The Virus-Like Particles Capture Assay: A Method to Isolate Antigen-Displaying VLPs from a Sample Using Neutralizing Antibody-Conjugated Magnetic Beads
Abstract
Source: Rosengarten, J. F., et al. Detection of Neutralization-sensitive Epitopes in Antigens Displayed on Virus-Like Particle (VLP)-Based Vaccines Using a Capture Assay. J. Vis. Exp. (2022).
In this video, we demonstrate an immunoprecipitation assay, called the VLP capture assay, to isolate antigen-displaying virus-like particles, or VLPs, from a sample. The antigen binds with specific antibodies bound to magnetic beads, forming an immobilized antigen-antibody complex that is later separated and stored for further analysis.
Protocol
1. Sample preparation
- Seed the VLP producer suspension cell lines derived from 293-F cells expressing HIV structural genes gag alone or in concert with env at a low cell density of 0.5 x 106 cells per mL in 293-F Expression Medium.
- Let them expand for 3-4 days at 37 °C and 8% CO2 in a shaker incubator rotation with an orbit of 5 cm and 135 rounds per minute (rpm).
- Pellet the producer cells by centrifugation at 100 x g for 5 min. Filter the clarified supernatant to remove residual cells and cell debris using 0.45 µm polyvinylidene fluoride (PVDF) membrane syringe filters to obtain cell-free cell culture supernatant (CFSN).
NOTE: The protocol can be paused here. CFSN can be stored overnight at 4 °C. - Either use the CFSN directly for the experiment or pellet VLPs from 35 mL of CFSN employing ultracentrifugation (112,700 x g, 4 °C, 1.5 h).
- Upon ultracentrifugation, discard the supernatant and suspend the VLP pellets in 200 µL of 15% (w/v) trehalose solution per centrifuge tube.
NOTE: The VLP pellet is often not visible to the naked eye. The protocol can be paused here. VLPs can be stored at –80 °C. - Prior to the VLP capture assay, determine the viral core protein concentrations in the VLP-containing samples using an ELISA. This step is important to standardize the capture assay input.
2. VLP capture assay
NOTE: An overview of the workflow is depicted in Figure 1.
- Suspend the magnetic beads by either pipetting up and down or mixing on a rotator at 50 rpm for at least 5 min.
- Meanwhile, prepare the antibody solution containing the bNAbs. Use 10 µg of each bNAb in 200 µL of antibody binding and washing buffer (see Table of Materials) per reaction.
NOTE: Antibody amounts may vary depending on the affinity of the bNAb and antigen decoration density on the VLPs used. - Use 50 µL of the magnetic bead solution (see Table of Materials) per reaction and transfer the beads into a 1.5 mL reaction tube. Place the tubes on the magnetic separation rack.
NOTE: Alternatively, a strong single magnet can be used for each tube to separate the beads from the supernatant. - Wait a few minutes until the beads gather at the tube wall to ensure that all beads are collected. Remove the supernatant.
- Remove the magnet and suspend the beads in 200 µL of the bNAb solution prepared in step 2.2. Incubate for 30 min to 3 h mixing on a rotator at 50 rpm at room temperature.
- Place the reaction tubes in the magnetic separation rack again, wait and remove the supernatant.
- Remove the tubes from the magnet and wash the beads by resuspending in 200 µL of antibody binding and washing buffer.
- Repeat step 2.4. Remove as much washing buffer as possible.
- Add the samples to the bead-bound bNAbs.
NOTE: The VLP input for the capture assay using HIV-derived particles should be at least 15 ng of viral core protein per reaction. VLP amounts may vary depending on the affinity of the bNAb, and the antigen decoration density on the VLPs used. - If the sample volume added in step 2.9 is below 1 mL, add PBS to adjust the sample volume to 1 mL. Suspend the beads gently by pipetting.
- Incubate the samples and beads for 2.5 h on a rotator at room temperature. Ensure that the beads stay in suspension and the solution is thoroughly mixed during incubation.
- Place the tubes on the magnet and remove the supernatant.
- Wash the magnetic beads by suspending them in 200 µL of washing buffer (see Table of Materials). Repeat the washing step three times.
- Suspend the beads in 100 µL of washing buffer and transfer the suspension to a clean (heat-resistant) reaction tube.
- Place the tube on the magnetic separation rack (see Table of Materials) and remove the supernatant completely.
- Prepare denatured SDS-PAGE samples following steps 2.16.1-2.16.2 or non-denatured samples by eluting the VLPs from the magnetic beads following steps 2.16.3-2.16.5.
- To prepare denatured SDS-PAGE samples, suspend the beads in 20-80 µL of Laemmli buffer (0.8 µL of Laemmli buffer per 1 ng of p55 Gag assay input) and incubate at 95 °C for 5 min.
- Proceed directly with SDS-PAGE or store the samples at -20 °C.
CAUTION: Laemmli buffer contains 2-mercaptoethanol and sodium dodecyl sulfate. Wear protective gloves and eye protection. Avoid contact with skin, eyes, and clothes. Do not inhale vapors. Work in a well-ventilated space, e.g., a fume hood.
NOTE: The protocol can be paused here. - Alternatively, perform a non-denaturing elution step to obtain VLPs with preserved native protein structure.
- Add 25 µL of elution buffer (frequently provided with the beads) to the magnetic beads and incubate for 2-5 min at room temperature.
- Remove the beads and transfer the eluate to a clean tube. Use the eluate for subsequent assays or store it at 4 °C.
NOTE: The protocol can be paused here.
Representative Results
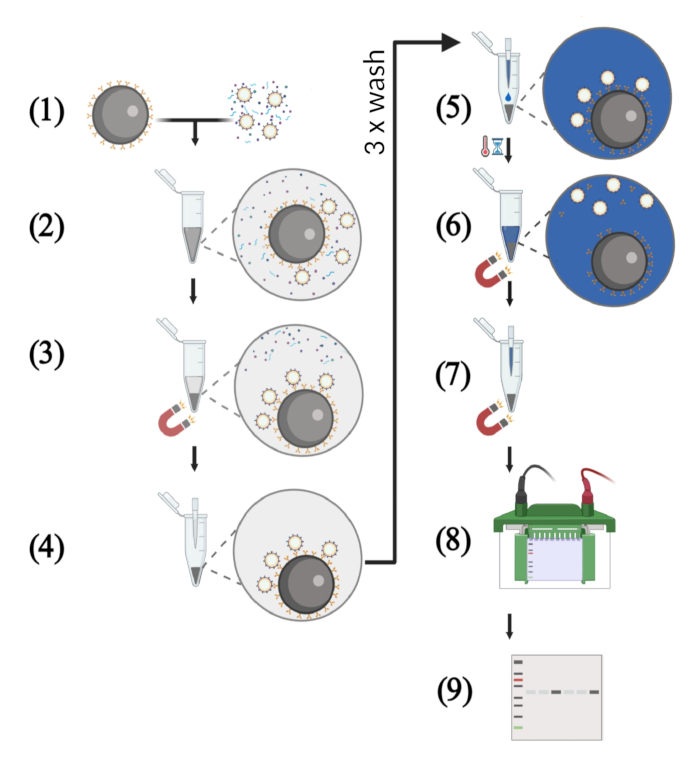
Figure 1: Schematic illustration of key steps of the VLP capture assay using cell-free supernatants. (1) The VLP capture assay starts with the binding of the HIV-1 bNAbs to the protein G-coupled magnetic beads. Meanwhile, the VLP solution with a defined p55 concentration is prepared. The cell-free culture supernatant consists of a mixture of p55 Gag VLPs, host cell proteins, and nucleic acids. (2) The magnetic beads coupled to bNAbs and the VLP solution are transferred into a 1.5 mL reaction tube followed by incubation under rotation. (3) Antigen-displaying VLPs are captured by the bNAbs-coated beads. Separation of these immune complexes from host cell-derived contaminants is performed in a magnetic field. (4) The supernatant is removed by pipetting, and the beads are washed three times to remove unbound VLPs. (5) In the next step, reducing protein loading buffer is added to the immune complexes consisting of magnetic beads coated with bNAbs and captured VLPs. (6) Boiling the sample dissociates bNAbs and target antigen from the magnetic beads and lysis the VLPs. (7) Magnetic beads are separated from the solution in a magnetic field. (8) The protein samples are subjected to SDS-PAGE. (9) Western blot analysis is performed in order to detect viral p55 Gag core proteins.
Declarações
The authors have nothing to disclose.
Materials
| 1.5 mL reaction tubes | Eppendorf | ||
| 10x PBS | gibco | 70011044 | |
| Antibodies (bnAbs) | Polymun Scientic | ||
| Dynabeads Protein G Immunoprecipitation Kit |
invitrogen (Thermo Fisher Scientific) | 10007D | includes buffers and washing solutions |
| FreeStyle 293-F cells | invitrogen (Thermo Fisher Scientific) | R790-07 | |
| FreeStyle 293 Expression Medium | invitrogen (Thermo Fisher Scientific) | 12338026 | |
| Magnetic separation rack | New England Biolabs | S1509S | for 12 x 1.5 mL or 6 x 1.5 mL tubes |
| Optima XE-90 ultracentrifuge | Beckman Coulter | ||
| Polyvinylidene fluoride (PVDF) syringe filters, 0.45 µm |
Carl Roth | KC89.1 | |
| PVDF transfermembrane, 0.45 µm | Carl Roth | T830.1 | |
| QuickTiter HIV p24 ELISA | Cell Biolabs | VPK-108-H | |
| Rotator | Heidolph | REAX2 | |
| SW28 rotor | Beckman Coulter | ||
| Thermomixer | Cel Media | basic | |
| Trehalose dihydrate | Carl Roth | 8897.2 | |
| Ultra Clear centrifuge tubes | Beckman Coulter | 344058 |

