In Vitro and In Vivo Models to Study Corneal Endothelial-mesenchymal Transition
Instructor Prep
concepts
Student Protocol
All the procedures followed in this study accorded with the Association for Research in Vision and Ophthalmology Statement for Use of Animals in Ophthalmic and Vision Research and were approved by the Institutional Animal Care and Use Committee of National Taiwan University Hospital.
1. Isolation, Primary Culture Preparation, and Immunostaining of Bovine CECs
- Acquire fresh bovine eyes from a local abattoir.
- Disinfect the eyes in a 10% w/v povidone-iodine solution for 3 min. Wash them with phosphate-buffered saline (PBS) solution.
- Harvest the corneal button with a scalpel and scissors under sterile conditions. Peel the Descemet's membrane with forceps under a dissecting microscope (note that in this study, the bovine eyes were enucleated by a local abattoir; therefore, the first study procedure was the disinfection of the preenucleated eyes in the laboratory).
- Incubate the Descemet's membrane in 1 ml of trypsin at 37 °C for 30 min. Collect the bovine CECs by centrifugation at 112 x g for 5 min. Resuspend the cells in 1 ml of supplemented hormonal epithelial medium (SHEM) containing equal volumes of HEPES-buffered Dulbecco's modified Eagle medium and Ham's F12 medium, supplemented with 5% fetal bovine serum, 0.5% dimethyl sulfoxide, 2 ng/ml of human epidermal growth factor, 5 mg/ml of insulin, 5 mg/ml of transferrin, 5 ng/ml of selenium, 1 nmol/l of cholera toxin, 50 mg/ml of gentamicin, and 1.25 mg/ml of amphotericin B.
- Seed the cells (approximately 1 x 105 cells per eye) into a 6 cm dish. Culture them in the SHEM. Incubate the dish at 37 °C in an atmosphere of 5% CO2 in air. Change the culture medium every 3 days.
- When the cells reach confluence, wash them in PBS and incubate them in 1 ml of trypsin at 37 °C for 5 min. Collect them by centrifugation at 112 x g for 5 min. Re-suspend the cell pellet in 1 ml of the SHEM.
- Count the cells in a hemocytometer. Seed the cells on cover slides at a density of 1 x 104 per well in a 24-well plate, and culture the cells in the SHEM. For investigating the EnMT-suppressing effect of marimastat, incubate the cells in SHEM with 10 µM of marimastat added into the culture medium, and change the culture medium every 3 days.
- Fix the cells at an indicated time point with 250 µl of 4% paraformaldehyde, pH 7.4, for 30 min at room temperature. Permeabilize with 250 µl of 0.5% Triton X-100 for 5 min. Block with 10% bovine serum albumin for 30 min.
- Incubate the cells with primary antibodies against active beta-catenin (ABC), snail, and slug overnight at 4 °C. The dilution of the primary antibodies used is 1:200. The antibodies are diluted in antibody diluent composed of 10 mM PBS, 1% w/v bovine serum albumin, and 0.09% w/v sodium azide.
- Wash the cells twice with PBS for 15 min, and incubate them with the Alexa Fluor-conjugated secondary antibody (1:100 in antibody diluent) at room temperature for 1 hr.
- Counterstain the cell nucleus by covering the cells with 2 µg/ml of 4',6-diamidino-2-phenylindole, wash the cells twice with PBS for 15 min, and mount them with anti-fading mounting solution.
- Obtain immunofluorescent images at the excitation wavelengths of 405 and 488 nm by using a laser scanning confocal microscope with 20X objective magnification.
2. Rat Corneal Endothelium Cryoinjury Model and Intracameral Injection
- Anesthetize 12 week-old male Sprague-Dawley rats with intramuscular injections of 2% xylazine (5.6 mg/kg) and tiletamine plus zolazepam (18 mg/kg). Gently pinch the skin of the animals to confirm proper anesthesia in the absence of skin twitching.
- Instill one drop of 0.5% proparacaine hydrochloride to the right eye of each rat to minimize eye pain and the blink reflex. Instill tetracycline ointment to the left eye to prevent corneal dryness.
- Cool a stainless steel probe (diameter = 3 mm) in liquid nitrogen. Apply the stainless steel probe to the central cornea of the right eye for 30 sec. Frequently instill PBS to the right eye during the procedure to prevent corneal dryness.
- Instill 0.1% atropine and 0.3% gentamicin sulfate immediately after cryoinjury and once daily to relieve ocular pain resulting from ciliary spasm and to prevent infection.
- After the procedure, keep the rats warm using a heat lamp, and observe their recovery every 15 min after anesthesia until they regain motor control. Additionally, apply tetracycline ointment to the right eye to prevent corneal dryness during the recovery period.
- Repeat the corneal cryoinjury for 3 consecutive days.
- For delivering marimastat or bFGF into the anterior chamber of the rat eyes, anesthetize the rats as described previously. Instill one drop of 0.5% proparacaine hydrochloride into the right eye to minimize eye pain and the blink reflex.
- Irrigate the ocular surface with sterile PBS. Perform anterior chamber paracentesis under an operating microscope by inserting a 30 G needle attached to a 1 ml syringe at the paralimbal clear cornea in a plane above and parallel to the iris.
- Turn the needle bevel up, and slightly depress the corneal wound to drain some aqueous humor and reduce the intraocular pressure. Inject 0.02 ml of the drug intracamerally. Gently compress the needle tract with a cotton tip during the needle withdrawal.
- Photograph the external eye at an indicated time point under the operating microscope.
3. Harvesting the Rat Corneal Button and Immunostaining
- To euthanize the rats, place them in a euthanasia chamber and infuse 100% CO2 at a fill rate of 10-30% of the chamber volume per minute. Maintain CO2 infusion for an additional minute after lack of respiration and faded eye color.
- Penetrate the rat eye at the limbus with a sharp blade. Cut the corneas with corneal scissors along the limbus. Flatten the corneas on a slide. Make additional radial incisions if the corneas curl up.
- Fix the corneas with 250 µl of 4% paraformaldehyde, pH 7.4, for 30 min at room temperature. Permeabilize with 250 µl of 0.5% Triton X-100 for 5 min, and block with 10% bovine serum albumin for 30 min.
- Incubate the corneas with primary antibodies against ABC overnight at 4 °C with a dilution of 1:200 in antibody diluent. Wash the corneas twice with PBS for 15 min, and incubate them with the secondary antibody (1:100 in antibody diluent) at room temperature for 1 hr. Counterstain the cell nucleus by covering the cells with 2 µg/ml of 4',6-diamidino-2-phenylindole, and wash the cells twice with PBS for 15 min.
- Mount the corneas in anti-fading mounting solution. Obtain the immunofluorescent images at excitation wavelengths of 405 and 561 nm with a laser scanning confocal microscope at 20X objective magnification.
In Vitro and In Vivo Models to Study Corneal Endothelial-mesenchymal Transition
Learning Objectives
After the isolation of bovine CECs, the cells were cultured in vitro. Figure 1 presents the phase contrast images of the bovine CECs. The hexagonal shape of the cells at confluence indicated that the cells were not contaminated by corneal stromal fibroblast during cell isolation. Figure 2 depicts the immunostaining that was performed using antibodies against ABC, snail, and slug at an indicated time point. Apart from phenotypic changes in the in vitro culture, a corresponding nuclear translocation of ABC and EMT regulators was observed. Figure 3 illustrates the effect of marimastat, a broad-spectrum MMP inhibitor, on the EnMT process of the in vitro cultured bovine CECs. Figure 4 comprises the external eye photographs of rats after cryoinjury followed by intracameral injection. Figure 5 shows the immunostaining of the rat corneal button that was performed using antibodies against ABC after cryoinjury followed by intracameral injection. The nuclear translocation of ABC was observed in the PBS group and was significantly increased in the bFGF group, indicating activation of the Wnt/β-catenin signaling and EnMT process. After intracameral injection of bFGF followed by marimastat injection, the nuclear staining of ABC was diminished, suggesting the EnMT-inhibiting effect of marimastat.
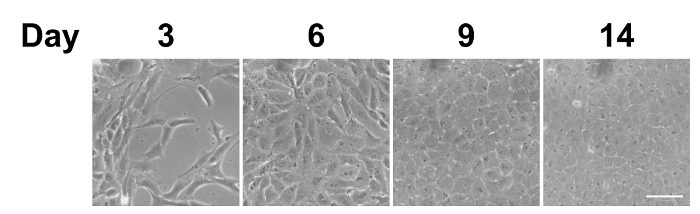
Figure 1: Phase contrast images of in vitro cultured bovine CECs. After being seeded on the culture plate, the bovine CECs initially appeared fibroblast-like on days 3 and 6. They became more hexagonal upon reaching complete confluence on day 9. Scale bar = 50 µm. Representative images of 3 replicates. Please click here to view a larger version of this figure.
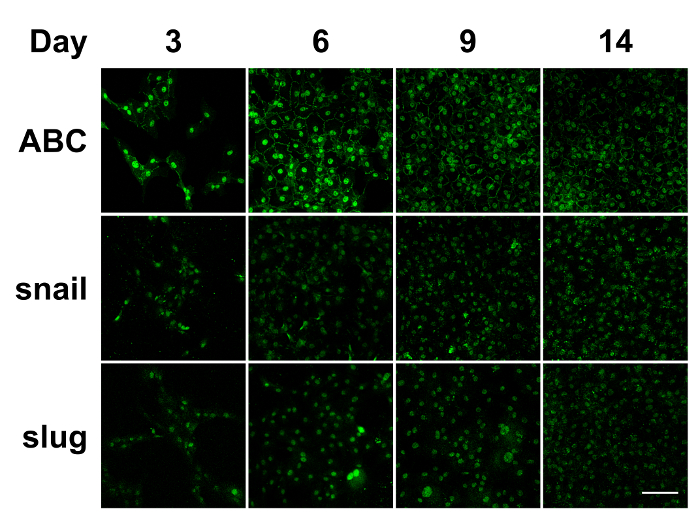
Figure 2: Immunostaining of in vitro cultured bovine CECs. During the in vitro culture of the bovine CECs, the nuclear translocation of ABC, snail, and slug was detected through day 14. ABC: active β-catenin. Scale bar = 100 µm. Representative images of 3 replicates. Please click here to view a larger version of this figure.
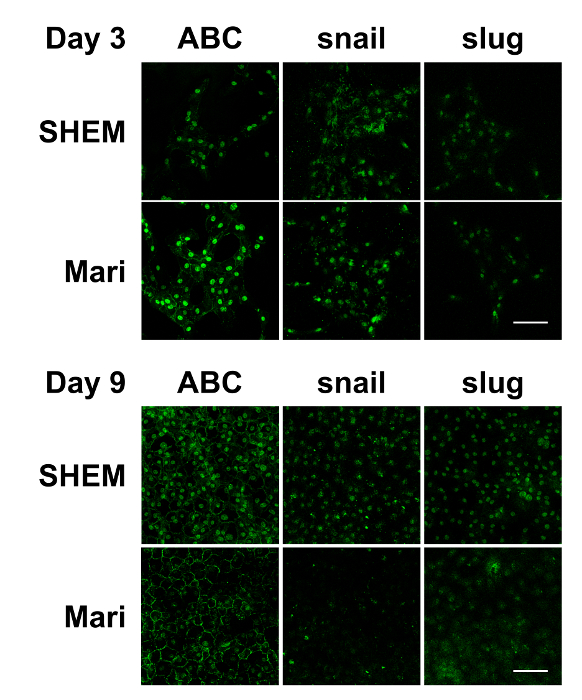
Figure 3: Immunostaining of in vitro cultured bovine CECs with or without marimastat at different cell confluence levels. Immunostaining demonstrated that ABC, snail, and slug were evident in the nucleus of the bovine CECs with or without 10 µM of marimastat on day 3. However, marimastat significantly reduced the nuclear staining of ABC, snail, and slug on day 9 when the bovine CECs became fully confluent. Scale bar = 100 µm. Representative images of 3 replicates. Please click here to view a larger version of this figure.
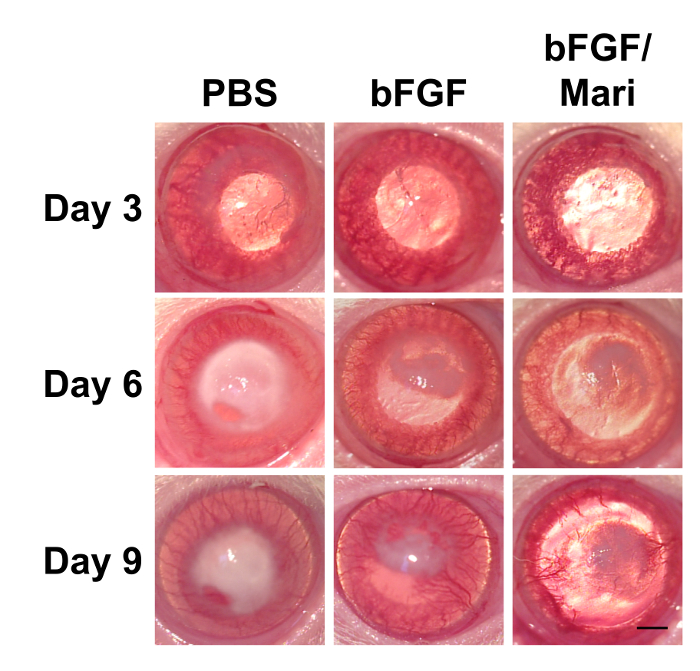
Figure 4: External eye photographs of rats at indicated time points after cryoinjury. Following cryoinjury for 3 consecutive days, rats were subjected to intracameral injection of 0.02 ml of PBS or 50 ng/ml bFGF on day 3. On day 6, 0.02 ml of 10 µM marimastat was injected intracamerally in the bFGF/Mari group, whereas PBS was injected in the other 2 groups (n = 9 in each group). External eye photographs revealed reduced corneal edema after bFGF injection, whereas marimastat further reduced corneal edema compared with bFGF alone. n = 9 in each group. Scale bar = 1 mm. Please click here to view a larger version of this figure.
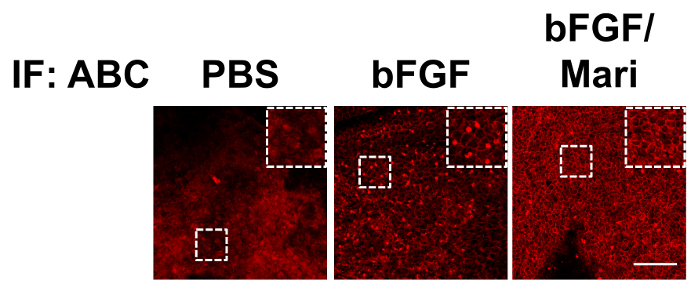
Figure 5: Immunostaining of rat corneal buttons. Immunostaining of the rat corneal buttons that were harvested on day 9 revealed little nuclear staining of ABC in the PBS group. In the bFGF group, there was extensive nuclear staining of ABC, which was significantly reduced in the bFGF/Mari group. Scale bar = 100 µm. Please click here to view a larger version of this figure.
List of Materials
| trypsin | ThermoFisher Scientific | 12604-013 | |
| Dulbecco’s modified Eagle medium and Ham's F12 medium | ThermoFisher Scientific | 11330 | |
| fetal bovine serum | ThermoFisher Scientific | 26140-079 | |
| dimethyl sulfoxide | Sigma | D2650 | |
| human epidermal growth factor | ThermoFisher Scientific | PHG0311 | |
| insulin, transferrin, selenium | ThermoFisher Scientific | 41400-045 | |
| cholera toxin | Sigma | C8052-1MG | |
| gentamicin | ThermoFisher Scientific | 15750-060 | |
| amphotericin B | ThermoFisher Scientific | 15290-026 | |
| paraformaldehyde | Electron Microscopy Sciences | 111219 | |
| Triton X-100 | Sigma | T8787 | |
| bovine serum albumin | Sigma | A7906 | |
| marimastat | Sigma | M2699-25MG | |
| anti-active beta-catenin antibody | Millpore | 05-665 | |
| anti-snail antibody | Santa cruz | sc28199 | |
| anti-slug antibody | Santa cruz | sc15391 | |
| goat anti-mouse IgG (H+L) secondary antibody | ThermoFisher Scientific | A-11001 | for staining of ABC of bovine CECs |
| goat anti-mouse IgG (H+L) secondary antibody | ThermoFisher Scientific | A-11003 | for staining of ABC of rat corneal endothelium |
| goat anti-rabbit IgG (H+L) secondary antibody | ThermoFisher Scientific | A-11008 | for staining of snail and slug of bovine CECs |
| antibody diluent | Genemed Biotechnologies | 10-0001 | |
| 4',6-diamidino-2-phenylindole | ThermoFisher Scientific | D1306 | |
| mounting medium | Vector Laboratories | H-1000 | |
| laser scanning confocal microscope | ZEISS | LSM510 | |
| xylazine | Bayer | N/A | |
| tiletamine plus zolazepam | Virbac | N/A | veterinary drug |
| proparacaine hydrochloride ophthalmic solution | Alcon | N/A | veterinary drug |
| 0.1% atropine | Wu-Fu Laboratories Co., Ltd | N/A | clinical drug |
| 0.3% gentamicin sulfate | Sinphar Group | N/A | clinical drug |
| basic fibroblast growth factor | ThermoFisher Scientific | PHG0024 | clinical drug |
Lab Prep
Corneal endothelial cells (CECs) play a crucial role in maintaining corneal clarity through active pumping. A reduced CEC count may lead to corneal edema and diminished visual acuity. However, human CECs are prone to compromised proliferative potential. Furthermore, stimulation of cell growth is often complicated by gradual endothelial-mesenchymal transition (EnMT). Therefore, understanding the mechanism of EnMT is necessary for facilitating the regeneration of CECs with competent function. In this study, we prepared a primary culture of bovine CECs by peeling the CECs with Descemet’s membrane from the corneal button and demonstrated that bovine CECs exhibited the EnMT process, including phenotypic change, nuclear translocation of β-catenin, and EMT regulators snail and slug, in the in vitro culture. Furthermore, we used a rat corneal endothelium cryoinjury model to demonstrate the EnMT process in vivo. Collectively, the in vitro primary culture of bovine CECs and in vivo rat corneal endothelium cryoinjury models offers useful platforms for investigating the mechanism of EnMT.
Corneal endothelial cells (CECs) play a crucial role in maintaining corneal clarity through active pumping. A reduced CEC count may lead to corneal edema and diminished visual acuity. However, human CECs are prone to compromised proliferative potential. Furthermore, stimulation of cell growth is often complicated by gradual endothelial-mesenchymal transition (EnMT). Therefore, understanding the mechanism of EnMT is necessary for facilitating the regeneration of CECs with competent function. In this study, we prepared a primary culture of bovine CECs by peeling the CECs with Descemet’s membrane from the corneal button and demonstrated that bovine CECs exhibited the EnMT process, including phenotypic change, nuclear translocation of β-catenin, and EMT regulators snail and slug, in the in vitro culture. Furthermore, we used a rat corneal endothelium cryoinjury model to demonstrate the EnMT process in vivo. Collectively, the in vitro primary culture of bovine CECs and in vivo rat corneal endothelium cryoinjury models offers useful platforms for investigating the mechanism of EnMT.
Procedure
Corneal endothelial cells (CECs) play a crucial role in maintaining corneal clarity through active pumping. A reduced CEC count may lead to corneal edema and diminished visual acuity. However, human CECs are prone to compromised proliferative potential. Furthermore, stimulation of cell growth is often complicated by gradual endothelial-mesenchymal transition (EnMT). Therefore, understanding the mechanism of EnMT is necessary for facilitating the regeneration of CECs with competent function. In this study, we prepared a primary culture of bovine CECs by peeling the CECs with Descemet’s membrane from the corneal button and demonstrated that bovine CECs exhibited the EnMT process, including phenotypic change, nuclear translocation of β-catenin, and EMT regulators snail and slug, in the in vitro culture. Furthermore, we used a rat corneal endothelium cryoinjury model to demonstrate the EnMT process in vivo. Collectively, the in vitro primary culture of bovine CECs and in vivo rat corneal endothelium cryoinjury models offers useful platforms for investigating the mechanism of EnMT.
