Identifying Kinase Inhibitors that Modulate the Thymocyte Response to Strong TCR Signals
Abstract
Source: Chen, E. W., et al. Identification of Mediators of T-cell Receptor Signaling via the Screening of Chemical Inhibitor Libraries. J. Vis. Exp. (2019).
In this video, we describe a method to identify the small-molecule kinase inhibitors that modulate the apoptosis of self-reactive CD4+CD8+ double-positive immature thymocytes. Apoptosis is induced in the double-positive thymocytes by activating them with anti-CD3- and anti-CD28-coated magnetic beads; this is followed by a small-molecule inhibitor treatment and flow cytometry analysis to detect if the inhibitors modulate the apoptotic marker expression.
Protocol
All procedures involving animal models have been reviewed by the local institutional animal care committee and the JoVE veterinary review board.
1. Kinase Inhibitor Library Screening (Centrifuge-independent Assay)
- Treatment of thymocytes with kinase inhibitors
- Prepare a thymocyte suspension.
- Dilute the thymocytes in complete RPMI to obtain a thymocyte suspension of 25 x 106 cells/mL.
- Add 40 μL of thymocytes to each well of a small-volume plate, using a multichannel pipette. Place the plate on ice.
- Dilute the inhibitors from the stock plate, DMSO, and dexamethasone in complete RPMI at a ratio of four parts of complete RPMI to one part of inhibitor/DMSO/dexamethasone (dilution factor of 5).
NOTE: As the volumes used in this small-volume plate are 5x smaller than in the conventional method, the inhibitors and the control reagents are diluted fivefold before adding them to the thymocytes in the plate. - Add 0.5 μL of kinase inhibitors to the 96-well plate from the corresponding wells of the inhibitor plate prepared in step 1.1.4.
- Prepare eight wells of untreated controls. Prepare four wells of vehicle-treated controls by adding 0.5 μL of the DMSO prepared in step 1.1.4. Prepare four wells of 5 μM dexamethasone-treated controls, using the diluted dexamethasone prepared in step 1.1.4 (Figure 1).
2. Stimulation of thymocytes using anti-CD3/CD28 beads
- Make sure that the beads are uniformly resuspended. Take 1 mL of beads and wash them with 2 mL of PBS. Separate the beads using a magnetic stand and aspirate the solution. Resuspend the beads in 1 mL of complete RPMI.
NOTE: The ratio of beads to cells is 1 to 2.5. Adjust the amount of beads, depending on the number of wells to stimulate and the number of thymocytes used. - Add 10 μL of bead suspension to each inhibitor-treated sample, the four DMSO-treated samples, and four of the eight untreated samples. Add 10 μL of complete RPMI to the remaining four untreated wells. Figure 1 shows the general plate layout.
NOTE: The final volume of the wells is 50 μL, which is within the maximum capacity of the wells. It is important to exercise caution and to hold the plates upright, to avoid cross-well spillage. - To mix, agitate the plate using a microplate orbital shaker. Alternatively, mix the contents of the wells using a multichannel pipette.
- Incubate the thymocytes in a 37 °C, 5% CO2 incubator for 17 – 20 h (or overnight) with an anti-evaporation lid.
3. Setup of the plate washer
NOTE: The instructions for setting up the plate washer are provided by the manufacturer. The steps are mentioned in brief below. Roughly 150 mL of solution is needed for each priming step.
- Prime the wash system with 70% ethanol containing 1% Tween 20.
- Prime the wash system with deionized water containing 1% Tween 20.
- Prime the wash system with FACS wash buffer.
4. Staining of surface antigens
- Prepare an antibody staining mixture containing anti-TCRβ, anti-CD4, anti-CD8, and anti-CD69 antibodies. Dilute the antibodies in FACS wash buffer at a ratio of 1:100 (v/v).
- Wash the plate 9x, using 55 μL of FACS wash buffer per wash, using the automated laminar flow washing system.
NOTE: At the end of the washes, there will be 25 μL of residual volume in each well. - Resuspend the cells in 25 μL of the staining antibody mixture prepared in step 1.4.1.
- If the samples are transferred from a 96-well plate, resuspend the cells in 50 μL of the antibody mixture, and transfer the samples to the small-volume plate. This step corresponds to method number 2, as depicted in Figure 2A.
- To mix, agitate the plate with a microplate orbital shaker or mix the samples using a multichannel pipette, and incubate on ice for 30 min.
5. Fixation of cells
- Wash the plate 9x, using 55 μL of FACS wash buffer per wash, using the automated laminar flow washing system.
- Add fixation/permeabilization buffer (comes with the active caspase-3 apoptosis kit; same with the 10x perm/wash buffer mentioned in step 1.6.1 and the anti-caspase-3 antibody in step 1.6.2) at 50 μL per well.
- Incubate on ice for 30 min.
6. Intracellular staining for active caspase 3
- Prepare 1x perm/wash buffer by diluting 25 mL of 10x perm/wash buffer in 225 mL of ultrapure water.
- Prepare intracellular active caspase stain by adding 1 mL of anti-caspase-3 antibody to 2 mL of 1x perm/wash buffer. The ratio of antibody to perm/wash buffer is 1:2.
- Prime the wash system with 1x perm/wash buffer.
- Wash the plate 9x with 1x perm/wash buffer, at 55 μL for each wash.
- Add 25 μL of the intracellular caspase stain prepared in step 1.6.2 to all wells.
- To mix, agitate the plate with a microplate orbital shaker or mix the samples using a multichannel pipette, and incubate on ice for 1 h.
- Wash the plate 9x with 1x perm/wash buffer, at 55 μL for each wash.
- Add 25 μL of FACS wash buffer to all wells.
- Transfer the samples to microtiter tubes after adequate mixing via pipetting.
- Add another 50 μL of FACS wash buffer to the empty wells and repeat step 1.6.9.
- Repeat steps 1.6.9 and 1.6.10 2x until 200 μL of the samples are collected in the microtiter tubes.
NOTE: The purpose of the procedures described in steps 1.6.10 and 1.6.11 is to ensure maximum recovery of the cells from the small-volume plate. If cell numbers are not a concern, after step 1.6.10, simply top up the microtiter tubes to 200 μL with FACS wash buffer. - Run a flow cytometric analysis of the samples and analyze the results with a FACS analysis program. Caspase-3 activation and CD69 expression are analyzed in the gate containing CD4+CD8+DP thymocytes.
Representative Results
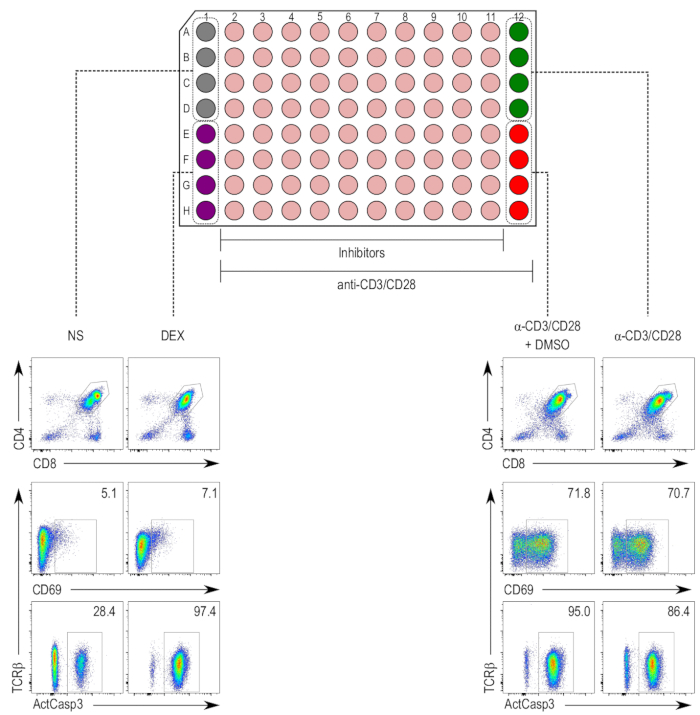
Figure 1: Plate layout of the thymocyte activation assay. (Top) Columns 1 and 12 are reserved for controls, while columns 2 to 11 are inhibitor-treated samples (beige). The negative control (nonstimulated [NS]; grey) occupies wells A1 to D1, and the positive control for cell death (dexamethasone-treated [DEX]; purple) occupies wells E1 to H1. Columns 2 to 12 contain thymocytes stimulated with anti-CD3/CD28 beads. The positive control for thymocyte activation (stimulated samples [α-CD3/CD28]; green) occupies wells A12 to D12, and the vehicle control (stimulated and DMSO-treated [α-CD3/CD28 + DMSO]; red) occupies wells E12 to H12. (Bottom) Flow cytometry plots of active caspase-3 (ActCasp3), CD69, and TCRβ staining of thymocytes gated within the double-positive (DP) gate. Representative plots of the different controls are shown. NS = nonstimulated; DEX = dexamethasone-treated samples; α-CD3/CD28 + DMSO = samples stimulated with CD3/CD28-coated beads and treated with DMSO; α-CD3/CD28 = samples stimulated with CD3/CD28-coated beads.
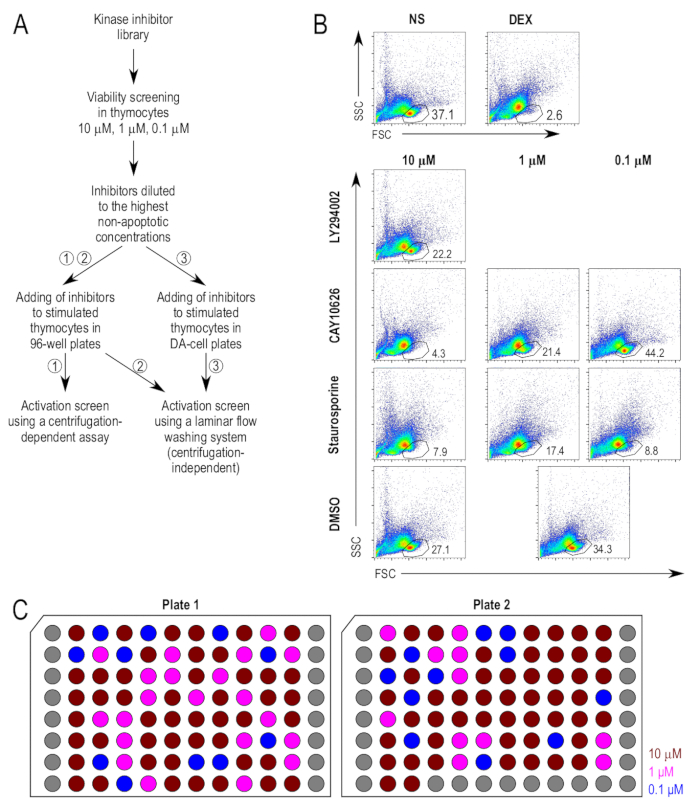
Figure 2: Thymocyte viability after treatment with inhibitors. (A) Experimental outline of the major steps in the screening assay. There are three proposed methods for the stimulation and staining of the thymocytes used in the activation assay, namely (1) the culturing of thymocytes in standard 96-well plates, followed by staining using a conventional centrifugation-based protocol, (2) the culturing of thymocytes in standard 96-well plates, followed by staining using a centrifugation-independent washing protocol, and (3) the culturing of thymocytes in small-volume plates, followed by staining in the same plates using a centrifugation-independent washing protocol. (B) Gating strategies used in the viability assays. The live cell gate was derived from the forward scatter (FSC) and side scatter (SSC) plots. Inhibitors that were deemed to be too toxic at the tested concentration were subject to further viability assays at 10-fold lower concentrations. Representative inhibitor-treated samples are shown. Note the common control (DMSO-treated [DMSO]) used for the 1 µM and 0.1 µM samples. (C) Plate layout of diluted inhibitors. A schematic representation of the plates of inhibitors diluted in DMSO to a concentration of 500x the intended final concentration. Each well represents one unique inhibitor; the grey wells are empty. The concentrations shown are the final concentration when added to the cell cultures, namely 10 µM (dark red), 1 µM (fuchsia), and 0.1 µM (blue).
Declarações
The authors have nothing to disclose.
Materials
| RPMI | HyClone | SH30027FS | |
| FBS | HyClone | SH3007103 | |
| L-Glutamine | HyClone | SH3003401 | |
| Sodium pyruvate | HyClone | SH3023901 | |
| 10X PBS | Vivantis | PB0344 – 1L | |
| Kinase Screening Library (96-Well) | Cayman Chemical | 10505 | Exact contents of the library may vary |
| DMSO | Sigma Aldrich | D2650 | |
| Dexamethasone | Sigma Aldrich | D4902 | |
| anti-CD3/CD28 beads | Thermo Fisher Scientific | 11452D | |
| FITC Active Caspase-3 Apoptosis Kit | BD Pharmingen | 550480 | Contains Fixation/Permeabilization buffer, 10X Perm/Wash buffer and anti-caspase 3 antibody |
| DA-Cell Washer | CURIOX | HT1000 | |
| 96-well DA-Cell Plate | CURIOX | 96-DC-CL-05 | |
| Antibodies | |||
| CD3e | BioLegend | 100236 | |
| TCRb | BD Biosciences | 553174 | |
| CD4 | BD Biosciences | 740007 | |
| CD8 | BD Biosciences | 563786 | |
| CD69 | eBioscience | 25-0699-42 | |
| Inhibitors | |||
| TG003 | Cayman Chemical | From the Kinase Screening Library | |
| TG003 | Cayman Chemical | – | From the Kinase Screening Library |
| PKC 412 | Cayman Chemical | – | From the Kinase Screening Library |
| Doramapimod | Cayman Chemical | – | From the Kinase Screening Library |
| Paclitaxel | Cayman Chemical | – | From the Kinase Screening Library |
| Erlotinib | Cayman Chemical | – | From the Kinase Screening Library |
| Necrostatin-5 | Cayman Chemical | – | From the Kinase Screening Library |
| NVP-BEZ235 | Cayman Chemical | – | From the Kinase Screening Library |
| Phthalazinone pyrazole | Cayman Chemical | – | From the Kinase Screening Library |
| AG-879 | Cayman Chemical | – | From the Kinase Screening Library |
| 1-NA-PP1 | Cayman Chemical | – | From the Kinase Screening Library |
| Torin 1 | Cayman Chemical | – | From the Kinase Screening Library |
| Bisindolylmaleimide II | Cayman Chemical | – | From the Kinase Screening Library |
| BIBF 1120 | Cayman Chemical | – | From the Kinase Screening Library |
| SMI-4a | Cayman Chemical | – | From the Kinase Screening Library |
| Bisindolylmaleimide XI (hydrochloride) | Cayman Chemical | – | From the Kinase Screening Library |
| CAY10657 | Cayman Chemical | – | From the Kinase Screening Library |
| AS-703026 | Cayman Chemical | – | From the Kinase Screening Library |
| Chelerythrine chloride | Cayman Chemical | – | From the Kinase Screening Library |
| Tunicamycin | Cayman Chemical | – | From the Kinase Screening Library |
| GSK 1059615 | Cayman Chemical | – | From the Kinase Screening Library |
| Ruxolitinib | Cayman Chemical | – | From the Kinase Screening Library |
| Necrostatin-1 | Cayman Chemical | – | From the Kinase Screening Library |
| SB 505124 | Cayman Chemical | – | From the Kinase Screening Library |
| INK128 | Cayman Chemical | – | From the Kinase Screening Library |
| Canertinib (hydrochloride) | Cayman Chemical | – | From the Kinase Screening Library |
| SB 431542 | Cayman Chemical | – | From the Kinase Screening Library |
| PD 173074 | Cayman Chemical | – | From the Kinase Screening Library |
| Valproic Acid (sodium salt) | Cayman Chemical | – | From the Kinase Screening Library |
| PD 0325901 | Cayman Chemical | – | From the Kinase Screening Library |
| SB 203580 | Cayman Chemical | – | From the Kinase Screening Library |
| VX-702 | Cayman Chemical | – | From the Kinase Screening Library |
| Emodin | Cayman Chemical | – | From the Kinase Screening Library |
| CHIR99021 | Cayman Chemical | – | From the Kinase Screening Library |
| BIO | Cayman Chemical | – | From the Kinase Screening Library |
| Imatinib (mesylate) | Cayman Chemical | – | From the Kinase Screening Library |
| Sunitinib Malate | Cayman Chemical | – | From the Kinase Screening Library |
| Gefitinib | Cayman Chemical | – | From the Kinase Screening Library |
| PP2 | Cayman Chemical | – | From the Kinase Screening Library |
| 3-Methyladenine | Cayman Chemical | – | From the Kinase Screening Library |
| Bisindolylmaleimide I | Cayman Chemical | – | From the Kinase Screening Library |
| Bisindolylmaleimide IV | Cayman Chemical | – | From the Kinase Screening Library |
| Bisindolylmaleimide V | Cayman Chemical | – | From the Kinase Screening Library |
| NSC 663284 | Cayman Chemical | – | From the Kinase Screening Library |
| D 4476 | Cayman Chemical | – | From the Kinase Screening Library |
| NU 7026 | Cayman Chemical | – | From the Kinase Screening Library |
| H-9 (hydrochloride) | Cayman Chemical | – | From the Kinase Screening Library |
| Indirubin-3'-monoxime | Cayman Chemical | – | From the Kinase Screening Library |
| KN-62 | Cayman Chemical | – | From the Kinase Screening Library |
| KN-93 | Cayman Chemical | – | From the Kinase Screening Library |
| CGP 57380 | Cayman Chemical | – | From the Kinase Screening Library |
| Iso-Olomoucine | Cayman Chemical | – | From the Kinase Screening Library |
| (S)-Glycyl-H-1152 (hydrochloride) | Cayman Chemical | – | From the Kinase Screening Library |
| Bisindolylmaleimide VIII (acetate) | Cayman Chemical | – | From the Kinase Screening Library |
| ST638 | Cayman Chemical | – | From the Kinase Screening Library |
| SU 6656 | Cayman Chemical | – | From the Kinase Screening Library |
| LY364947 | Cayman Chemical | – | From the Kinase Screening Library |
| SB 203580 (hydrochloride) | Cayman Chemical | – | From the Kinase Screening Library |
| CAY10621 | Cayman Chemical | – | From the Kinase Screening Library |
| YM-201636 | Cayman Chemical | – | From the Kinase Screening Library |
| ZM 447439 | Cayman Chemical | – | From the Kinase Screening Library |
| AS-041164 | Cayman Chemical | – | From the Kinase Screening Library |
| NVP-AEW541 (hydrochloride) | Cayman Chemical | – | From the Kinase Screening Library |
| PP242 | Cayman Chemical | – | From the Kinase Screening Library |
| ABT-869 | Cayman Chemical | – | From the Kinase Screening Library |
| CAY10622 | Cayman Chemical | – | From the Kinase Screening Library |
| 17β-hydroxy Wortmannin | Cayman Chemical | – | From the Kinase Screening Library |
| CAY10626 | Cayman Chemical | – | From the Kinase Screening Library |
| SU 6668 | Cayman Chemical | – | From the Kinase Screening Library |
| CAY10572 | Cayman Chemical | – | From the Kinase Screening Library |
| N,N-Dimethylsphingosine | Cayman Chemical | – | From the Kinase Screening Library |
| LY294002 | Cayman Chemical | – | From the Kinase Screening Library |
| U-0126 | Cayman Chemical | – | From the Kinase Screening Library |
| Staurosporine | Cayman Chemical | – | From the Kinase Screening Library |
| KN-92 (hydrochloride) | Cayman Chemical | – | From the Kinase Screening Library |
| AS-605240 (potassium salt) | Cayman Chemical | – | From the Kinase Screening Library |
| O-1918 | Cayman Chemical | – | From the Kinase Screening Library |
| Y-27632 (hydrochloride) | Cayman Chemical | – | From the Kinase Screening Library |
| Leelamine | Cayman Chemical | – | From the Kinase Screening Library |
| PD 98059 | Cayman Chemical | – | From the Kinase Screening Library |
| PD 169316 | Cayman Chemical | – | From the Kinase Screening Library |
| TGX-221 | Cayman Chemical | – | From the Kinase Screening Library |
| (S)-H-1152 (hydrochloride) | Cayman Chemical | – | From the Kinase Screening Library |
| AS-605240 | Cayman Chemical | – | From the Kinase Screening Library |
| D-erythro-Sphingosine C-18 | Cayman Chemical | – | From the Kinase Screening Library |
| OSU03012 | Cayman Chemical | – | From the Kinase Screening Library |
| JNJ-10198409 | Cayman Chemical | – | From the Kinase Screening Library |
| Leelamine (hydrochloride) | Cayman Chemical | – | From the Kinase Screening Library |
| Arachidonic Acid Leelamide | Cayman Chemical | – | From the Kinase Screening Library |
| Lauric Acid Leelamide | Cayman Chemical | – | From the Kinase Screening Library |
| AS-252424 | Cayman Chemical | – | From the Kinase Screening Library |
| CAY10505 | Cayman Chemical | – | From the Kinase Screening Library |
| PI-103 | Cayman Chemical | – | From the Kinase Screening Library |
| PIK-75 (hydrochloride) | Cayman Chemical | – | From the Kinase Screening Library |
| Sphingosine Kinase Inhibitor 2 | Cayman Chemical | – | From the Kinase Screening Library |
| Piceatannol | Cayman Chemical | – | From the Kinase Screening Library |
| SC-1 | Cayman Chemical | – | From the Kinase Screening Library |
| (R)-Roscovitine | Cayman Chemical | – | From the Kinase Screening Library |
| BAY-43-9006 | Cayman Chemical | – | From the Kinase Screening Library |
| CAY10561 | Cayman Chemical | – | From the Kinase Screening Library |
| AS-604850 | Cayman Chemical | – | From the Kinase Screening Library |
| PI3-Kinase α Inhibitor 2 | Cayman Chemical | – | From the Kinase Screening Library |
| ML-9 | Cayman Chemical | – | From the Kinase Screening Library |
| Triciribine | Cayman Chemical | – | From the Kinase Screening Library |
| Erbstatin Analog | Cayman Chemical | – | From the Kinase Screening Library |
| Kenpaullone | Cayman Chemical | – | From the Kinase Screening Library |
| Olomoucine | Cayman Chemical | – | From the Kinase Screening Library |
| AG-494 | Cayman Chemical | – | From the Kinase Screening Library |
| AG-825 | Cayman Chemical | – | From the Kinase Screening Library |
| AG-1478 | Cayman Chemical | – | From the Kinase Screening Library |
| SB 216763 | Cayman Chemical | – | From the Kinase Screening Library |
| SB 415286 | Cayman Chemical | – | From the Kinase Screening Library |
| AG-17 | Cayman Chemical | – | From the Kinase Screening Library |
| H-8 (hydrochloride) | Cayman Chemical | – | From the Kinase Screening Library |
| LFM-A13 | Cayman Chemical | – | From the Kinase Screening Library |
| SC-514 | Cayman Chemical | – | From the Kinase Screening Library |
| Apigenin | Cayman Chemical | – | From the Kinase Screening Library |
| AG-18 | Cayman Chemical | – | From the Kinase Screening Library |
| CAY10554 | Cayman Chemical | – | From the Kinase Screening Library |
| DRB | Cayman Chemical | – | From the Kinase Screening Library |
| RG-13022 | Cayman Chemical | – | From the Kinase Screening Library |
| RG-14620 | Cayman Chemical | – | From the Kinase Screening Library |
| AG-490 | Cayman Chemical | – | From the Kinase Screening Library |
| AG-82 | Cayman Chemical | – | From the Kinase Screening Library |
| AG-99 | Cayman Chemical | – | From the Kinase Screening Library |
| AG-213 | Cayman Chemical | – | From the Kinase Screening Library |
| AG-183 | Cayman Chemical | – | From the Kinase Screening Library |
| Lavendustin C | Cayman Chemical | – | From the Kinase Screening Library |
| ZM 336372 | Cayman Chemical | – | From the Kinase Screening Library |
| 5-Iodotubercidin | Cayman Chemical | – | From the Kinase Screening Library |
| SB 202190 | Cayman Chemical | – | From the Kinase Screening Library |
| CAY10571 | Cayman Chemical | – | From the Kinase Screening Library |
| Nilotinib | Cayman Chemical | – | From the Kinase Screening Library |
| SP 600125 | Cayman Chemical | – | From the Kinase Screening Library |
| L-threo-Sphingosine C-18 | Cayman Chemical | – | From the Kinase Screening Library |
| H-89 | Cayman Chemical | – | From the Kinase Screening Library |
| HA-1077 (hydrochloride) | Cayman Chemical | – | From the Kinase Screening Library |
| AG-370 | Cayman Chemical | – | From the Kinase Screening Library |
| Wortmannin | Cayman Chemical | – | From the Kinase Screening Library |
| AG-1296 | Cayman Chemical | – | From the Kinase Screening Library |
| KT 5823 | Cayman Chemical | – | From the Kinase Screening Library |
| Janex 1 | Cayman Chemical | – | From the Kinase Screening Library |
| CAY10574 | Cayman Chemical | – | From the Kinase Screening Library |
| CAY10575 | Cayman Chemical | – | From the Kinase Screening Library |
| CAY10576 | Cayman Chemical | – | From the Kinase Screening Library |
| NH125 | Cayman Chemical | – | From the Kinase Screening Library |
| TWS119 | Cayman Chemical | – | From the Kinase Screening Library |
| NSC 210902 | Cayman Chemical | – | From the Kinase Screening Library |
| CAY10577 | Cayman Chemical | – | From the Kinase Screening Library |
| CAY10578 | Cayman Chemical | – | From the Kinase Screening Library |
| PD 184161 | Cayman Chemical | – | From the Kinase Screening Library |
| CCT018159 | Cayman Chemical | – | From the Kinase Screening Library |
| Myricetin | Cayman Chemical | – | From the Kinase Screening Library |

