Intraperitoneal Injection into Adult Zebrafish
Summary
We demonstrate intraperitoneal injection into adult zebrafish. We use a 10 μl NanoFil microsyringe controlled by a Micro4 controller and UltraMicroPump III. This demonstration includes the use of cold water as an anesthetic.
Abstract
A convenient method for chemically treating zebrafish is to introduce the reagent into the tank water, where it will be taken up by the fish. However, this method makes it difficult to know how much reagent is absorbed or taken up per fish. Some experimental questions, particularly those related to metabolic studies, may be better addressed by delivering a defined quantity to each fish, based on weight. Here we present a method for intraperitoneal (IP) injection into adult zebrafish. Injection is into the abdominal cavity, posterior to the pelvic girdle. This procedure is adapted from veterinary methods used for larger fish. It is safe, as we have observed zero mortality. Additionally, we have seen bleeding at the injection site in only 5 out of 127 injections, and in each of those cases the bleeding was brief, lasting several seconds, and the quantity of blood lost was small. Success with this procedure requires gentle handling of the fish through several steps including fasting, weighing, anesthetizing, injection, and recovery. Precautions are required to minimize stress throughout the procedure. Our precautions include using a small injection volume and a 35G needle. We use Cortland salt solution as the vehicle, which is osmotically balanced for freshwater fish. Aeration of the gills is maintained during the injection procedure by first bringing the fish into a surgical plane of anesthesia, which allows slow operculum movements, and second, by holding the fish in a trough within a water-saturated sponge during the injection itself. We demonstrate the utility of IP injection by injecting glucose and monitoring the rise in blood glucose level and its subsequent return to normal. As stress is known to increase blood glucose in teleost fish, we compare blood glucose levels in vehicle-injected and non-injected adults and show that the procedure does not cause a significant rise in blood glucose.
Protocol
1. Pre-injection Preparations
- Fast the fish for at least 24 hours prior to injection. This will empty the intestinal bulb (stomach) contents. The basic fasting protocol is to transfer the fish, at their normal density, to a clean tank, then withhold food. For longer-term fasting that requires more rigorous conditions (e.g., for blood glucose studies), see additional considerations in the Discussion.
- Prepare Cortland salt solution (Perry et al., 1984).
For a 100 mL volume, dissolve the following in distilled water:
725 mg NaCl (124.1 mM)
38 mg KCl (5.1 mM)
41 mg Na2HPO4 (2.9 mM)
24 mg MgSO4∙7H2O (1.9 mM)
16 mg CaCl2∙2H2O (1.4 mM)
100 mg NaHCO3 (11.9 mM)
4 g Polyvinylpyrrolidone (PVP) (4%)
1,000 USP units Heparin
Filter, sterilize and store at 4°C. - Prepare the microscope.
- Cover the microscope base with plastic wrap for protection in case of spills.
- Put a paper towel on top of the plastic wrap. The surgical table will sit on top of the paper towel.
- Pre-adjust focus by viewing the surgical table and focusing on the sponge.
- Weigh the fish.
- Fill a 500 mL beaker about 1/3 full with fish facility water.
- Tare the balance.
- Collect the fish using a net. Wick excess water away from the net and fish by briefly dabbing the net on paper towels. Transfer the fish to the beaker.
- Weigh the fish.
- Transfer the fish to a clean tank.
- Transfer each weighed fish to its own labeled tank.
- Calculate the injection volume for each fish based on fish weight.
- Prepare the syringe and related injection equipment. For injection, we recommend a 35G beveled steel needle and a 10 μl NanoFil microsyringe. Prepare the NanoFil syringe and silflex tubing following the manufacturer’s instructions. It is important to remove any bubbles from the syringe and tubing. After filling the syringe and tubing, mount the syringe on the pump, and program the injection volume for the first fish.
- Prepare the surgical table.
- Cut a soft sponge (such as #L800-D, Jaece Industries) so that it is approximately 20 mm in height. On the flat face, make a cut that is 10-15 mm deep. This cut is the trough that will hold the fish for injection.
- Set the sponge into a 60 mm Petri dish.
- Set the Petri dish with sponge into a suitably-sized pipette tip box lid. The lid needs to be large enough to hold water to help maintain sponge temperature, but it should be shallow enough to not get in the way. We use a lid from a P200 tip box that is 11.4 cm L x 7.7 cm W x 1.5 cm D.
- Prepare the anesthetic.
- Make crushed ice using cubes made from fish facility water.
- Fill a clean ice bucket with the crushed ice.
- Put the surgical table into a larger container such as a 2.4 liter Rubbermaid food storage container.
- Pour some facility water (warm) into the outer container and the surgical table. Keep a reserve of warm facility water nearby.
- Put a thermometer into the outer container.
2. Anesthesia, Injection and Recovery
- Place the anesthetic outer container plus surgical table adjacent to the microscope. Have the bucket of ice chips nearby.
- Bring the water temperature down to 17°C by adding ice chips. Important: Don’t go below 17°C for this step.
- Use a net to transfer the fish to the outer container.
- Slowly add ice chips to the container to bring the temperature down to 12°C, over the course of several minutes.
- Monitor fish behavior: At 17°C or slightly lower, the fish typically will spread its pectoral fins horizontally, gasp, and have rapid operculum movements. As the temperature drops, the fish will swim more slowly and finally stop swimming. As the surgical plane of anesthesia is approached, gasping will stop and operculum movements will slow. The fish is ready for injection when it does not react to being handled. For most fish, 12°C is sufficient. Larger fish may require colder water.
- As the required temperature is reached (~12°C or colder), press on the sponge to saturate it.
- Keep your fingers in the cold water sufficiently so that they will not warm up the fish and bring it out of anesthesia during handling.
- With cold fingers, gently transfer the fish to the trough of the sponge. Position the fish with the abdomen up and the gills in the trough.
- Quickly transfer the surgical table to the microscope stage.
- Working quickly, carefully insert the needle into the midline between the pelvic fins. The needle should point cranially and be inserted closer to the pelvic girdle than to the anus. You should be able to feel when the needle is deep to the body wall. Inject the appropriate volume and withdraw the needle.
- After injection, immediately transfer the fish back to its warm-water (~28.5°C) tank for recovery by releasing the fish from the sponge over the tank water.
Tip: If the fish does not begin swimming immediately, help it to recover by gently swirling water towards the gills. - Check the needle. Occasionally a scale may be attached and should be removed prior to the next injection.
- For subsequent injections, use warm facility water to bring the anesthetic chamber water temperature back up to 17°C before introducing the next fish.
3. Representative Results:
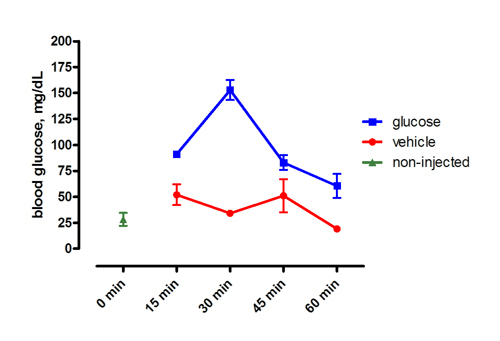
Figure 1. Representative results following intraperitoneal injection of 0.5 mg/g glucose or vehicle. Fish were fasted for 72 hours prior to injection. The x-axis shows time, post-injection. Mean ± SEM.
Discussion
Intraperitoneal injection involves five steps: fasting, weighing, anesthetizing, injection, and recovery. For each step there are best practices that can ensure success. Success includes a healthy fish patient as well as a good experimental outcome.
Fasting: A 24-hour fast should empty the intestinal bulb. This practice is taken from the fish veterinary literature (e.g., Brown 1993). Additional fasting considerations are discussed below.
Longer-term fasting: We have found that a 72-hour fast is required to decrease blood glucose to a baseline level prior to injection (Eames et al., 2010). We have also found that for glucose studies there are several procedures that are required to ensure that the fish are fasted properly. Start with a clean tank (no debris on the bottom). Tanks should be offline, clearly labeled as ‘fasting’, and in a location where enthusiastic fish care personnel will not feed them. Evaluate the external environment of the tank and take steps to prevent the fish from being stressed from disturbances, as stress is known to raise blood glucose (Chavin and Young, 1970; Groff et al., 1999). For example, we had a fasting experiment in which a radio was operated daily on the bench that was holding the fish tanks. We found that blood glucose was unusually high and concluded that the fish were stressed by the vibrations. Another stressor is overcrowding. Fish should be kept at a density that conforms with good fish husbandry practices. For recommendations, see Brand et al. (2002) and Westerfield (1995). We have had good results fasting our fish at a density of 10-12 fish in a 9 liter tank (with 3 layers of marbles taking up some of that volume). Separating the sexes may cause stress, so we recommend maintaining a mixed-sex population during the fast. This means that eggs can be laid, and the eggs need to be sequestered so that they will not be eaten. A simple way to sequester eggs is to cover the tank bottom with 2-3 layers of marbles. Water quality needs to be maintained by removing eggs and waste and by replacing about 10-15% of the tank water, daily. For removing eggs and waste, siphoning works well.
Weighing: When weighing fish that are not anesthetized, care should be taken to minimize water transfer from the net into the beaker, to ensure accurate weighing. If the net (with fish) is blotted on paper towels, the majority of the excess water can be removed, and the weight can be accurately measured. It may be easier to anesthetize the fish prior to weighing, but we have not tested the possible effects of anesthetizing a fish twice in one day. We have tested our technique by weighing the fish first with the netting/blotting method and then re-weighing the fish after it has been anesthetized, and gently blotted dry. We found no significant difference in weight between the methods (P = 0.7927, t-test). Additionally, we tested whether this netting/blotting method affected blood glucose, in comparison with simply transferring the fish to the beaker as soon as it is netted (no blotting). We found no significant difference in blood glucose level between the two transfer methods (P = 0.2241, t-test).
Anesthetizing: Chemical anesthesia may be suitable for many studies. Here we have demonstrated cold water anesthesia as an alternative, because many anesthetics (including tricaine/MS-222 (Brown, 1993)), raise blood glucose. In previous studies, we have determined that cold water does not raise blood glucose in zebrafish (Eames et al., 2010).
For cold water anesthesia, the temperature should be decreased slowly. The rate of decrease seems to depend on the size of the fish, with smaller fish going under faster than larger fish. Following injection, you may observe that the fish is recovering too slowly from the anesthetic (see below). This can result when either the starting temperature is too low, or when the temperature is decreased too rapidly. The starting temperature is too low if the fish bends laterally upon entering the water. If the starting temperature is correct, the fish will maintain its balance initially. It will rotate its pectoral fins to a horizontal position, gasp, and have rapid operculum movements. Typically, it will swim. As temperature decreases, movements will decrease and the fish will lose equilibrium. A surgical plane of anesthesia is reached when the fish can be handled without reacting. To maintain the fish under surgical anesthesia, your fingers must be cold, so keep them in the water prior to handling the fish. The sponge must also be kept cold at the same temperature as the water used for anesthetizing the fish. It is important to saturate the sponge with water that is sufficiently cold to maintain anesthesia once the fish is placed onto it.
Injection: Prior to undertaking injections, you may want to dissect at least one fish to get a sense of body wall thickness. This can help you to judge how far the needle needs to insert to enter the abdominal cavity. Additionally, as you insert the needle, you can feel the body wall “give” when the needle enters the abdominal cavity. During the injection, take steps to keep the patient happy. Make sure the sponge is saturated with the correct temperature cold water to prevent the fish from reviving during injection. A well-saturated and soft sponge is important for minimizing damage to the scales and mucus covering of the skin. A well-saturated sponge is also important for keeping the gills aerated. We highly recommend the foam sponge listed below under Materials. Finally, once the fish is anesthetized, work quickly to minimize the time that the fish is under.
Recovery: The fish should recover from the anesthesia virtually upon entering the warm tank water. If the fish does not begin swimming immediately, gently swirl the water towards its gills to speed recovery. If recovery is slow, then the fish went under too quickly and you should adjust the anesthesia procedure appropriately. The possible causes of slow recovery are discussed under Anesthetizing.
Declarações
The authors have nothing to disclose.
Acknowledgements
This study was supported by Juvenile Diabetes Research Foundation grant 5-2007-97 (to V.E.P.), by National Institute of Diabetes and Digestive and Kidney Diseases grants R01DK064973 (to V.E.P.), R01DK48494 (to L.H.P.), T32DK07074 (supporting S.C.E.), K01DK083552 (to M.D.K), and by P60DK20595 to The University of Chicago Diabetes Research and Training Center. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIDDK or the NIH.
Materials
| Material Name | Tipo | Company | Catalogue Number | Comment |
|---|---|---|---|---|
| Foam Sponge | Jaece Industries | L800-D | ||
| 60 mm Petri dish | ||||
| Pipet tip box lid | not too deep, e.g. 1.5 cm | |||
| Plastic storage container | deep, e.g. 7 cm | |||
| Thermometer | ||||
| Crushed ice | made from facility water | |||
| Warm facility water | 1 liter or more | |||
| 500 ml beaker | for weighing | |||
| NanoFil syringe | World Precision Instruments (WPI) | NANOFIL | or Hamilton syringe | |
| 35 gauge needle | WPI | NF35BV-2 | beveled | |
| Silflex tubing | WPI | SILFLEX-2 | ||
| UltraMicroPump III and Micro4 controller | WPI | UMPS-1 | ||
| Foot switch | WPI | 15867 | ||
| Dissecting microscope | ||||
| Plastic wrap | ||||
| Paper towels | ||||
| Cortland salt solution |
Referências
- Perry, S. F., Davie, P. S., Daxboeck, C., Ellis, A. G., Smith, D. G., Hoar, W. S., Randall, D. J. Perfusion methods for the study of gill physiology. Fish Physiology Volume X: Gills, Part B: Ion and Water. , 325-388 (1984).
- Brown, L. A., Stoskopf, M. K. Anesthesia and restraint. Fish Medicine. , 79-90 (1993).
- Eames, S. C., Philipson, L., Prince, V. E., Kinkel, M. D. Blood sugar measurement in zebrafish reveals dynamics of glucose homeostastis. Zebrafish. 7, 205-213 (2010).
- Chavin, W., Young, J. E. Factors in the determination of normal serum glucose levels of goldfish Carassius auratus L. Comp Biochem Physiol. 33, 629-653 (1970).
- Groff, J. M., Zinkl, J. G. Hematology and clinical chemistry of cyprinid fish. Common carp and goldfish. Vet Clin North Am Exot Anim Pract. 2, 741-776 (1999).
- Brand, M., Granato, M., Nusslein-Volhard, C., Nusslein-Volhard, C., Dahm, R. Keeping and raising zebrafish. Zebrafish: A Practical Approach. , 7-37 (2002).
- Westerfield, M. . The Zebrafish Book. , (1995).
- Iwama, G. K., Ackerman, P. A., Hochachka, P. W., Mommsen, T. P. Anaesthetics. Biochemistry and Molecular Biology of Fishes, Volume 3: Analytical Techniques. , 1-15 (1994).
- Reavill, D. R. Common diagnostic and clinical techniques for fish. Vet Clin North Am Exot Anim Pract. 9, 223-235 (2006).
- Stoskopf, M. K., Stoskopf, M. K. Surgery. Fish Medicine. , 91-97 (1993).

