In vivo Imaging and Therapeutic Treatments in an Orthotopic Mouse Model of Ovarian Cancer
Summary
Orthotopic animal models of ovarian cancer replicate better human disease and therefore enhance our understanding of cancer progression and tumor response to therapy. A mouse model receives an intrabursal injection of luciferase-expressing ovarian tumor cells. Treatment is administered via oral gavage. Tumor growth is monitored by in vivo imaging system.
Abstract
Human cancer and response to therapy is better represented in orthotopic animal models. This paper describes the development of an orthotopic mouse model of ovarian cancer, treatment of cancer via oral delivery of drugs, and monitoring of tumor cell behavior in response to drug treatment in real time using in vivo imaging system. In this orthotopic model, ovarian tumor cells expressing luciferase are applied topically by injecting them directly into the mouse bursa where each ovary is enclosed. Upon injection of D-luciferin, a substrate of firefly luciferase, luciferase-expressing cells generate bioluminescence signals. This signal is detected by the in vivo imaging system and allows for a non-invasive means of monitoring tumor growth, distribution, and regression in individual animals. Drug administration via oral gavage allows for a maximum dosing volume of 10 mL/kg body weight to be delivered directly to the stomach and closely resembles delivery of drugs in clinical treatments. Therefore, techniques described here, development of an orthotopic mouse model of ovarian cancer, oral delivery of drugs, and in vivo imaging, are useful for better understanding of human ovarian cancer and treatment and will improve targeting this disease.
Protocol
I. Preparation of Ovarian Tumor Cells
- Grow ovarian cancer cell lines expressing luciferase in culture. Sources of luciferase can be Firefly, Renilla, or other species. Cells expressing fluorescent proteins can also be used. For this demonstration we use an ovarian cancer cell line, OVCAR5, expressing firefly luciferase.
- Harvest cells using routine cell culture technique.
- Keep the cell suspension (10,000 cells/μL) in phosphate buffered saline (PBS) on ice until time of injection.
II. Intrabursal Injection
This procedure requires assistance from a second person. All surgical procedures are conducted under aseptic conditions. This includes wearing surgical attire and using sterile surgical instruments, syringe, and needles.
- Prepare the anesthetic solution by mixing 3 mL of ketamine hydrochloride (100 mg/mL), 1.6 mL of xylazine hydrochloride (100 mg/mL), 1.5 mL of acepromazine (10 mg/mL), and 20 mL of 0.9% sodium chloride.
- Check the animal’s identification number and observable health. Anesthetize the animal with prepared ketamine-xylazine-acepromazine anesthesia via intraperitoneal (i.p.) injection with a dose volume of 8~9 mL/kg body weight (BW).
- Confirm that the animal is under an acceptable plain of anesthesia by performing a toe pinch with forceps or fingers to the animal’s hind paws. If there is pedal reflex, wait for a deeper plain of anesthesia until the animal is unresponsive to this procedure.
- Lay the animal dorsal side up on a sterile gauze pad with its head facing away and its tail facing towards you. The point of incision is located to the left or right of the midline and above the ovaries. Shave or wet the fur with 70% alcohol at the incision point.
- Lift the wetted skin using forceps and make a small incision with the scissor at the dorsomedial position and directly above the ovarian fat pad. The ovarian fat pad should be visible beneath the surface of the peritoneal wall. Fat pad is easily recognizable by its white color in contrast to the dark pink tissue surrounding it.
- Gently lift the peritoneal wall lining and make a small incision as described above (5).
- Place a sterile soaked saline gauze pad on the midline adjacent to the incision. Locate the ovarian fat pad and gently pull it out and rest it onto the gauze. Stabilize the ovary by clamping the fat pad with a bulldog clip. Under a dissecting microscope, position the ovary as to allow for the insertion of the needle (30 gauge, G) into the oviduct tubule bend leading to the bursa. When the needle is inserted into the proper position, it should be visible under the bursa.
- Gently push the plunger of the syringe to inject 5 μL of cell suspension between the bursa and the ovary while the syringe is positioned to injection site. This step requires two people. One person pushes the plunger while the other person maintains positioning of needle. Remove the needle quickly to seal the puncture site but gently enough not to tear the bursa and tubule. The bursa should appear to be slightly distended with proper injection.
- Release the fat pad from the bulldog clip and gently replace the reproductive tract and fat pad back into the peritoneal cavity. Gently close the body wall by pulling the upper peritoneal lining over the lower lining. Close the skin with surgical staples or wound clips.
- Place the recovering animal back in its cage and provide a safe heat source to avoid hypothermia and speed up recovery. Monitor the breathing rate and ease, the return of muscle tone, and the ability to voluntarily move. These are all good indicators of the progression towards recovery. Staples or wound clips can be removed 7 or more days post surgery.
III. Oral Gavage Administration
- Use an 18~20 G gavage needle or feeding tube with a rounded tip. The gavage needle should be no longer than the distance from the tip of the animal’s head to its last rib.
- Check the animals’ identification number and observable health. Gently scruff the animal by grasping the skin over the shoulders with thumb and fingers. The restraint should be just firm enough for the fore limbs to be extended to each side and out of the way. The animal should not be able to grasp at the needle.
- Gently pull back the animal’s head with your index finger, forming a straight line through the neck and esophagus. Support the animal’s back with the inside of your thumb.
- Gently and without force, insert the gavage needle at either side of the mouth and over the tongue. In one smooth motion, the needle should pass against the roof of the mouth and down the esophagus. Gravity should guide the needle down without meeting resistance. If there is any resistance, remove the needle and start again. The animal’s gag and swallow reflex may be triggered during insertion. However, there should be no resistance or gasping from the animal. It is important to make sure that the animal is breathing when the needle is in place by checking the movement of the nostrils and chest.
- Slowly push plunger of the syringe to dispense the dose volume.
- Gently remove the gavage needle at the same angle and pathway as the way it was inserted down the esophagus.
- Return the animal to its cage and monitor breathing and behavior for 5~10 minutes.
IV. In Vivo Imaging
We use the Caliper Life Sciences to monitor the behavior of cells injected into intrabursal cavity. Experiments using this system typically have a timeframe of 4~16 weeks from the time of tumor implantation.
- Prepare the solution of D-luciferin (substrate of firefly luciferase) by dissolving 5~20 mg in 1 mL PBS and filtering through 0.22 μm membrane for sterilization.
- Charge the induction chamber by turning on both the oxygen and the isoflurane gas. Turn ON the gas flow to the induction chamber only. The isoflurane flow rate is maintained at a low level until animals are ready to be imaged.
- Check the animals’ identification number and observable health. Place the animal in the induction chamber charged with isoflurane gas.
- Remove the animal from the chamber when the animal appears to be anesthetized. Deliver 200 μL of prepared luciferin solution via i.p. injection using 30 G needle. It is important to record the time of injection in order to keep the time consistent between luciferin injection and imaging performance throughout the study.
- Place the luciferin-injected animal back into the induction chamber.
- Set the imaging system to the appropriate settings. Be sure to initialize the system before acquiring the first animal image.
- Turn OFF the gas flow to the induction chamber and turn ON the gas flow to the imaging chamber.
- Place the anesthetized animal dorsal or ventral side up. Be sure to place the animals’ nose in a nose cone to maintain proper anesthesia induction. Make sure the animal is within the “point of interest grid” as indicated by green illumination.
- Close the door of the chamber and acquire the image at the appropriate time. Time can be determined by kinetics.
- Remove the animal from the imaging chamber and place it back into its cage. Monitor the animals’ recovery as described in “Intrabursal Injection”.
V. Representative Results
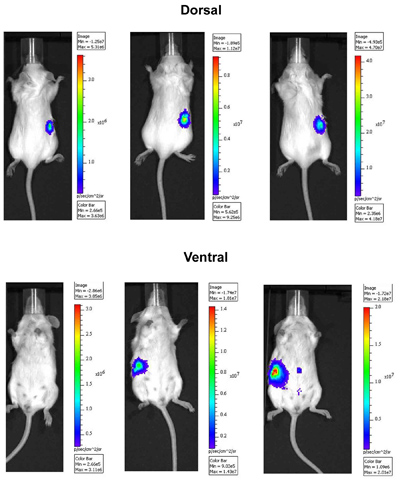
Figure 1. In Vivo imaging of ovarian tumor cells in an orthotopic mouse model. OVCAR5 cells expressing luciferase were injected intrabursally into right ovary and imaged over time. Images were taken 13 (left), 17 (middle), and 22 (right) days after the injection using the IVIS Spectrum imaging system. Dorsal side is shown in upper panel and ventral side is in lower panel. Note that the peritoneal spread of tumor cells 22 days following injection.
Experiments on animals were performed in accordance with the guidelines and regulations set forth by Fox Chase Cancer Center’s Institutional Animal Care and Use Committee.
Discussion
Ovarian cancer is the leading cause of death among all gynecologic malignancies 1. The high mortality rate of this disease is largely due to its late diagnosis and the lack of reliable diagnostic methods 2. Furthermore, conventional chemotherapy often encounters chemoresistance and relapse of cancer 3. Therefore, novel therapeutics is required to effectively target this disease. In development of new therapy targeting human ovarian cancer, it is critical to develop a representative animal model.
An orthotopic animal model has advantages over conventional xenograft models (e.g. subcutaneous or intraperitoneal injections of tumor cells) in that 1) it reproduces the primary site of tumor formation, 2) it represents common site of metastases, and 3) it provides tumor cells to interact with appropriate microenvironment 4, 5, 6, 7, 8. Rodents have a unique bursal membrane that surrounds the ovary and is continuous with the oviduct. This unique anatomy of rodents allows injection of ovarian tumor cells orthotopically. Intrabursally injected ovarian tumor cells behave similar to human disease, thereby growing within intrabursal membrane and spreading into peritoneal cavity as tumor progresses. Injection of cells stably expressing luciferases or fluorescent proteins also allows tracking behavior of tumor cells in real time. Bioluminescent and/or fluorescent imaging technology makes it possible to repeatedly image tumor cells over an extended period of time and study tumor growth, distribution, and regression in non-invasive manner 9, 10, 11.
In establishing the orthotopic model, it is critical to quickly remove the needle from the bursa upon injection. Abrupt removal of the needle seals the puncture site and prevents the leakage of injected cells. However, at the same time, the movement should be gentle not to tear the bursa. If leakage occurs during injection, it can cause cells to seed in the abdomen and potentially confound the study, i.e., premature spreading outside the ovary. In this event, the specific animal should be recorded and followed for the development of multiple tumor sites in the peritoneum prior to treatment or alternatively eliminated from the subsequent analyses. Prior to performing oral gavage, it is critical to check the length of the gavage tube by measuring from the tip of the animal’s head to the last rib. It is helpful to mark the tube at the animal’s nose and do not pass the tube past this mark. Insertion of the tube past this mark can result in a perforation to the stomach. Determining the length of the tube is especially important with younger animals or animals weighing under 20 g. Upon insertion of the gavage needle down the esophagus, there should be no resistance or struggle from the animal. It is important not to administer the solution or suspension too fast as this can lead to reflux and subsequently deliver inaccurate dose volume, as well as adding stress to the animal. For comparable imaging results from time to time, it is desirable to keep the time consistent between injection of luciferase substrate and imaging. The time lapse can be determined by taking series of images after substrate injection and observe the kinetics of signals. Development of an orthotopic mouse model of ovarian cancer, oral delivery of drugs, and in vivo imaging are necessary techniques for better understanding of development and spread of ovarian cancer and assessing novel therapeutic regimens that may ultimately improve the outcome of patient with this deadly disease.
Declarações
The authors have nothing to disclose.
Acknowledgements
Materials
| Material Name | Tipo | Company | Catalogue Number | Comment |
|---|---|---|---|---|
| Ketamine Hydrochloride | Vedco, Inc. | Not Available | ||
| Xylazine Hydrochloride | Lloyd, Inc. | Not Available | ||
| Acepromazine | Vedco, Inc. | Not Available | ||
| D-Luciferin Potassium Salt | Caliper Life Sciences | 122796 |
Referências
- Jemal, A. Cancer statistics. CA Cancer J Clin. 59, 225-249 (2009).
- Lynch, H. T. Hereditary ovarian carcinoma: heterogeneity, molecular genetics, pathology, and management. Mol Oncol. 3, 97-137 (2009).
- Yap, T. A., Carden, C. P., Kaye, S. B. Beyond chemotherapy: targeted therapies in ovarian cancer. Nat Rev Cancer. 9, 167-181 (2009).
- Killion, J. J., Radinsky, R., Fidler, I. J. Orthotopic models are necessary to predict therapy of transplantable tumors in mice. Cancer Metastasis Rev. 17, 279-284 (1998).
- Bibby, M. C. Orthotopic models of cancer for preclinical drug evaluation: advantages and disadvantages. Eur J Cancer. 40, 852-857 (2004).
- Shaw, T. J., Senterman, M. K., Dawson, K., Crane, C. A., Vanderhyden, B. C. Characterization of intraperitoneal, orthotopic, and metastatic xenograft models of human ovarian cancer. Mol Ther. 10, 1032-1042 (2004).
- Greenaway, J., Moorehead, R., Shaw, P., Petrik, J. Epithelial-stromal interaction increases cell proliferation, survival and tumorigenicity in a mouse model of human epithelial ovarian cancer. Gynecol Oncol. 108, 385-394 (2008).
- Connolly, D. C. Animal models of ovarian cancer. Cancer Treat Res. 149, 353-391 (2009).
- Sadikot, R. T., Blackwell, T. S. Bioluminescence imaging. Proc Am Thorac Soc. 2, 537-540 (2005).
- Lehmann, S. Longitudinal and multimodal in vivo imaging of tumor hypoxia and its downstream molecular events. Proc Natl Acad Sci U S A. 106, 14004-14009 (2009).
- Connolly, D. C., Hensley, H. H. Xenograft and Transgenic Mouse Models of Epithelial Ovarian Cancer and Non-Invasive Imaging Modalities to Monitor Ovarian Tumor Growth In Situ: Applications in Evaluating Novel Therapeutic Agents. Current Protocols in Pharmacoogy. 45, (2009).

