Abstract
Source: Gervaise, A. L. and Arur, S. Spatial and Temporal Analysis of Active ERK in the C. elegans Germline. J. Vis. Exp. (2016).
This video describes a method to dissect gonads from adult C. elegans hermaphrodites, resulting in tissues that are suitable for immunostaining and fluorescent imaging.
Protocol
This protocol is an excerpt from Gervaise and Arur, Spatial and Temporal Analysis of Active ERK in the C. elegans Germline. J. Vis. Exp. (2016).
1. Dissection of Adult Worms for Obtaining Gonads
- Pick 100 – 150 WT (N2) or desired genotype of worms at the desired and specific developmental stage (L1, L2, L3, L4, adult, etc.) in 100 µL of M9 buffer in a 1.5 mL microcentrifuge tube.
- Fill the microcentrifuge tube with 900 µL of M9 buffer (see Materials Table). Centrifuge the tubes at 1,000 x g in a microcentrifuge for 1 min at ambient temperature.
- Gently remove 900 µL of the M9 buffer and discard. Remove the buffer under a dissecting microscope to ensure that the worms are not accidently removed.
- Repeat steps 1.2 – 1.3 two more times with fresh M9 buffer each time. This ensures that any bacteria that were carried over with the worms are effectively washed away.
- After the final wash remove 900 µL of M9 buffer from the tube and discard.
- Transfer the remaining 100 µL of M9 buffer containing the 100 – 150 worms with a 200 µL-micropipette tip to a flat bottom glass watch dish. Ensure that the micropipette tip is cut at the 10 µL mark before use. Not cutting the tip will result in shearing of the worms.
- Add 1 – 3 µL of 0.1 M levamisole to the glass watch dish containing the worms in M9 buffer. Gently swirl the levamisole and the M9 to enable even mixing until the worms stop moving.
NOTE: Levamisole stocks can be stored at -20 °C for long-term storage. Here, use the higher concentration of 1 – 3 mM than what has been described in literature23 of 0.2 – 0.25 mM of levamisole in order to obtain rapid loss of mobility in the animals. If the animals flay around for too long in the liquid suspension, the resulting patterns of dpERK in the gonads can be variable (due to stress or lack of nutrition or both). More reproducible staining patterns have been achieved with 1 – 3 mM levamisole for dissection. - Attach two 25 G needles to two 1 mL syringes (the size of the syringe does not matter, the gauge of the needle is critical). Position one syringe in each hand (Figure 1).
- Under a dissecting microscope, position each needle under and over each worm as shown in Figure 1 and place a fine cut on the worm near the second pharyngeal bulb in a scissor-like motion. After one cut in the worm move on to the next animal until all the animals in the dish have been cut at least once.
NOTE: Be mindful that steps 1.8 – 1.9 are time sensitive. Dissect all worms in the dish in under five min for a reproducible dpERK pattern. Longer dissection times will result in a turning off of the dpERK signal, especially in Zone 1. Adjust the number of worms per round of dissection (i.e., 50 worms vs. 150) if necessary to stay in the allotted time frame.- In case an extruded gonad is not visible during the dissection, do not attempt another cut in the same animal. Usually that will only shear the animal and not result in extrusion of the gonad. Instead, move on to the next animal. Worms where the gonads do not extrude will appear as such on the slides that will be made in section 6 and can be ignored during imaging
NOTE: Through all of the dissection and processing steps, it is important to bear in mind that the gonad will remain attached to the body/carcass. Do not force the gonad and the body apart with the needle. Often times an aberrant tear in the gonadal tissue in an attempt to sever the gonad from the body can result in spurious antibody signal. The body/carcass can be ignored during the imaging steps, and only the gonad imaged. Additionally, sometimes the intestinal cells can also serve as internal controls/somatic controls for the antibody being assayed.
- In case an extruded gonad is not visible during the dissection, do not attempt another cut in the same animal. Usually that will only shear the animal and not result in extrusion of the gonad. Instead, move on to the next animal. Worms where the gonads do not extrude will appear as such on the slides that will be made in section 6 and can be ignored during imaging
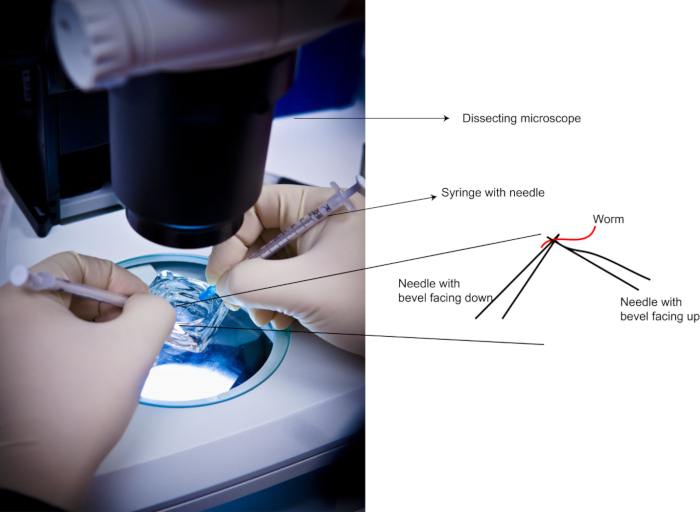
Figure 1: Demonstration of Needle Positions during dissections. Left: Photograph of a dissection in process. Right: Needle positions with reference to the worm. Please click here to view a larger version of this figure.
Materials
| Flat bottom glass watch dish | Agar Scientific | AGL4161 | We use glass because dissected gonads often stick to plastic |
| 25 G needles | BD PrecisionGlide | 305122 | |
| Syringes (could be 1 or 5 mL) | BD Syringes | 1 mL: BD 309659 | |
| Microscope slides (25 x 75 x 1.0 mm) | Fisherbrand | 12-550-343 | |
| Clinical bench top centrifuge | |||
| *M9 solution | |||
| Levamisole | Sigma | L9756 | |
| *M9 Buffer Recipe | 3 g KH2PO4, 6 g Na2HPO4, 5 g NaCl, 1 mL 1 M MgSO4, H2O to 1 L. |
Cite This Article
C. elegans Gonad Extrusion: A Rapid Dissection Technique. J. Vis. Exp. (Pending Publication), e20131, doi: (2023).

