Acinar-to-Ductal Metaplasia Induction: A Method to Study Acinar Cell to Ductal Cell Differentiation in 3D Ex Vivo Culture
Abstract
Source: Fleming Martinez, A. K. et. al. Mimicking and Manipulating Pancreatic Acinar-to-Ductal Metaplasia in 3-dimensional Cell Culture. J. Vis. Exp. (2019).
This video describes the technique of differentiating pancreatic acinar cells to ductal cells in ex vivo 3D culture. This helps to understand the mechanisms regulating the acinar-to-ductal metaplasia (ADM), a key process in the early development of pancreatic cancer.
Protocol
All procedures involving animal models have been reviewed by the local institutional animal care committee and the JoVE veterinary review board.
1. Preparation of Materials, Solutions, and 3-dimensional Matrix Bases
- Cut 500 µm and 105 µm polypropylene meshes into 76 mm by 76 mm squares. Fold each square in half twice to create a smaller folded square. Place one 500 µm and one 105 µm mesh square into an autoclavable pouch.
- Autoclave polypropylene mesh squares, as well as two pairs of scissors and forceps.
- Make 100 mL of 10x Waymouth's solution. Stir 1 vial (14 g) of Waymouth's powder into 50 mL of deionized (DI) water and, when dissolved, add 30 mL of 7.5% (75 g/L) sodium bicarbonate. Bring the final volume to 100 mL using DI water and filter sterilize.
- Store 10x Waymouth's media at 4 °C and use to prepare collagen gels and 1x Waymouth's media. When precipitates form, discard.
- Make 50 mL of 1x Waymouth's complete media per pancreas by adding 125 µL of 40 mg/mL soybean trypsin inhibitor, 12.5 µL of 4 mg/mL dexamethasone, and 500 µL of fetal bovine serum (FBS). Sterilize by filtration and use within 48 h.
- Prepare 3-dimensional matrix bases using either collagen or basement membrane matrix (see Table of Materials for product information). When pipetting the base, place the plate on ice, avoid bubbles, and rotate the plate immediately after pipetting each well's volume to ensure full coverage.
- Create collagen bases by mixing the following components on ice in a tissue culture hood: 5.5 mL of type I rat tail collagen, 550 µL of 10x Waymouth's Media, and 366.6 µL of 0.34 M NaOH (filtered).
NOTE: This is enough solution to make collagen bases for one 24-well, 12-well, or 6-well plate. One plate is sufficient for acinar isolation from one pancreas. - Use the following volumes to plate the collagen base: 80 µL (8-well glass slide), 200 µL (24-well plate), 400 µL (12-well plate), or 800 µL (6-well plate).
- For the basement membrane matrix bases, pipet the matrix without any additional components. Use the following volumes for each well: 50 µL (8-well glass slide), 120 µL (24-well plate), 240 µL (12-well plate), or 600 µL (6-well plate).
- Let the bases solidify for at least 30 min in a cell culture incubator (37 °C, 5% CO2) before creating a second layer of matrix with embedded cells on top of these bases.
- Create collagen bases by mixing the following components on ice in a tissue culture hood: 5.5 mL of type I rat tail collagen, 550 µL of 10x Waymouth's Media, and 366.6 µL of 0.34 M NaOH (filtered).
- In preparation for acinar isolation keep one 600 mL beaker, three 50 mL tubes, an autoclaved pair of scissors, an autoclaved pair of forceps, and an ice bucket under the hood with three weigh boats. Then, make 30 mL of HBSS with 300 µL of 100x penicillin-streptomycin, putting 10 mL into each of two weigh boats and keeping the final 10 mL in a 50 mL tube (for use in steps 1.8.2 and 2.1.4).
- Make 40 mL of HBSS + 5% FBS, 20 mL of HBSS + 30% FBS, and 5 mL of 2 mg/mL collagenase in HBSS. Sterilize the collagenase by filtration (0.22 µm pore) and keep at room temperature. Keep each of the HBSS solutions on ice.
- Assemble a workspace for pancreas dissection, which can be done on a lab bench.
- Place an absorbent pad on the table, along with a polystyrene lid covered in foil. Then place a paper towel with 4 pins on top of the foil.
- Keep the following items within reach of the dissection workspace: an incineration bag, one set of autoclaved scissors and forceps, a spray bottle with 70% ethanol, and an ice bucket (with the 50 mL tube of HBSS containing penicillin-streptomycin from step 1.6).
- Set a centrifuge to 4 °C and a shaker to 37 °C.
2. Acinar Cell Isolation
- Sacrifice the mouse via CO2 induction, and perform cervical dislocation. Immediately dissect the pancreas. To dissect the pancreas, first pin the paws of the mouse to the polystyrene lid, orient the mouse such that the tail is facing the researcher, and spray the abdomen with 70% ethanol.
NOTE: The mouse used in the representative results was a 12-week old non-transgenic female with C57BL/6 background. Mouse selection is further discussed in the discussion section.- Using a set of autoclaved scissors and forceps, lift the fur/skin with the forceps at the midline and use the scissors to make an incision through the fur and skin from the urethral opening to the diaphragm/ribcage area.
- Make additional incisions to the left and right such that the fur/skin is cut away to create a clear view of the abdominal cavity. Then, cut into the peritoneal lining (down the middle and to the right and left) and pull it away from the organs, as was done with the fur/skin.
- Lift the intestines with the forceps and put it to the left side of the mouse creating space to see the pancreas, which is light pink in color, and attached to the spleen, which is a dark red oval. The pancreatic tissue is distinguished by its soft, spongy texture. Cut out the pancreas which will run along the stomach and intertwine with the intestines.
- Separate the spleen from the pancreas. Then, put the pancreas in a 50 mL tube containing 10 mL of HBSS with 1x penicillin-streptomycin and bring this to the laminar flow hood.
NOTE: The remaining steps of the protocol should be done utilizing sterile technique in a laminar flow hood.
- Pour the pancreas and HBSS (with penicillin-streptomycin) into an empty weigh boat and, using forceps, wash the pancreas by swirling it. Then, move the pancreas with the forceps to a second weigh boat containing HBSS (with penicillin-streptomycin). Again, wash the pancreas by swirling.
- Move the pancreas to the third HBSS (with penicillin-streptomycin)-containing weigh boat and begin cutting the pancreas into small pieces of 5 mm or less. Next, pour the pancreas pieces and HBSS into an empty 50 mL tube.
- To do this, use the forceps to move the pancreas pieces into the liquid as the weigh boat is being tipped. Pour once all the pieces are no longer attached to the weigh boat. Pick up any remaining pieces with the forceps and wash the forceps in the 50 mL tube containing the pancreas in HBSS with penicillin-streptomycin.
- Centrifuge at 931 x g for 2 min at 4 °C and then remove the HBSS (and any fat that is floating) by pipetting it off with a 5 mL pipette.
- Add 5 mL of collagenase (diluted in HBSS in step 1.7) to the pancreas. Ensure a sealed lid by wrapping the 50 mL tube in plastic paraffin film and then place it in an incubator shaking at 220 rpm for 20 min at 37 °C.
NOTE: The collagenase digestion time may vary, as noted in the discussion. At the end of incubation, no large pieces of tissue should remain. - To stop the dissociation, place the pancreas-containing tube on ice and add 5 mL of cold HBSS + 5% FBS. Centrifuge at 931 x g for 2 min at 4 °C and then pipet off the supernatant using a 5 mL pipet.
- Resuspend in 10 mL of HBSS + 5% FBS and centrifuge at 931 x g for 2 min at 4 °C. Pipet off the supernatant using a 5 mL pipet and repeat this step with another 10 mL of HBSS + 5% FBS.
- Resuspend in 5 mL of HBSS + 5% FBS and, using a P1000, transfer 1 mL at a time through a 500 µm mesh into a 50 mL tube. Add an additional 5 mL of HBSS + 5% FBS through the mesh with a P1000 to wash any remaining pancreatic cells through the mesh.
- Put the cell suspension from the 500 µm mesh through the 105 µm mesh by pipetting 1 mL at a time using a P1000. Next, gently pipet the cell suspension into a tube containing HBSS + 30% FBS. A layer of cell suspension will form at the top; upon centrifugation, the acinar cells will sink to form a pellet.
- Centrifuge at 233 x g for 2 min at 4 °C. Remove the supernatant and resuspend the pellet in 1x Waymouth's complete media.
- If proceeding directly to embedment of cells in collagen or basement membrane matrix, resuspend in a volume of 7 mL per pancreas. If proceeding to adenoviral or lentiviral infection, resuspend in 4 mL per pancreas.
3. Viral Infection
- As one pancreas is sufficient for two viral infections (control and experimental, e.g., null and cre), split the cell suspension between the lids of two 35 mm plates. Using the lid of the plates allows for infection in suspension and therefore easy movement onto the final substrate later.
- When working with virus, soak all used pipette tips in 10% bleach for at least 15 minutes.
NOTE: Utilization of the 35 mm plates and the 4 mL volume works well for cells from non-transgenic mice between 8 and 16 weeks old. The plate size and volume may need to be adjusted for animals with smaller or larger pancreata.
- When working with virus, soak all used pipette tips in 10% bleach for at least 15 minutes.
- For adenoviral infection, add the virus (with a titer of at least 107 TU/mL) at a 1:1,000 dilution to the lid of each plate and swirl (Figure 1, GFP adenovirus). Place the plates in a cell culture incubator (37 °C, 5% CO2) and swirl every 15 minutes for 1 h. Continue incubation for an additional 2 h and then proceed to embedment of cells in collagen or basement membrane matrix.
- After completing viral work in the hood, soak all components in the hood with 10% bleach for at least 15 minutes and then thoroughly clean the bleach off using 70% ethanol.
4. Embedment of cells in Collagen or Basement Membrane Matrix
- Make collagen gel on ice by combining 7 mL type I rat tail collagen (3.3 mg/mL), 700 µL of 10x Waymouth's media, and 466.6 µL of 0.34 M NaOH.
- Taking equal volumes of collagen gel and cell suspension, gently mix. If adding a stimulus or inhibitor, combine this with the collagen-cell suspension. Plate one well at a time (plates from step 1.5.3); keeping the plate on ice, swirl to evenly distribute the collagen-cell layer.
- In each well, pipet the following volumes of collagen-cell suspension: 200 µL (8-well glass slide), 500 µL (24-well plate), 1000 µL (12-well plate), or 2000 µL (6-well plate). Note that ADM is induced via stimulation with TGF-α (50 ng/mL) in Figure 1.
- For embedment of cells in basement membrane matrix, mix one-part basement membrane matrix with two-parts cell suspension. As with collagen, keep the matrix-cell suspension as well as the plate on ice. Pipet one well at a time using the same volumes noted in 4.2.1 and swirl for even distribution.
- Place the plate in a cell culture incubator (37 °C, 5% CO2) for 30 min to solidify and then add 1x Waymouth's complete media with any stimulus or inhibitor (Figure 1 uses TGF-α at 50 ng/mL). Change the media (with stimulus or inhibitor) the next day and then every other day. Depending on the stimulus, ADM can be observed between day 3 and day 5.
Representative Results
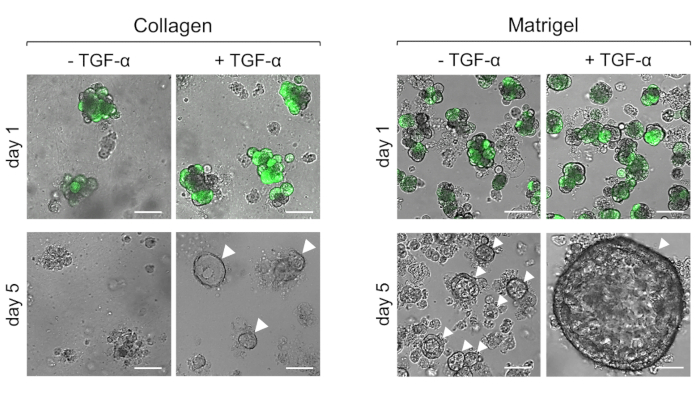
Figure 1: Representative results of primary acinar cells plated within collagen after stimulation with TGF-α. Primary acinar cells from a non-transgenic mouse were infected with GFP-adenovirus, embedded in collagen I or basement membrane matrix, and stimulated with or without TGF-α (50 ng/mL). Twenty-four hours post-infection with GFP adenovirus, images were captured to show infection efficiency. At five days post-infection, the images denote the differences in cells stimulated with or without TGF-α in collagen or basement membrane matrix. Duct-like structures are indicated by white arrowheads and scale bars represent 50 µm. Images were obtained using a confocal microscope with the EC Plan-Neofluar 10x/0.3 M27 objective. Please click here to view a larger version of this figure.
Declarações
The authors have nothing to disclose.
Materials
| 5% CO2, 37 °C Incubator | NUAIRE | NU-5500 | |
| 50 ml tubes | Falcon | 352070 | |
| Beaker, 600 mL | Fisherbrand | FB-101-600 | |
| Adenovirus, Ad-GFP | Vector Biolabs | 1060 | |
| Dexamethasone | Sigma | D1756 | Create a 4 mg/ml solution by dissolving powder in methanol, aliquoting and storing at -20 °C |
| Fetal Bovine Serum | Sigma | F0926-100mL | |
| Forceps | Fine Science Tools | 11002-12 | |
| Glass slide, 8-well | Lab-Tek | 177402 | |
| Ice bucket, rectangular | Fisher Scientific | 07-210-103 | |
| Matrigel | Corning | 356234 | Referred to as 'basement membrane matrix' in the manuscript. |
| PBS | Fisher Scientific | SH30028.02 | |
| Penicillin-Streptomycin | ThermoFisher Scientific | 15140122 | |
| Plate, 12-well | Corning Costar | 3513 | |
| Plate, 24-well plate | Corning Costar | 3524 | |
| Plate, 6-well | Falcon | 353046 | |
| Polypropylene Mesh, 105 µm | Spectrum Labs | 146436 | |
| Polypropylene Mesh, 500 µm | Spectrum Labs | 146418 | |
| Scissors | Fine Science Tools | 14568-12 | |
| Sodium Bicarbonate (Fine White Powder) | Fisher Scientific | BP328-500 | |
| Sodium Hydroxide | Fisher Scientific | S318-500 | |
| Soybean Trypsin Inhibitor | Gibco | 17075029 | |
| Type I Rat Tail Collagen | Corning | 354236 | |
| PIPETMAN Classic P10, 1-10 μl | Gilson | F144802 | |
| Pipettes, 5 ml | Falcon | 357543 | |
| Pipet tips, 10 µl | USA Scientific | 1110-3700 | |
| Pipet tips, 1000 µl | Olympus Plastics | 24-165RL | |
| Pipet tips, 200 µl | USA Scientific | 1111-1700 | |
| Pipettes, 25 ml | Falcon | 357525 | |
| Pipet-Aid | Drummond | ||
| Pipettes, 10 ml | Falcon | 357551 | |
| PIPETMAN Classic P200, 20-200 μl | Gilson | F123601 | |
| PIPETMAN Classic P1000, 200-1000 μl | Gilson | F123602 | |
| PIPETMAN Classic P20, 2-20 μl | Gilson | F123600 | |
| Waymouth MB 752/1 Medium (powder) | Sigma | W1625-10X1L | |
| Weigh boat, hexagonal, medium | Fisherbrand | 02-202-101 |

