Recording Behavioral Responses to Reflection in Crayfish
Summary
We have developed two methods for studying effects of visual cues on behavior in the absence of tactile and chemical cues. One method involves videotaping responses of crayfish to reflective walls in an aquarium; the other examines effects of visual inputs provided by a live crayfish behind a transparent partition.
Abstract
Social behavior depends on sensory input from the visual, mechanical and olfactory systems. One important issue concerns the relative roles of each sensory modality in guiding behavior. The role of visual inputs has been examined by isolating visual stimuli from mechanical and chemosensory stimuli. In some studies (Bruski & Dunham, 1987: Delgado-Morales et al., 2004) visual inputs have been removed with blindfolds or low light intensity, and effects of remaining sensory modalities have been elucidated. An alternative approach is to study the effects of visual inputs in the absence of any appropriate mechanical and chemosensory cues. This approach aims to identify the exclusive role of visual inputs.
We have used two methods to provide visual stimuli to crayfish without providing chemical and mechanical cues. In one method, crayfish are videotaped in an aquarium where half of the walls are covered in mirrors to provide a reflective environment, and the other half are covered in a non-reflective (matte finish) plastic. This gives the crayfish a choice between reflective and non-reflective environments. The reflective environment provides visual cues in the form of reflected images of the crayfish as it moves throughout half of the tank; these visual cues are missing from the non-reflective half of the tank. An alternative method is to videotape the behavior of crayfish in an aquarium separated by a smaller chamber at each end, with a crayfish in one small chamber providing visual cues and an inert object in the opposite small chamber providing visual input from a non-moving, non-crayfish source.
Our published results indicate that responses of crayfish to the reflective environment depend on socialization and dominance rank. Socialized crayfish spent more time in the reflective environment and exhibited certain behaviors more frequently there than in the non-reflective environment; isolated crayfish showed no such differences. Crayfish that were housed in same-sex pairs developed a social rank of either dominant or subordinate. Responses to reflection differed between dominant and subordinate crayfish (May & Mercier, 2006; May & Mercier, 2007). Dominant crayfish spent more time on the reflective side, entered reflective corners more frequently and spent more time in reflective corners compared to the non-reflective side. Subordinate crayfish walked in reverse more often on the reflective side than on the non-reflective side. Preliminary data suggest similar effects from visual cues provided by a crayfish in a small adjoining chamber (May et al., 2008).
Protocol
1. Socialization
- To extinguish existing social ranks, each crayfish is housed for 7 – 14 days in a separate plastic container (30 cm long x 17.5 cm wide x 13 cm deep), filled with filtered, aerated water and a small plastic flower pot which serves as a shelter. If transparent containers are used, a divider (e.g. paper towel) is placed in between containers to ensure crayfish are visually isolated.
- Crayfish in the “Isolated” group remain in isolation for an additional 3 – 14 days. Water is changed and crayfish are fed 3 times weekly.
- To establish a social rank, crayfish are placed in same-sex pairs following the initial isolation. Crayfish are weighed, and body length is measured (rostrum to telson); pairs are size-matched to within 10% weight and length. White acrylic paint is applied to the carapace of each crayfish to identify individuals. (Usually a dot is applied to one member and a line to the other.)
- After a 30 min resting period, each crayfish pair is allowed to interact in a clean, white plastic container (48 cm long x 25 cm wide x 13 cm deep), filled half-way with filtered, aerated water (room temperature). This encounter is either recorded onto VHS tape with a camera connected to a video cassette recorder, or it is recorded onto a computer with a webcam. This interaction allows dominance ranks to develop.
- Following the initial interaction, each crayfish pair is housed together in a larger plastic container (58 cm long x 30 cm wide x 35 cm deep) for an additional 3 – 14 days. This container receives circulating water from a filtration system. A single PVC tube (approx. 10 cm length) is provided for shelter.
2. Construction of Test Aquarium and Controls
Method I
- To examine responses to a reflective environment, a glass or Plexiglas aquarium (52 cm long x 25 cm wide x 30 cm high) is used (Figure 1).
- One mirror (25 cm long x 20 cm high) is placed at one end of the aquarium and secured with either glue or mounting wax. Two mirrors (26 cm long x 20 cm high) are placed on the sides, abutting the end mirror, and are secured with glue or wax.
- The remaining walls are covered in a matte finish window film. This film removes the reflection but does not interfere with lighting conditions. A light sanding to the film may be required to ensure that the film is completely non-reflective.
Method II
- To examine responses to visual stimuli provided by a live crayfish (“source crayfish”), a Plexiglas aquarium with two end chambers is used (Figure 2). The aquarium contains a large central compartment (48 cm long x 26 cm wide x 26 cm high) and two smaller end compartments of equal size (12 cm long x 26 cm wide and 26 cm high). The end compartments are separated from the center compartment by transparent Plexiglas, which is sealed to prevent water and chemical cues from passing between compartments.
- The outer perimeter of the entire aquarium is coated in a non-reflective matte plastic, leaving the two barriers at each end transparent.
- As a control, a cube constructed of modeling clay (e.g. Sculptee) is used to provide visual input from an inert object. The cube (3.5 cm x 3.0 cm x 3.3 cm) is the approximate volume of the source crayfish and is painted a similar color red.
3. Visual Training
Method I
- Crayfish are videotaped in the mirror / matte tank 3 – 14 days following the fight to determine dominance status.
- Crayfish are placed, one each, into a Tupperware container containing aerated, filtered water and a plastic flower pot used for shelter. Crayfish are taken to the testing room and given 30 min to acclimate before testing.
- The test aquarium, half-lined with mirrors and half-lined with non-reflective plastic, is filled with aerated, filtered water to a depth of approximately 20 cm.
- A digital camera is connected via audio/video cables to a VHS videocassette recorder and is placed above the aquarium high enough to view the entire aquarium. Videos are recorded onto VHS tape. Alternatively, a webcam connected to a computer can be used, and videos can be recorded with a movie-making program and stored on DVD.
- One crayfish of the pair is placed gently into the center of the aquarium using a flower pot, and its activity is recorded for 20 min. No person is present in the room during recording, to reduce interference from extraneous visual and auditory inputs. Every effort is made to ensure that the recording environment is quiet.
- Following testing, the crayfish is removed, and the tank is emptied, rinsed and refilled with water.
- To counter-balance experimental conditions, the aquarium is turned around to place the mirrors on the opposite side of the room. This helps to compensate for unknown factors that might cause crayfish to prefer one side of the aquarium.
- The second member of the pair is then tested in the same manner as the first.
Method II
- As described in Method I, crayfish are brought to the testing room and are videotaped using the same recording equipment.
- All chambers (the middle testing chamber and the two end chambers) are filled with aerated, filtered water to a height of approximately 20 cm.
- A crayfish, approximately the same size as the crayfish being tested, is placed in one end chamber, and the control cube is placed in the other.
- Using the flower pot shelter, the crayfish being tested is gently placed into the center of the aquarium and is videotaped for 20 min.
- Following testing, the aquarium is emptied, rinsed and refilled with water.
- Before testing the second member of a pair, the source crayfish and control cube are moved to the opposite end chambers, to counter balance conditions.
4. Analysis of Responses to Visual Stimuli
Method I
- So that the experimenter is blind to the social status of the crayfish, behavior in the mirror / matte aquarium is analyzed before analyzing fighting behavior trials.
- The amount of time the crayfish spends on each side of the aquarium is recorded. The crayfish is considered to pass from one side to the next when the coxae of the chelipeds pass the midline of the tank (Figure 3).
- The frequency of the behaviors cornering, turning, crossing, freezing and reverse walking (see Table 1 for descriptions) are also recorded.
- Behaviors exhibited on the reflective side of the tank are compared to behaviors exhibited on the non-reflective side using a paired t-Test for each social group.
Method II
- As in Method I, videotapes of crayfish behavior are analyzed before analyzing fight trials.
- The amount of time the crayfish spends on either side of the aquarium is recorded as described in Method I.
- The frequency of cornering, end facing, turning, crossing, rearing up and reverse walking (Table 1) are recorded.
- Behaviors performed on the source crayfish side are compared to behaviors performed on the control cube side using a paired t-Test for each social group.
5. Analysis of Fights
- Recordings of fight behavior during the initial 30 min of pairing are viewed and scored. Each 30 min recording session includes several encounters in which the crayfish make contact using their claws and/or antennae. Within these encounters, the chelae are usually used for pushing, striking or grasping the opponent, and the antennae are sometimes used for whipping the opponent. One crayfish may attempt to flip its opponent and hold him to the floor. Each encounter ends when one crayfish retreats by reverse walking or by flipping the tail to achieve “escape swimming.”
- The number of agonistic encounters is recorded, and the identity of the crayfish (determined by the paint marking on the thorax) that retreats from each encounter is also recorded. The crayfish that loses the majority of encounters in each 30 min session is deemed the loser, and the other crayfish is deemed the winner.
6. Representative Results
During the 30 minute fighting period, all pairs exhibit agonistic behaviors. Such behaviors include antenna tapping, lunging, pushing, grasping, chelae striking, and flipping the opponent, as reported elsewhere (e.g. May & Mercier 2006, 2007). Eventually one crayfish in each pair consistently retreats and avoids further contact. The retreating crayfish is deemed the loser and is ranked as subordinate; the winning crayfish is ranked as dominant.
Rank can also be monitored throughout the paired housing period by observing crayfish behavior in the holding tank, which contains only one plastic tube for shelter. The dominant crayfish invariably occupies the shelter, and the subordinate crayfish does not. The subordinate crayfish often walks with the chelae held close together and out in front, and the dominant crayfish typically walks with the chelae raised and further apart (Guiasu & Dunham, 1998). When new food is added to the holding tank, the dominant crayfish usually takes the food first. In our studies with P. clarkii (e.g. May & Mercier 2006, 2007), there are typically no rank reversals after three days of continuous pairing.
In rare cases, one member of the pair dies before the pairing period is complete. This is more likely to occur if the pairing period is long (e.g. 14 days) and if the crayfish are too closely matched by size or weight. Thus, deaths can be avoided by allowing approximately a 10% difference in weight. If one member of a pair dies, data from the other member are not included in the study.
Dominant and subordinate crayfish typically show different responses to the reflective environment. Dominant crayfish consistently spend more time on the reflective side, but subordinate and isolated crayfish do not (May & Mercier, 2006, 2007). Dominant crayfish also exhibit cornering and turning more frequently on the reflective side than on the non-reflective side, but isolates and subordinates typically do not. Such differences between dominant and subordinate crayfish, however, are not established immediately but develop over the 3-14 day pairing period (May & Mercier, 2007).
Preliminary evidence indicates that dominant and subordinate crayfish respond differently to visual cues provided by a live crayfish (source crayfish) after 3 days of pairing (May et al., 2008). Dominant crayfish spend more time on the side of the aquarium with the source crayfish and corner for longer on that side, but subordinate crayfish do not exhibit such differences between the two sides.
| Comportamento | Description |
| cornering | facing the corner of the aquarium and remaining there for at least 5 s with the tips of both claws touching different walls (Figure 4) |
| end facing | facing the wall at one end of the tank for at least 5 s with the tip of at least one claw touching the end wall (Figure 5) |
| turning | changing the walking path direction from clockwise to counter-clockwise or vice versa (Figure 6) |
| crossing | leaving the perimeter of the aquarium and walking at least one body length to any other wall (Figure 7) |
| reverse walking | walking backwards for at least one body length |
| freezing | abrupt cessation of all visible movement, including that of appendages and antennae, for at least 5 s |
| rearing up | standing on the fourth and fifth walking legs while lifting the ventral surface of the thorax against a wall and placing at least one walking leg on the wall |
Table 1. Behaviors exhibited by P. clarkii in the central compartment of the test tank.
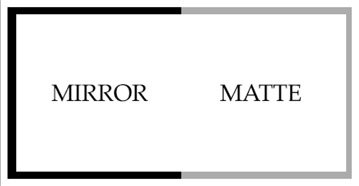
Figure 1. Schematic of aquarium used in Method I. Half of the aquarium is lined with mirrors, while the other half is lined with non-reflective plastic.
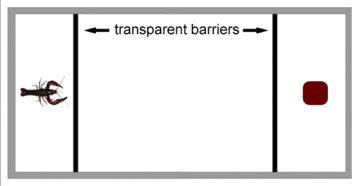
Figure 2. Schematic of aquarium used in Method II. This tank is comprised of a center area, where the test crayfish is placed, and two end chambers, divided by an intact Plexiglas barrier. A live crayfish is placed in one end chamber, and a cube is placed in the other end chamber as a control.
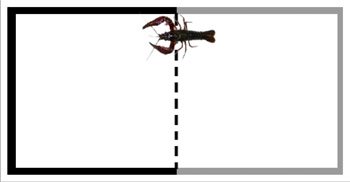
Figure 3. Schematic of test aquarium with the midline depicted as a vertical dotted line. The crayfish passes from one side to the other when the coxae of the chelipeds pass the midline.

Figure 4. Diagram indicating the position of the crayfish while cornering. Note that the tips of both chelae are touching different walls.

Figure 5. Diagram indicating the position of the crayfish while facing the end wall. Note that both chelae are touching the same wall.

Figure 6. Schematic diagram indicating turning. Reversing the direction of movement constitutes a turn. Turns can occur in a corner (e.g. direction 1 to direction 2 and from direction 4 to direction 5) or at the side (not shown). Entering the cornering and leaving it in the same direction (e.g. direction 1 to direction 3) is not a turn.

Figure 7. Schematic showing crossing behavior. Crossing occurs when the crayfish leaves one wall and travels to another wall, at a distance of at least one body length from the nearest wall (arrow).

Figure 8. Photograph depicting rearing up behavior. The crayfish stands on the last two walking legs and lifts the body against a wall, making contact with at least one walking leg.
Discussion
The observation that dominant crayfish spend more time in a reflective environment suggests that they recognize a reflected image as a conspecific, and that visual cues are sufficient to elicit approach behavior. Similar results using a live crayfish instead of a reflected image support such an interpretation. Subordinate and isolated crayfish do not show such a preference for the reflective environment, nor do subordinate crayfish appear to prefer a live crayfish over a cube. Thus, responses to visual cues depend on dominance rank. It is not yet clear whether crayfish are attracted specifically by a crayfish image or by movement. Nonetheless, it is possible to explore the role of visual inputs and to identify visual cues using mirrors and images provided across a transparent partition.
Previous results indicate that dominant and subordinate crayfish respond to reflective surfaces in essentially the same manner after only a 30 min pairing session, and that differences in behavior emerge with 3 – 14 days of pairing because of changes in behavior of the losing crayfish (May & Mercier, 2007). Thus, the behaviors one observes in response to visual cues depend on both the outcome of the encounter and on the duration of socialisation. Winning or losing agonistic encounters is known to affect success of future encounters, with winning increasing the likelihood of winning again, and losing increasing the probability of subsequent loses (Hsu & Wolf, 1999). The mechanisms underlying behavioral plasticity exhibited by losing crayfish in response to visual cues might be related to changes in the nervous system that accompany reinforcement of subordinate status (May & Mercier, 2006; 2007).
The experiments described here can be modified to assess the complexity of sensory cues required to elicit approach behavior. This could be achieved by altering various aspects of the visual cues provided behind a transparent partition and by combining such visual cues with chemical and tactile factors. Conditioned water could be added to the mirror / matte aquarium to add a chemical component. Alternatively, holes could be added to the transparent barrier in the end chamber tank to allow for transfer of chemical cues from the live source crayfish.
Declarações
The authors have nothing to disclose.
Acknowledgements
Research supported by NSERC.
Materials
| Material Name | Tipo | Company | Catalogue Number | Comment |
|---|---|---|---|---|
| Single chamber test tank | Brock University Machine Shop | |||
| End-chamber test tank | Brock University Machine Shop | |||
| Isolation container | Rubbermaid | 30 cm x 17.5 cm x 13 cm holes in lid to allow air and light to pass through when lid is on | ||
| Fighting chamber | 48 cm x 25 cm x 13 cm | |||
| Social housing chamber | 58 cm x 30 cm x 35 cm | |||
| small plastic flower pot | to serve as shelter | |||
| Coolpix 4500 camera | Nikon | |||
| T120 video cassette recorder | RCA | |||
| Computer | Windows Movie Maker or other video acquiring software | |||
| webcam | Logitech | |||
| modelling clay | Sculptee | to make control cube | ||
| red latex paint | FolkArt | to make control cube | ||
| waterbase varnish | FolkArt | to make control cube |
Referências
- Bruski, C. A., Dunham, D. W. The importance of vision in agonistic communication of the crayfish Orconectes rusticus. I: an analysis of bout dynamics. Behav. 103, 83-107 (1987).
- Delgado-Morales, G., Hernandez-Falcon, J., Ramon, F. Agnostic behavior in crayfish:the importance of sensory inputs. Crustaceana. 77 (1), 1-24 (2004).
- Guiasu, R. C., Dunham, D. W. Inter-form agonistic contests in male crayfishes, Cambarus robustus (Decapoda, Cambaridae). Invert. Biol.. 117 (2), 144-154 (1998).
- May, H. Y., Mercier, A. J. Responses of crayfish to a reflective environment depend on dominance status. Can. J. Zool. 84 (8), 1104-1111 (2006).
- May, H. Y., Mercier, A. J. Joffre Duration of socialization influences responses to a mirror: responses of dominant and subordinate crayfish diverge with time of pairing. J. Exp. Biol. 210, 4428-4436 (2007).
- May, H. Y., Luebbert, J., Mercier, A. J. Responses of Crayfish to a Visual Stimulus Provided by a Conspecific. , (2008).

