliably characterizing the dynamics of this tissue over time. This is useful in tracking disease progression in animal models.
Another application is in the characterization of intestinal tissue. In vivo imaging of the intestines is challenging because of the effects from surrounding structures. However, calculating strain from images of intestinal fibrosis could be particularly useful to provide early detection of problematic areas that require surgical intervention.
At a much smaller scale, this DDE method is also applied to the cellular level by using higher resolution imaging techniques such as confocal microscopy. It serves, for example, in the characterization of extracellular matrix to understand how cells communicate under mechanical changes.
You’ve just watched JoVE’s introduction to quantitative strain visualization. You should now understand how to measure three-dimensional strain in biological tissues and how that is used in early disease detection. Thanks for watching!
