In Vitro Characterization of the Electrophysiological Properties of Colonic Afferent Fibers in Rats
Instructor Prep
concepts
Student Protocol
The experimental protocol reported here has been approved by the Animal Ethical Committee of Shanghai Jiaotong University School of Medicine (# SYXK2013-0050). The dissection of the colorectum with intact ganglion and nerve trunk takes a minimum of 15 minutes for a person quite experienced in this technique. It is therefore necessary to keep the animal alive but under deep anesthesia whilst performing the dissections, to ensure viability of the tissue for subsequent electrophysiological recording.
1. Preparation of Perfusion Solution and Test Compounds
- Prepare 5 L of Krebs solution: 113 mM NaCl, 5.9 mM KCl, 1.2 mM NaH2PO4, 1.2 mM MgSO4, 25 mM NaHCO3, 1.2 mM CaCl2, and 11.5 mM glucose. Saturate the solution with a 95% O2 + 5% CO2 gas mixture. Pre-cool ~500 mL of oxygenated Krebs in the fridge.
- Prepare aliquots of stock test compounds (1 mM capsaicin in ethanol and 10 mM 5-hydroxytryptamine (5-HT) in saline) as needed. Dilute the stock in Krebs (for bath application) and in saline (for intraluminal administration) to the final concentration just prior to use.
2. Preparation of the Recording Electrode
- Pull the recording electrode from standard glass tubes without inner filaments (1.5 mm outer diameter) using a conventional electrode puller. Adjust the puller settings for heat and pull so that the shank of the pulled electrodes is between 20 and 25 mm.
3. Tissue Collection
- Anaesthetize the rat deeply using sodium pentobarbital (80 mg/kg, i.p.).
- While ensuring sterility, expose the abdominal cavity by performing a midline incision on the abdominal wall using a scalpel. Pull the mesentery and other tissues aside to expose the colorectum.
- Place the animal under the dissecting microscope. Through careful dissection, locate the left PG and identify the PN and the LSN joining it. Cut these nerves a few millimeters away from the PG.
NOTE: The PG lies close to the colorectal junction. Typically, 3 – 4 PN from the lumbosacral spinal cord and an LSN project to the PG (Figure 1). - Cut the symphysis bone to expose the rectum. Remove the tissues (i.e., urinary bladder, etc.) above the colorectum; be careful to leave the PG intact.
- Sacrifice the rat by the intravenous injection of an overdose of pentobarbital. Transect the colon about 3 cm above the PG and remove the colorectum from the animal using forceps.
- Transfer the colorectum to a Petri dish filled with pre-cooled Krebs solution. Remove the feces by gently flushing the colon. Remove the remnant urinary bladder and other tissues carefully, without compromising the PG.
4. Dissection of the Colonic Afferent Nerves
- Place the colon into a recording chamber (20 mL) and perfuse the tissue continuously with carbogenated Krebs solution. Set the perfusion rate at 15 mL/min.
- Cannulate the colorectum at both the oral and anal ends. Start the intraluminal infusion of the colon with saline at a rate of 10 mL/h in the oral to anal direction.
- Locate the major PG under the dissecting microscope. Use insect pins to expose the ganglion. With careful dissection, find a fine branch of nerve emanating from the ganglion and running towards the colon (Figure 1). Cut the nerve close to the ganglion.
NOTE: Figure 1 illustrates a recording from a nerve branch distal to the PG. The nerve presumably contains a mixture of pelvic and lumbar splanchnic afferent fibers. Alternatively, a recording can be made from the PN and/or the LSN proximal to the PG. - Turn on the heating bath and warm the Krebs solution to keep the chamber temperature at 34 ± 0.5 °C.
5. Preparation of the Suction Electrode
- Take a pre-pulled glass pipette (step 2) and inspect it under the dissecting microscope. Break the tip of the electrode with a pair of forceps so that it is of a size compatible with the diameter of the nerve to be recorded.
- Bevel the tip by placing it close to a lighter flare.
6. Electrophysiological Recording
- Connect the beveled electrode to the electrode holder. Connect a 10-mL syringe to the side port on the holder to apply negative or positive pressure to the electrode.
- Connect the holder to the headstage of the bioamplifier and mount the headstage onto a manipulator.
- Move the electrode to the tissue bath and fill the electrode with the Krebs solution by applying gentle suction until it contacts the silver wire of the holder. Place the electrode tip close to the cut end of the nerve and apply negative pressure to suck the nerve into the electrode. Apply more negative pressure so that ~1 mm of the nerve is pulled into the electrode and forms a tight seal.
- Turn on the bioamplifier and set the filter to 300 – 3,000 Hz. Monitor the signal on the oscilloscope and record the nerve signal (20-kHz sampling rate) and the intraluminal pressure signal (100-Hz sampling rate) using a computer with a spike data processing software.
7. Testing the Colonic Afferent Sensitivity
- Apply ramp distension of the colon by closing the three-way tap on the outlet cannula while continuously infusing intraluminally. Monitor the intraluminal pressure until it reaches 60 mmHg, at which time open the three-way tap on the draining cannula.
- Repeat this procedure at regular intervals of 15 min. Apply drugs extra- or intraluminally to test the chemical sensitivity of the afferent nerves.
In Vitro Characterization of the Electrophysiological Properties of Colonic Afferent Fibers in Rats
Learning Objectives
Figure 1 is the schematic illustration of the experimental setup for the ex vivo "tube" colorectum preparation, with a representative recording from a nerve distal to the PG. The nerve presumably contained a mixture of pelvic and lumbar splanchnic afferents. In preparations from normal rats, the colonic afferent nerves typically have a low level of irregular spontaneous activity. Ramp distension of the colon induces a gradual increase in the firing rate (Figure 1B). The mechanosensory property of each nerve is represented by plotting the intraluminal pressure-afferent nerve response curve (Figure 1C). Averaged pressure-response curves can be compared between groups (i.e., control versus treated) to reveal whether a treatment alters the mechanosensory property of the colonic afferent nerves (see Rong et al.13 for details on the pressure-afferent response curve).
Alternatively, recordings can be made from the PN or the LSN proximal to the PG. Figure 2 is a representative recording from a PN bundle that exhibited a mechanosensory response qualitatively similar to that of the bundle distal to the PG, shown in Figure 1B. Principal component analysis of the neural signal allows for the identification of single-unit activity. The discriminated single units are classified as low-threshold (LT) fibers, wide-dynamic range (WDR) fibers, and high-threshold (HT) fibers, according to the profiles of the responses to distension. LT units are activated at a low distension pressure, and the firing rate reaches a maximum at about 20 mmHg. WDR units are also activated at a low distension pressure, and the firing rate continues to increase as the intraluminal pressure increases (Figure 2A and C). HT units are activated at a distension pressure >20 mmHg (see Dong et al.)4.
The chemical sensitivity of the colonic afferents can be tested by superfusion (bath application) or intraluminal infusion. For example, the bath application of 10 µM 5-HT caused a modest increase, whereas capsaicin at 0.3 µM evoked robust increases in pelvic afferent discharge that were followed by a period of decreased activity due to desensitization (Figure 3).
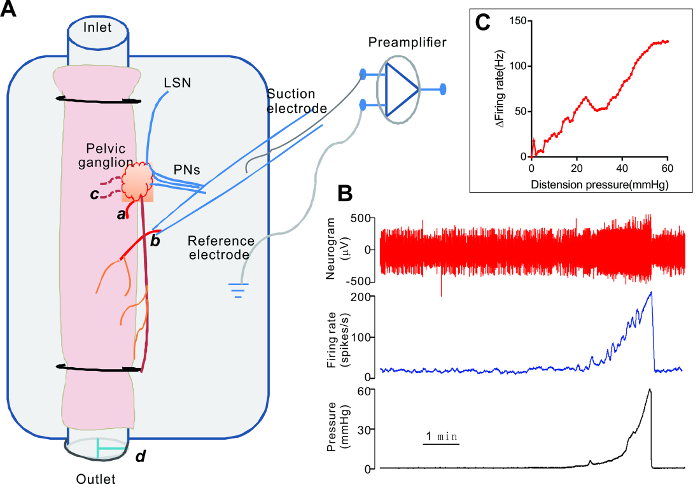
Figure 1. Electrophysiological Recording of Rat Colonic Afferent Nerves In Vitro. A. A schematic illustration of the ex vivo "tube" colon preparation of the rat. The colorectum, with the attached PG, is superfused in the recording chamber and is cannulated at both ends. Intraluminal infusion is achieved using a syringe pump and moves in the oral to anal direction. Distension of the colon is effected by closing the three-way tap (d) on the outlet cannula. A fine nerve branch from the PG is cut and recorded using a suction electrode. A branch of the LSN and three branches of the PN joining the PG are also shown. a. The proximal cut end of a fine nerve projecting to the colon. b. The distal cut end of the nerve sucked into the electrode. c. The cut end of two fine nerves projecting to the urinary bladder. LSN: lumbar splanchnic nerves, PN: pelvic nerves. B. The representative traces of the colonic afferent nerve signal and intraluminal pressure. C. A plot of the pressure-response curve of this nerve. Please click here to view a larger version of this figure.
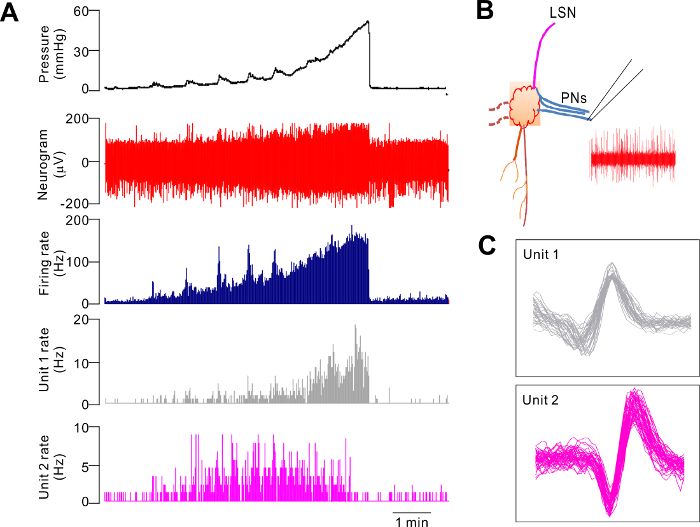
Figure 2. Representative Electrophysiological Recording from a Branch of the Pelvic Nerves during Ramp Distension of the Ex Vivo Colorectum. A. The response pattern of two single units (WDR and LT units), together with the multiunit mechanosensory response during ramp distension. B. A schematic illustration of the nerve bundle being recorded. LSN: lumbar splanchnic nerves, PN: pelvic nerves. C. Superimposed waveform of the two single units. Please click here to view a larger version of this figure.
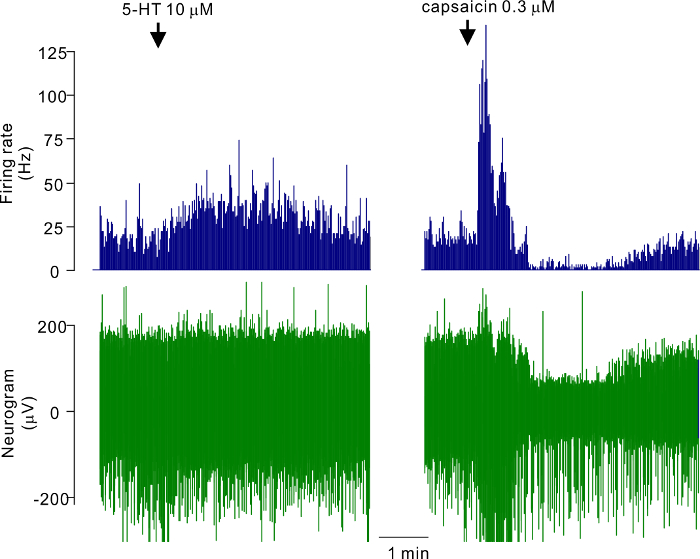
Figure 3. Representative Electrophysiological Recording from a Branch of the Pelvic Nerves During the Bath Application of 5-HT and Capsaicin. (Left) Application of 10 µM 5-HT. (Right) Application of 0.3 µM capsaicin. Please click here to view a larger version of this figure.
List of Materials
| Sodium Pentobarbital | Shanghai Westang Bio-Tech | B558 | |
| Capsaicin | Sigma | M2028 | |
| Electrode puller | MicroData Instrument Inc | PMP107 | |
| Neurolog System (Bioamplifier) | Digitimer, Ltd | Neurolog System | |
| A/D converter | Cambridge Electronic Design | Micro1401 | |
| Data processing software | Cambridge Electronic Design | Spike2 version 6 | |
| Silver wire | World Precision Instruments | EP12 | |
| Glass tubes | World Precision Instruments | 1B150-4 | |
| Electrode holder | World Precision Instruments | MEH3SBW | |
| Heating bath | Grant | GR150 | |
| Dissecting microscope | Leica | Zoom2000 | |
| Dissecting microscope | World Precision Instruments | PZMIII-BS | |
| Cigarette lighter | any | NA | |
| Surgical tools | World Precision Instruments | NA | |
| Insect pins | home-made from 0.1 mm stainless steel wire | NA | |
| Three way manipulator | World Precision Instruments | KITF-R | |
| Rats | Any | NA | Any strain/sex can be used. |
Lab Prep
Dysfunction of the colonic sensory nerves has been implicated in the pathophysiology of several common conditions, including functional and inflammatory bowel diseases and diabetes. Here, we describe a protocol for the in vitro characterization of the electrophysiological properties of colonic afferents in rats. The colorectum, with the intact pelvic ganglion (PG) attached, is removed from the rat; superfused with carbogenated Krebs solution in the recording chamber; and cannulated at the oral and anal ends to allow for distension. A fine nerve bundle emanating from the PG is identified, and the multiunit afferent nerve activity is recorded using a suction electrode. Distension of the colonic segment elicits gradual increases in multiunit discharge. A principal component analysis is conducted to differentiate the low-threshold, the high-threshold, and the wide-dynamic range afferent fibers. Chemical sensitivity of colonic afferents can be studied through the bath or intraluminal administration of test compounds. This protocol can be modified for application to other species, such as mice and guinea pigs, and to study the differences in the electrophysiological properties of thoracolumbar/hypogastric and lumbosacral/pelvic afferents of the descending colon in normal and pathological conditions.
Dysfunction of the colonic sensory nerves has been implicated in the pathophysiology of several common conditions, including functional and inflammatory bowel diseases and diabetes. Here, we describe a protocol for the in vitro characterization of the electrophysiological properties of colonic afferents in rats. The colorectum, with the intact pelvic ganglion (PG) attached, is removed from the rat; superfused with carbogenated Krebs solution in the recording chamber; and cannulated at the oral and anal ends to allow for distension. A fine nerve bundle emanating from the PG is identified, and the multiunit afferent nerve activity is recorded using a suction electrode. Distension of the colonic segment elicits gradual increases in multiunit discharge. A principal component analysis is conducted to differentiate the low-threshold, the high-threshold, and the wide-dynamic range afferent fibers. Chemical sensitivity of colonic afferents can be studied through the bath or intraluminal administration of test compounds. This protocol can be modified for application to other species, such as mice and guinea pigs, and to study the differences in the electrophysiological properties of thoracolumbar/hypogastric and lumbosacral/pelvic afferents of the descending colon in normal and pathological conditions.
Procedure
Dysfunction of the colonic sensory nerves has been implicated in the pathophysiology of several common conditions, including functional and inflammatory bowel diseases and diabetes. Here, we describe a protocol for the in vitro characterization of the electrophysiological properties of colonic afferents in rats. The colorectum, with the intact pelvic ganglion (PG) attached, is removed from the rat; superfused with carbogenated Krebs solution in the recording chamber; and cannulated at the oral and anal ends to allow for distension. A fine nerve bundle emanating from the PG is identified, and the multiunit afferent nerve activity is recorded using a suction electrode. Distension of the colonic segment elicits gradual increases in multiunit discharge. A principal component analysis is conducted to differentiate the low-threshold, the high-threshold, and the wide-dynamic range afferent fibers. Chemical sensitivity of colonic afferents can be studied through the bath or intraluminal administration of test compounds. This protocol can be modified for application to other species, such as mice and guinea pigs, and to study the differences in the electrophysiological properties of thoracolumbar/hypogastric and lumbosacral/pelvic afferents of the descending colon in normal and pathological conditions.
