A Luciferase-Fluorescent Reporter Influenza Virus to Track Viral Infections
Abstract
Source: Chiem, K., et al. A Luciferase-fluorescent Reporter Influenza Virus for Live Imaging and Quantification of Viral Infection. J. Vis. Exp. (150), (2019).
This video demonstrates a method to track viral infections in mouse models using a recombinant luciferase and fluorescence protein-expressing bi-reporter Influenza A virus. This method enables both in vivo and ex vivo studies, enabling researchers to gain insights into viral dynamics and the effects of interventions.
Protocol
All procedures involving animal models have been reviewed by the local institutional animal care committee and the JoVE veterinary review board.
1. In Vivo Characterization of BIRFLU (Figure 1 and Figure 2)
- Mouse infection
NOTE: Intranasal infection of mice was done as previously described. For a more detailed protocol of IAV infection in vivo using the mouse model of infection, we recommend viewing the video associated with the previous publication. This section only summarizes the steps required for mouse infection with BIRFLU.- Inspect the mice to evaluate their health and overall physical appearance. Prepare the dilution of BIRFLU in 1x PBS to inoculate mice with 1 x 106 PFU of BIRFLU in a total volume of 30 μL/mouse. Maintain the virus on ice until mouse inoculation.
NOTE: Mock-infected (1x PBS) mice will be needed as an internal control for imaging. Place mock-infected mice in a different cage than BIRFLU-infected animals. - Anesthetize five-to-seven-week-old female BALB/c mice intraperitoneally with 240–250 mg/kg of tribromoethanol (TBE) by inserting the needle into the caudal 2/3 of the right side of the abdomen. Then, return the mouse to the cage and wait for around 5 min. Check that the mouse is anesthetized before virus inoculation by the absence of the toe-pinch reflex.
NOTE: Injectable sedatives are recommended over inhaled sedatives for influenza infections in vivo, as the latter can modify the behavior and uptake of the virus by the airway epithelium. Moreover, they can also affect local immune responses of the lung and interfere with the in vivo imaging of infected mice. Finally, inhaled sedatives have a reduced duration of effect, which would be a problem for in vivo imaging of the infected animals. - Inoculate the mice intranasally with the 30 μL of the prepared BIRFLU dilution. Check that the mice are breathing properly before returning them to the cage.
- Inspect the mice to evaluate their health and overall physical appearance. Prepare the dilution of BIRFLU in 1x PBS to inoculate mice with 1 x 106 PFU of BIRFLU in a total volume of 30 μL/mouse. Maintain the virus on ice until mouse inoculation.
- Bioluminescence monitoring of mice infected with BIRFLU (Figure 2A)
NOTE: In this manuscript reporter expression of Nluc or Venus and viral replication in the lungs of mice infected with BIRFLU are determined at 3 days post-infection. However, the nature of bioluminescence imaging allows repeated monitoring of Nluc expression in individual BIRFLU-infected animals without the need to euthanize them for each experimental time point. An in vivo imaging system with an isoflurane anesthesia manifold is required to perform the described experimental procedures. See the Table of Materials for details regarding the imaging instrument and image software used for the image acquisition and data analysis.- Shave the mice's chest to improve the bioluminescence signal. Open the imaging software and press Initialize. Next, set the parameters that will be used, including setting the imaging mode to bioluminescence, auto-saving, exposure time to auto, open filter, etc.
- Once the machine is fully initialized, turn on the isoflurane anesthesia system. Place animals in the anesthesia chamber. Mice are simultaneously and lightly anesthetized with a mixture of oxygen gas and vaporized 1–2% isoflurane.
- Once mice are anesthetized, administer the Nluc substrate (see Table of Materials) diluted 1:10 in 1x PBS (final volume 100 µL/mouse) via retro-orbital route using a syringe with a 22 G needle.
- Immediately after Nluc reagent administration, place the animals in the imaging instrument with their chests facing up and snouts inside the manifold cone to keep the animal anesthetized during imaging. Immediately after closing the imager door, click Acquire in the software program (Figure 2A, top).
- After imaging, return the mice to their cages monitoring them until they have fully recovered, and turn off the isoflurane vaporizer. Then, proceed with the ex vivo imaging of mice lungs to evaluate Venus reporter gene expression (section 1.3).
- Use the imaging software tools to analyze the acquired bioluminescence data. Utilize the tool ROI (region of interest) to designate the specific signal and perform flux measurements in the region of interest (usually around the chest) (Figure 2A, bottom). Although the ROI shape is irrelevant, larger ROIs are generally preferred to capture the entire signal diffusion area.
- Click Measure. Assess the bioluminescence in photons since it provides an absolute photon emission measurement that is comparable to output measurements provided by different parameters or imaging instruments.
- Fluorescence analysis in mice infected with BIRFLU (Figure 1 and Figure 2B)
- Collect the mouse lungs after in vivo imaging, as previously described.
- Briefly, euthanize mice with a lethal dose of TBE (500 mg/kg). Disinfect the incision site with 70% ethanol. With a scalpel, make an incision from the sternum to the base of the abdomen and then cut from the bottom of the incision to the sides with scissors. Next, cut the hepatic vein (second physical euthanasia method) to bleed the animal.
NOTE: To avoid high background signals during imaging, it is important to minimize the amount of blood in the lungs (see below).
- Briefly, euthanize mice with a lethal dose of TBE (500 mg/kg). Disinfect the incision site with 70% ethanol. With a scalpel, make an incision from the sternum to the base of the abdomen and then cut from the bottom of the incision to the sides with scissors. Next, cut the hepatic vein (second physical euthanasia method) to bleed the animal.
- Place the mouse in dorsal recumbency, and use the scissors to cut the pleura and open the rib cage. Subsequently, remove the lungs by snipping the end of the trachea with scissors while gently holding the lungs with forceps.
- Place the lungs in a six-well plate with 2 mL of 1x PBS and wash the lungs three times with 1x PBS.
NOTE: To avoid contamination between samples, clean and disinfect the dissecting tools between each animal.
- Place the lungs in a six-well plate with 2 mL of 1x PBS and wash the lungs three times with 1x PBS.
- Start the image acquisition software by clicking initialize and setting the parameters for imaging, including setting imaging mode to fluorescence, auto-saving, exposure time to auto, excitation (500 nm), and emission (540 nm) filters.
- When the machine is initialized, place the lungs in a black background tray, ensuring that the tissues are separated from one another, introduce the tray into the imager, and click Acquire on the imaging system program after closing the imager door (Figure 2B, top).
- After imaging, remove the tissues immediately and store them on ice (4 °C) if the samples are processed the same day; or on a tube and dry ice to freeze them quickly, before storing them at -80 °C, if the samples will be processed later on a different day.
- For image processing, select the ROI tool and draw ROIs around each of the individual lungs. Click Measure. Then, using the resulting average radiant efficiency measurements, subtract the values from the mock-infected mice (Figure 2B, bottom).
NOTE: With BIRFLU, there is a good correlation of the levels of Nluc and Venus expression, and signal distribution. Therefore, it is important to analyze Nluc (whole mouse) and Venus (excised lungs) expression from the same animal and maintain the same orientation.
- Collect the mouse lungs after in vivo imaging, as previously described.
Representative Results
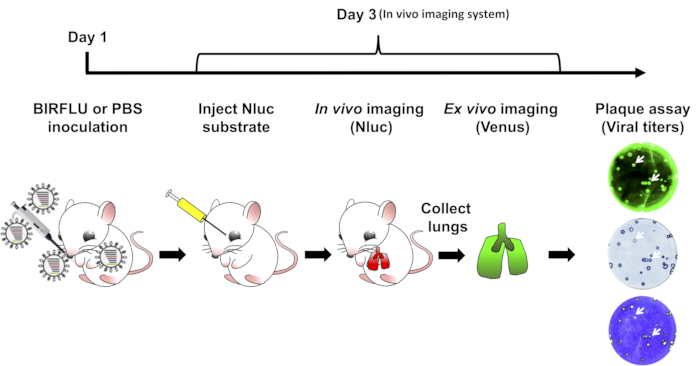
Figure 1: Schematic representation for the study of BIRFLU in mice. Expression of Nluc and Venus reporter genes was evaluated in mice infected with 1 x 106 PFU of BIRFLU using in vivo or ex vivo imaging. Briefly, on day 1, 5 to 7-week-old female BALB/c mice were mock-infected (1x PBS) or inoculated with 1 x 106 PFU of BIRFLU intranasally. At day 3 post-infection, mice were mildly anesthetized using isoflurane, and Nluc substrate was injected retro-orbitally. Nluc signal was directly assessed using in vivo imaging. Immediately after imaging, mice were euthanized and expression of Venus in whole excised lungs was analyzed using ex vivo imaging. Recovered mice lungs were homogenized to evaluate viral replication and stability by plaque assay. Arrows indicate the correlation between fluorescence (Venus), immunostaining (Nluc), and crystal violet staining.
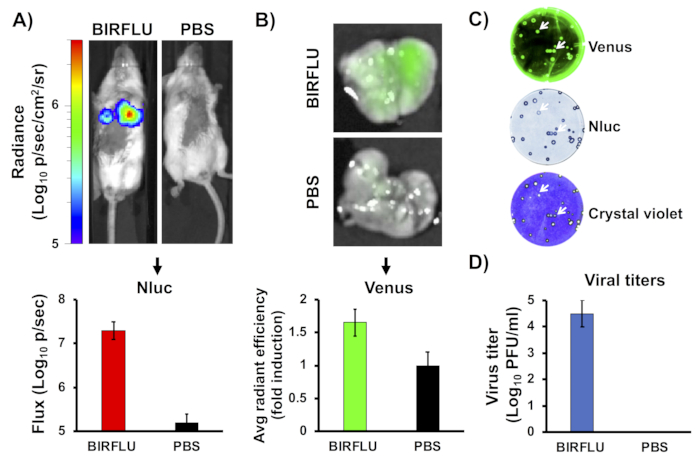
Figure 2: In vivo bioluminescence and fluorescence expression. Female five-to-seven-week-old BALB/c mice were mock-infected (1x PBS) or inoculated with 1 x 106 PFU of BIRFLU intranasally. At day 3 post-infection, Nluc activity (A) in the whole mouse was determined. Representative images of a single mouse showing radiance scale (p/sec/cm2/sr). Bioluminescence radiance values were quantitated and the average total flux is shown (Flux (Log10 p/s). After Nluc imaging, lungs were harvested for ex vivo imaging (B). Representative pictures from whole lungs are shown. To quantify Venus expression, mean values of regions of interest (ROIs) were normalized to lung auto-fluorescence from mock-infected mice, and fold changes were calculated. To analyze the genetic stability of BIRFLU in vivo, viruses recovered from mice lungs were analyzed by plaque assay using fluorescent microscopy (Venus, top), immunostaining (Nluc, middle), and crystal violet staining (bottom) (C). Representative images from one mouse are shown. To evaluate virus replication, whole lungs were homogenized after imaging and used to infect MDCK cells and determine viral titers by plaque assay (PFU/mL) (D). Arrows indicate the correlation between fluorescence (Venus), immunostaining (Nluc), and crystal violet staining. Bars represent the mean ± SD of lung virus titers. This figure has been adapted from Logales et al.
Declarações
The authors have nothing to disclose.
Materials
| Five- to seven-week-old female BALB/c mice | National Cancer Institute (NCI) | 555 | |
| Isoflurane | Baxter | 1001936040 | Store at Room temperature |
| IVIS Spectrum | PerkinElmer | 124262 | This instrument is used for in vivo imaging |
| IX81 Motorized Inverted Microscope | Olympus | Olympus IX81 | |
| Living Image 4.7.2 software | PerkinElmer | This instrument is used for in vivo imaging | |
| Lumicount | Packard | This instrument is used for quantifying luciferase activity | |
| Nano-Glo Luciferase Assay Reagent | Promega | N1110 | This reagent is used to measure Nluc activity. Store at -20 °C |
| Retiga 20000R Fast1394 Camera | Qimaging | Retiga 2000R | |
| Scanner | HP |

