HSV-Mediated Transgene Expression of Chimeric Constructs to Study Behavioral Function of GPCR Heteromers in Mice
Instructor Prep
concepts
Student Protocol
NOTE: All procedures for animal breeding and cares were conducted according to the Institutional Animal Care and Use Committee (IACUC) regulation of Icahn School of Medicine at Mount Sinai. Be sure to use sterile gloves throughout the procedure.
1. Drug and Virus Preparation
- Drug Preparation
- Prepare 15.0 ml ketamine/xylazine anesthetic by dissolving 1.35 ml of 100 mg/ml Ketamine and 0.75 ml of 20 mg/ml xylazine in 12.9 ml of 0.9% Saline solution. Thoroughly mix solution.
- Virus Preparation
- Clone the mGlu2 and mGlu2ΔTM4N constructs into a bicistronic herpes simplex virus (HSV) vector following standard protocols previously described6. Package the viral particles as previously described6,13,14. Substitution of residues Ala-6774.40, Ala-6814.44 and Ala6854.48 in mGlu2 for Ser6864.40, Phe6904.44 and Gly-6944.48 in mGlu3 (HA-mGlu2ΔTM4N) have been described previously6.
NOTE: It was previously demonstrated that the chimeric construct HA-mGlu2ΔTM4N is expressed at the plasma membrane with intact G protein-dependent signaling6. - Store viral vectors in -80 °C when not in use. Thaw viral vector on ice, and then aliquot into 10 μl aliquots. For the surgical procedure, keep on ice.
- Clone the mGlu2 and mGlu2ΔTM4N constructs into a bicistronic herpes simplex virus (HSV) vector following standard protocols previously described6. Package the viral particles as previously described6,13,14. Substitution of residues Ala-6774.40, Ala-6814.44 and Ala6854.48 in mGlu2 for Ser6864.40, Phe6904.44 and Gly-6944.48 in mGlu3 (HA-mGlu2ΔTM4N) have been described previously6.
2. Surgery
- Surgery Preparation
- Weigh mouse and inject mouse with appropriate dose of ketamine/xylazine cocktail (for details, see 1.1.1).
- Check mouse to see if properly anesthetized, squeeze foot and tail for pain response, if unable to elicit a response, the mouse is properly anesthetized.
- Shave mouse head from the base of the skull to the tip of the nose using clippers. Apply ophthalmic gel to the mouse afterward to prevent blindness of the mouse.
- Load each syringe onto the stereotaxic frame. Then tilt perpendicular portion of each arm of the stereotaxic frame so that they are 10 degrees away from the normal. Ensure that the arms are tilted, such that the needles are facing each other.
- Clean each syringe by filling the needle with 70% ethanol. Fill the needle at least three times to ensure that the syringe is clean.
- Once the needle has been cleaned, flush the needle by filling the needle with double distilled H2O. Once flushed, fill each needle with 1.3 µl of double distilled H2O. Twist the plunger of the syringe to release 0.3 µl of double distilled H2O. If water beads at the tip of the needle, carefully wipe away water. If nothing comes out of the syringe, push the plunger completely down and then repeat cleaning of syringe.
- After filling with water, then pull up the syringe filling the syringe with 0.5 µl of air.
- Once the air and water are in the syringe, carefully fill the syringe with 1.3 µl of virus solution. At this point ensure that the total volume in the syringe is 2.8 µl. Again twist the tip of the syringe to release 0.3 µl of virus. If liquid beads at the tip of the needle, carefully wipe away liquid. If nothing comes out of the syringe, push the plunger completely down and then repeat cleaning of syringe.
- Surgery
- Attach the mouse to the stereotaxic frame, making sure to adjust the stereotaxic frame so that the skull is level and flat. Apply povodine-iodine to the exposed scalp. Using a scalpel, make a sagittal incision along the midline of the skull within the exposed shaved area. Then attach the buret clamps to the skin at the incision site to make sure that the skull remains exposed.
- Use H2O2 to dissolve away the periosteum to expose the sutures of the skull. Now that the bregma and sutures are visible, be sure to adjust the stereotaxic frame to make sure that the skull is level.
- Align the needle tips of the syringes with the bregma and record the coordinates of the bregma. Calculate the coordinates of the where the needles are going to be inserted.
- For the Rostral-Cauldal (R-C) plane, add 1.6 mm to the recorded R-C bregma coordinates (+1.6 from Bregma). For the Dorsal-Ventral (D-V) plane, subtract 2.4 mm from the recorded D-V bregma coordinates (-2.4 from Bregma).
- Finally, for the Medial Lateral (M-L) plane, add 2.6 mm to the recorded M-L bregma coordinates (+2.6 from Bregma). For all coordinates be sure to record both left and right coordinates, as this is a bilateral injection.
- Bring the needles to the desired coordinates. Mark the places of where the needles are going to be inserted and with a drill, drill the marked areas.
- With a cotton tip applicator wipe away any excess blood or bone fragment.
- Bring the needles to the skull where the tips of the needles are touching the surface of the brain. Then lower the needles to the desired coordinates slowly lowering them.
- Once the needles are at the desired coordinates, slowly inject the contents of the syringe by twisting the plunger of the needle 0.1 µl per minute over the course of 5 min (in total 0.5 µl).
- Once the injection has been made leave the syringe in cortex for another 5 min.
- Closing Up/Care
- Remove the needles from the mouse cortex steadily and slowly. Then remove the mouse from the stereotaxic frame.
- Apply cyanoacrylate (dermal adhesive) to the base flaps of skin from the incision and then with forceps grab the flaps of skin and place them together.
- Allow the cyanoacrylate to dry. Place the mouse in cage over a heating pad (heating pad is optional if the surgical suite is kept under RT of 37 °C – otherwise not necessary). Be sure to place the mouse on a paper towel to make sure that bedding does not adhere to surgical site.
- Depending on the length of the surgical procedure, ensure that the mice is out of anesthesia within 30 – 60 min after procedure. Following surgery, the animal is placed in its own cage and monitored until it becomes conscious before returning to its room to recover. No analgesics are used post-operatively, because they may alter the results of our experiments by modifying some of the brain biochemical pathways. However, animals are monitored until they recover from anesthesia on the day of surgery, and daily post-operation for signs of infection and evaluation of pain/distress.
3. Head Twitch Response Experiment
- Set-up
- Carry out all behavioral testing between 10:00 AM and 2:00 PM, 2 – 3 days after stereotactic injection of viral particles.
- Dissolve (±)-1-(2,5-dimethoxy-4-iodophenyl)-2-aminopropane hydrochloride (DOI) into a 0.9% saline solution to 2.0 mg/kg. Also prepare a 0.9% saline solution.
- Prepare a home cage (28 x 18 x 15 cm) without any bedding and using a tri-pod, adjust a camera so that the view of the video camera is directly above the home cage.
- Habituate the mice to the room for at least 4 hr prior to the beginning of the experiment.
- Set up a camcorder to record the head twitch.
- Experiment
- Position the camera so that it is directly over a home cage. Calibrate the camera so that the entire home cage is in the field of view.
- Weigh mouse and inject mouse intraperitoneally with appropriate dose of either 0.9% saline or DOI (0.01 ml/g). NOTE: If a mouse weighs 25 grams, administer dose to a total volume of 0.25 ml.
- Place each mouse back in their home cage for 10 min. After 10 min, place mouse into the center of the empty home cage and validate that there are no blind spots in the field of view of the camera. Press record on the camcorder. Leave the room.
NOTE: Mouse movements and various behavioral responses therein (head twitch, ear scratch, etc. Please refer to supplemental data table 1 of Gonzalez-Maeso et al. 2007 for full list of behavioral responses induced by DOI.)3, will be recorded for 30 min. Therefore, it is important there are no blind spots in the field of view recorded. - After 30 min stop recording on the camcorder and place mouse back in original home cage. Repeat this process for each mouse.
- Review
- Have each referee review the tapes blind to the experimental conditions of mouse (i.e., drugs used during head twitch experiment or virus used during intracranial injection)). Manually record every head twitch throughout the video.
NOTE: Head-twitch is defined as a rapid shaking head movement conducted by a mouse (supplemental video). - For each mouse, average the final HTR response from the three totals of the blind referees. Then group these values by experimental condition and carry out statistical analysis (i.e., t-test or ANOVA).
- Have each referee review the tapes blind to the experimental conditions of mouse (i.e., drugs used during head twitch experiment or virus used during intracranial injection)). Manually record every head twitch throughout the video.
HSV-Mediated Transgene Expression of Chimeric Constructs to Study Behavioral Function of GPCR Heteromers in Mice
Learning Objectives
Previous findings demonstrate that the head-twitch murine behavioral response is reliably and robustly elicited by hallucinogens, and it is absent in 5-HT2A-KO mice3. Furthermore, it has been shown that the head-twitch response elicited by the hallucinogenic 5-HT2A agonists DOI and LSD was significantly decreased in mGlu2-KO mice5. However, although previous findings convincingly demonstrate that 5-HT2A and mGlu2 are assembled as a heteromeric complex in vitro in transfected cells1,2,15, whether this structural arrangement behaves as such in living mice remained unsolved. To fully understand the role of the 5-HT2A-mGlu2 receptor heterocomplex in the psychoactive-like effects induced by hallucinogenic 5-HT2A receptor agonists, expression of either mGlu2 or mGlu2ΔTM4N in frontal cortex of mGlu2-KO mice to examine whether this manipulation regulates behavior.
Mice received intra-frontal cortical injections of bicistronic HSV expressing green fluorescent protein (GFP) and either mGlu2 or mGlu2ΔTM4N, or GFP alone. First, it was confirmed that the virus over-expresses mGlu2 or mGlu2ΔTM4N in mouse frontal cortex (Figures 1A and 1B). As previously demonstrated5, head-twitch behavior induced by DOI was absent in mGlu2-KO mice injected with the empty vector HSV-GFP. Notably, the head-twitch response induced by the hallucinogenic 5-HT2A agonist DOI was rescued in mGlu2-KO mice over-expressing mGlu2, but not mGlu2ΔTM4N, in frontal cortex as compared to that seen in animals expressing GFP (Figure 1C). Together, these findings suggest that the 5-HT2A-mGlu2 receptor complex in frontal cortex is critical for regulating psychosis-like states.
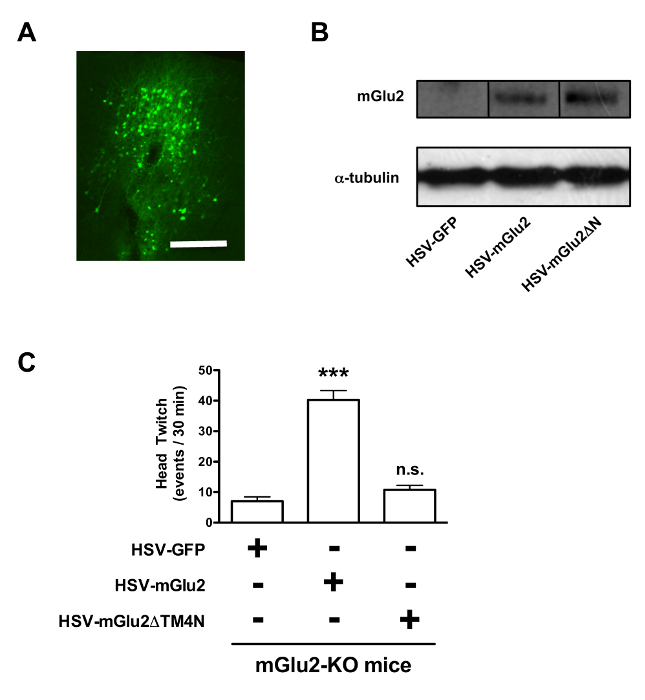
Figure 1. Expression of mGlu2 as a Receptor Heterocomplex. Expression of mGlu2 as a receptor with 5-HT2A is necessary for psychosis-like behavior induced by hallucinogenic drugs. (A) Representative image of HSV-mediated transgene expression in frontal cortex. HSV-mGlu2, which also expresses GFP, was injected into frontal cortex, and GFP expression was revealed by immunocytochemistry, scale bar, 200-um. (B) HSV-mediated transgene expression in mouse frontal cortex of mGlu2-KO mice, and anti-mGlu2 reactivity was measured by Western Blotting. Specificity of the primary antibody against the mGlu2 receptor has previously been confirmed in knockout mice6. Metabotropic glutamate receptors are GPCRs that form covalently linked homodimers. We measured immunoreactivity of mGlu2 as a monomer (100 kDa)6. (C) Viral-mediated expression of mGlu2, but not mGlu2ΔTM4N, in frontal cortex of mGlu2- KO mice significantly rescues the head-twitch response induced by the hallucinogenic 5-HT2A agonist DOI (n = 4 per group). ***p <0.001; n.s, not significant; Bonferroni's post hoc test of one-way ANOVA. Error bars represent S.E.M. Figure was modified from Moreno et al (2012)6. Please click here to view a larger version of this figure.
Supplemental Video 1. Head Twitch Response. (Right click to download). CD-1 WT mice were injected with 2.0 mg/kg DOI and placed in a cage (wall blacked out between the two cages) to elicit head-twitch response (behavior elicited after *).
List of Materials
| mGlu2 bicitronic herpes simplex virus (HSV) vector | MIT Core | mGlu2 and mGlu2DTM4N were subcloned into the bicistronic HSV-GFP virus vector p1005+ HSV expressing GFP under the control of the CMV promoter. Viral particles were produced by the Viral Core Facility at the McGovern Institute (MIT). For more information, please contact the director, Dr. Rachael Neve (rneve@mit.edu) | |
| mGlu2ΔTM4N bicitronic herpes simplex virus (HSV) vector | MIT Core | mGlu2 and mGlu2DTM4N were subcloned into the bicistronic HSV-GFP virus vector p1005+ HSV expressing GFP under the control of the CMV promoter. Viral particles were produced by the Viral Core Facility at the McGovern Institute (MIT). For more information, please contact the director, Dr. Rachael Neve (rneve@mit.edu) | |
| GFP bicitronic herpes simplex virus (HSV) vector | MIT Core | mGlu2 and mGlu2DTM4N were subcloned into the bicistronic HSV-GFP virus vector p1005+ HSV expressing GFP under the control of the CMV promoter. Viral particles were produced by the Viral Core Facility at the McGovern Institute (MIT). For more information, please contact the director, Dr. Rachael Neve (rneve@mit.edu) | |
| xylazine | Lloyd | List no. 4811-20ml, NADA #139-236, NDC Code(s): 61311-481-10 | 1.35 mL of 100mg/ml of ketamine+.75 mL of 20mg/ml of xylazine are diluted in 12.0 mL of .9% saline solution |
| ketamine | Vedco | KetaVed-10ml, NADA #200-029, NDC Code(s): 50989-161-06 | 1.35 mL of 100mg/ml of ketamine+.75 mL of 20mg/ml of xylazine are diluted in 12.0 mL of .9% saline solution |
| ophthalmic gel | Fisher Scientific | NC0550805 | |
| burret clips | Fisher Scientific | NC9268369 | |
| Feather surgical blade | Fisher Scientific | NC9032736 | |
| Hydrogen Peroxide | Fisher Scientific | 19-898-919 | |
| Hamilton syringe | Fisher Scientific | 14815203 | |
| Hamilton™ Small Hub Removable Needles (33 Ga) | Fisher Scientific | 14816206 | |
| Cordless Micro Drill | Fisher Scientific | NC9089241 | |
| Dermabond Dermal Adhesive | Fisher Scientific | NC0690470 | |
| (±)-1-(2,5-Dimethoxy-4-iodophenyl)-2-aminopropane hydrochloride (DOI) | Sigma-Aldrich | 42203-78-1 | Dissolved in .9% saline solution to the concentration of 2.0 mg/kg |
Lab Prep
The heteromeric receptor complex between 5-HT2A and mGlu2 has been implicated in some of the behavioral phenotypes in mouse models of psychosis1,2. Consequently, investigation of structural details of the interaction between 5-HT2A and mGlu2 affecting schizophrenia-related behaviors represents a powerful translational tool. As previously shown, the head-twitch response (HTR) in mice is elicited by hallucinogenic drugs and this behavioral response is absent in 5-HT2A knockout (KO) mice3,4. Additionally, by conditionally expressing the 5-HT2A receptor only in cortex, it was demonstrated that 5-HT2A receptor-dependent signaling pathways on cortical pyramidal neurons are sufficient to elicit head-twitch behavior in response to hallucinogenic drugs3. Finally, it has been shown that the head-twitch behavioral response induced by the hallucinogens DOI and lysergic acid diethylamide (LSD) is significantly decreased in mGlu2-KO mice5. These findings suggest that mGlu2 is at least in part necessary for the 5-HT2A receptor-dependent psychosis-like behavioral effects induced by LSD-like drugs. However, this does not provide evidence as to whether the 5-HT2A-mGlu2 receptor complex is necessary for this behavioral phenotype. To address this question, herpes simplex virus (HSV) constructs to express either mGlu2 or mGlu2ΔTM4N (mGlu2/mGlu3 chimeric construct that does not form the 5-HT2A-mGlu2 receptor complex) in the frontal cortex of mGlu2-KO mice were used to examine whether this GPCR heteromeric complex is needed for the behavioral effects induced by LSD-like drugs6.
The heteromeric receptor complex between 5-HT2A and mGlu2 has been implicated in some of the behavioral phenotypes in mouse models of psychosis1,2. Consequently, investigation of structural details of the interaction between 5-HT2A and mGlu2 affecting schizophrenia-related behaviors represents a powerful translational tool. As previously shown, the head-twitch response (HTR) in mice is elicited by hallucinogenic drugs and this behavioral response is absent in 5-HT2A knockout (KO) mice3,4. Additionally, by conditionally expressing the 5-HT2A receptor only in cortex, it was demonstrated that 5-HT2A receptor-dependent signaling pathways on cortical pyramidal neurons are sufficient to elicit head-twitch behavior in response to hallucinogenic drugs3. Finally, it has been shown that the head-twitch behavioral response induced by the hallucinogens DOI and lysergic acid diethylamide (LSD) is significantly decreased in mGlu2-KO mice5. These findings suggest that mGlu2 is at least in part necessary for the 5-HT2A receptor-dependent psychosis-like behavioral effects induced by LSD-like drugs. However, this does not provide evidence as to whether the 5-HT2A-mGlu2 receptor complex is necessary for this behavioral phenotype. To address this question, herpes simplex virus (HSV) constructs to express either mGlu2 or mGlu2ΔTM4N (mGlu2/mGlu3 chimeric construct that does not form the 5-HT2A-mGlu2 receptor complex) in the frontal cortex of mGlu2-KO mice were used to examine whether this GPCR heteromeric complex is needed for the behavioral effects induced by LSD-like drugs6.
Procedure
The heteromeric receptor complex between 5-HT2A and mGlu2 has been implicated in some of the behavioral phenotypes in mouse models of psychosis1,2. Consequently, investigation of structural details of the interaction between 5-HT2A and mGlu2 affecting schizophrenia-related behaviors represents a powerful translational tool. As previously shown, the head-twitch response (HTR) in mice is elicited by hallucinogenic drugs and this behavioral response is absent in 5-HT2A knockout (KO) mice3,4. Additionally, by conditionally expressing the 5-HT2A receptor only in cortex, it was demonstrated that 5-HT2A receptor-dependent signaling pathways on cortical pyramidal neurons are sufficient to elicit head-twitch behavior in response to hallucinogenic drugs3. Finally, it has been shown that the head-twitch behavioral response induced by the hallucinogens DOI and lysergic acid diethylamide (LSD) is significantly decreased in mGlu2-KO mice5. These findings suggest that mGlu2 is at least in part necessary for the 5-HT2A receptor-dependent psychosis-like behavioral effects induced by LSD-like drugs. However, this does not provide evidence as to whether the 5-HT2A-mGlu2 receptor complex is necessary for this behavioral phenotype. To address this question, herpes simplex virus (HSV) constructs to express either mGlu2 or mGlu2ΔTM4N (mGlu2/mGlu3 chimeric construct that does not form the 5-HT2A-mGlu2 receptor complex) in the frontal cortex of mGlu2-KO mice were used to examine whether this GPCR heteromeric complex is needed for the behavioral effects induced by LSD-like drugs6.
