Recombinant Retroviral Production and Infection of B Cells
Summary
An efficient system of structure and function analysis of a gene in an ex vivo culture of splenic B-lymphocytes is described. This method takes advantage of recombinant retroviral production in a helper free, ecotrophic packaging cell line. Stable, heritable expression of a gene of interest within primary lymphocytes is achieved leading to generation of surface antibodies on B cells undergoing class switch recombination.
Abstract
The transgenic expression of genes in eukaryotic cells is a powerful reverse genetic approach in which a gene of interest is expressed under the control of a heterologous expression system to facilitate the analysis of the resulting phenotype. This approach can be used to express a gene that is not normally found in the organism, to express a mutant form of a gene product, or to over-express a dominant-negative form of the gene product. It is particularly useful in the study of the hematopoetic system, where transcriptional regulation is a major control mechanism in the development and differentiation of B cells 1, reviewed in 2-4.
Mouse genetics is a powerful tool for the study of human genes and diseases. A comparative analysis of the mouse and human genome reveals conservation of synteny in over 90% of the genome 5. Also, much of the technology used in mouse models is applicable to the study of human genes, for example, gene disruptions and allelic replacement 6. However, the creation of a transgenic mouse requires a great deal of resources of both a financial and technical nature. Several projects have begun to compile libraries of knock out mouse strains (KOMP, EUCOMM, NorCOMM) or mutagenesis induced strains (RIKEN), which require large-scale efforts and collaboration 7. Therefore, it is desirable to first study the phenotype of a desired gene in a cell culture model of primary cells before progressing to a mouse model.
Retroviral DNA integrates into the host DNA, preferably within or near transcription units or CpG islands, resulting in stable and heritable expression of the packaged gene of interest while avoiding transcriptional silencing 8 9. The genes are then transcribed under the control of a high efficiency retroviral promoter, resulting in a high efficiency of transcription and protein production. Therefore, retroviral expression can be used with cells that are difficult to transfect, provided the cells are in an active state during mitosis. Because the structural genes of the virus are contained within the packaging cell line, the expression vectors used to clone the gene of interest contain no structural genes of the virus, which both eliminates the possibility of viral revertants and increases the safety of working with viral supernatants as no infectious virions are produced 10.
Here we present a protocol for recombinant retroviral production and subsequent infection of splenic B cells. After isolation, the cultured splenic cells are stimulated with Th derived lymphokines and anti-CD40, which induces a burst of B cell proliferation and differentiation 11. This protocol is ideal for the study of events occurring late in B cell development and differentiation, as B cells are isolated from the spleen following initial hematopoetic events but prior to antigenic stimulation to induce plasmacytic differentiation.
Protocol
1. Splenic B-Lymphocyte Isolation and Stimulation
- Harvest the spleens from AID-deficient mice 12 between 2-3 months of age. The isolation of spleen is performed in sterile conditions and the organs are temporarily kept in cold phosphate buffer saline (PBS) containing 15% fetal bovine serum (FBS).
- The spleen is then transferred to a sterile tissue culture hood and homogenized in 5 mL of PBS supplemented with 2.5% FBS. Transfer the homogenate to a 15 mL falcon tube.
- Centrifuge the homogenized splenic tissue sample at 1000rpm for 10 minutes at 4°C to pellet the cells.
- Decant the supernatant after centrifugation. Resuspend the cell pellet in 10 mL red blood cell (RBC) lysis buffer at room temperature for 7 minutes to avoid contamination by red blood cells.
- Centrifuge sample at 1000 rpm for 10 minutes and decant the supernatant and resuspend the cells in 10 mL PBS containing 2.5% FBS.
- Remove 100 μL aliquot and count the cells at a 1:1000 dilution using a standard hemacytometer using trypan blue to exclude any dead cells. Approximately 20-30 million cells can be obtained per spleen.
- Centrifuge sample at 1000rpm for 10 minutes and decant the supernatant.
- Resuspend the pellet in 0.5%BSA/PBS containing 2mM EDTA at a concentration of 107cells per 90 μL.
- Add anti-CD43 antibody coated magnetic beads to the cells to specifically bind non-B cells. Use 10 μL beads per 90 μL (107cells). Mix well and incubate at 4°C for 20 minutes.
- Once the cells have been labeled, wash them by adding 2.5% FBS/PBS to top of the tube. Centrifuge sample at 1000 rpm for 10 minutes.
- Decant the supernatant. Resuspend the cells in 0.1% BSA/PBS at a concentration of 10×107cells per ml.
- Mount a magnetic sorting column on a magnetic separator. Position a conical tube directly underneath the column to collect the flow-through and load the column with 3mL 0.5% BSA/PBS to prime and wash.
- Once the wash fluid has flowed through, replace with a new collection tube. If the sample is less than 1 mL, adjust sample volume to 1 mL with 0.5 %BSA/PBS/EDTA and load the sample in the column. Load only 1 mL maximum of cells per column and use multiple columns if necessary.
- Allow the cells to passively flow through the column and collect the unbound cells in the conical tube under the column. Wash the column 4 times with 3 mL 0.5% BSA/PBS while continuing to collect the flow-through.
- Collect all flow-through in which CD43 negative resting B cells will be enriched. Discard the column. Centrifuge flow-through at 1000 rpm for 10 minutes.
- Drain the supernatant and add 10 mL medium (15% FCS/ +glutamine, Pen/Strep, Non-Essential amino acids, 50 μM Β-ME)
- Count the cells and culture at 106 cells per ml.
- In order to stimulate B cell growth and proliferation, supplement the medium with recombinant IL-4 and anti-CD40 antibody such that the final concentrations are 20 μg/mL and 1 μg/mL, respectively, and transfer the cells to a culture flask.
- Culture the cells overnight. Count cells and dilute to 106 cells per ml, adding IL-4 and CD40 as appropriate.
- Culture cells for 72 hours before viral infection.
2. Transfection of Producer Cells
Use established protocols for transfection using either cationic lipids or calcium phosphate. Transfect 10cm3 plate per DNA construct, using 100 μg plasmid DNA per plate. We routinely use calcium phosphate transfection method 13, 14 and note high viral titer when supplemented with chloroquine.
- Use 60%-70% confluent Phoenix cells as producer cells to be transfected. At this confluence cell count should be 5 million cells per 10 cm plate.
- One hour prior to transfection, replace the media with fresh complete media for Phoenix cell growth.
- In a sterile eppendorpf tube, combine 100 μg of packaging construct DNA, 250 μL of 0.5M CaCl2, and adjust the final volume to 500 μL with sterile water
- Using a 1 mL pipette, vigorously mix this solution with 500 μL 2X HNP in a 15mL polypropylene tube
- Allow this mixture to rest at room temperature for 8-10 minutes. During this time a precipitate will form.
- Add the transfection mixture drop-wise to the cells while swirling the medium to evenly distribute the mixture throughout.
- Incubate the cells at 37°C for 12 hours.
- 12 hours post-transfection, replace the medium with 8ml complete B cell growth medium and return the cells to the 37°C incubator.
3. Infection of Stimulated Splenic B Cells
- Remove supernatant from Phoenix cells 48hr post-transfection and pass the supernatant through a 0.2 micron filter to remove any cell debris.
- Mix 3 mL of virus-containing supernatant with 3 mL of pre-activated B cells (as in step 1). In order to enhance viral adsorption to the cells, add polybrene at a final concentration of 16 μg per mL.
- Infect the cells by centrifugation at 2500 rpm for 90 minutes at 30°C.
- Immediately after the spin, incubate for a further 48-72 hrs at 37°C to allow for cell growth and proliferation.
4. Flow Cytometry Analysis
- Using a flow cytometer, analyze the cells for surface expression of B220 and surface IgG1. The presence of IgG1 at the surface of the cells indicates that class switch recombination has occurred as a result of successful rescue via the retroviral complementation of the AID gene.
5. Representative Results
We have successfully rescued the deficiency of the protein factor Activation Induced Cytidine Deaminase (AID) from AID-deficient (AID-/-) B-lymphocytes using retroviral transduction. Briefly, we generated recombinant retroviruses expressing mouse AID (mAID) protein by transfecting Phoenix cells with pMX-mAID-GFP 15-18. The harvested viruses were infected into AID-deficient B cells obtained from AID-deficient mice in ex vivo class switch recombination (CSR) stimulation conditions 19. The infected B cells were then analyzed for CSR to IgG1 after 72hrs in culture using flow cytometry analysis. Figure 2 shows the detection of IgG1 at the surface of B cells with AID but not the empty construct, indicating that CSR has occurred as a result of AID expression rescue.
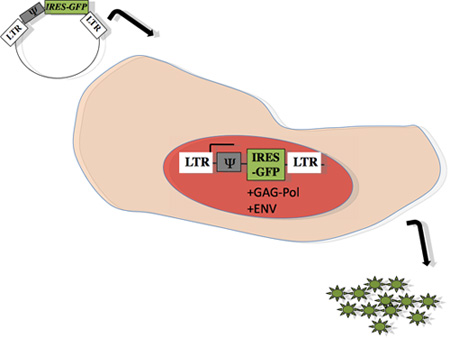
Figure 1: Scheme of retroviral packaging within producer cells: Gene of interest, represented by Ψ, was subcloned into pMX-IRES-GFP, as previously described 15. The plasmid was transfected into an ecotropic viral packaging cell line capable of producing gag-pol and envelope protein (Phoenix, Orbigen), using calcium phosphate method 13, 14. Forty-eight hours after transfection, supernatant containing viral particles was harvested for infection into target primary B cells obtained from mice.
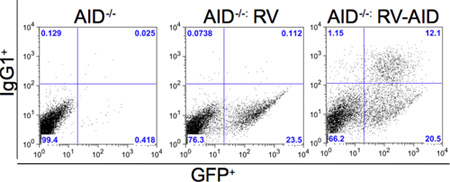
Figure 2: AID deficient B cells, stimulated with anti-CD40 and IL-4 were infected with a retrovirus containing pMX-mAID-GFP or a retrovirus expressing GFP alone. The level of CSR to IgG1 was evaluated by FACS analysis.
Discussion
Retroviral transduction of splenic B cells as described here and as depicted in Figure 1 is a genetic approach that is useful in the study of B-lymphocytes because many of the developmental events in lymphopoesis are controlled by transcriptional regulation 1, 2. In the later stages of B cell maturation, triggering via CD40L is essential for the induction of B cell growth, entry into the cell cycle, and proliferation 11, 20. As shown in Figure 2, B cells can be stimulated in vitro with anti-CD40 and IL4 to undergo this burst of proliferation, and retroviral vectors are useful tools to over-express proteins that may function during these stages of B cell development. In the example shown in Fig. 2, we demonstrate that AID-deficient B cells that are unable to undergo CSR (here to IgG1) can be rescued for this function by retrovirally introducing the mouse AID gene. This approach allows us to track for GFP-positive cells (see Figure 1) that have undergone CSR (IgG1+GFP+).
This method is advantageous over mouse modeling in that it can be completed in less than one week after splenic harvest as opposed to the time investment to create a transgenic mouse. The type of analysis can be tailored to the investigator’s applications; here we have presented analysis of class switch recombination by flow cytometry as in Figure 2. In addition, this method is suitable for a range of biochemical approaches following protein extraction from the retrovirally-transduced cells 15, 21.
Declarações
The authors have nothing to disclose.
Acknowledgements
C.K. is supported by Columbia University Graduate program. U.B. is Fellow of the Leukemia and Lymphoma Society of America, the recipient of New Investigator Award from the Leukemia Research Foundation and is supported by Columbia University New Faculty start-up funds.
Materials
| Material Name | Tipo | Company | Catalogue Number | Comment |
|---|---|---|---|---|
| FCS | Atlanta Biologicals | S11550 | ||
| RPMI | Invitrogen/Gibco | 22400 | ||
| PBS | Invitrogen/Gibco | 20012 | ||
| Red Blood Cell (RBC) lysis buffer | Sigma Aldrich | R7757 | ||
| CD43 beads | Miltenyi | |||
| B Cell Complete Media | Various components | Various Components | RPMI, 15% FCS, 1% Non-Essential Amino Acids, 1% Sodium Pyruvate, 1% HEPES, 1% Pen-Strep, 50μM β-Mercaptoethanol | |
| IL-4 | ||||
| Anti-CD40 | BD Pharmigen | 553787 | ||
| polybrene | Sigma Aldrich | 107689 | ||
| Chloroquine diphosphate salt | Sigma Aldrich | C6628 | Used at 100mM | |
| Phoenix Eco cells (Murine) | Orbigen | RVC-10002 | ||
| PE-Cy5-α-mouse-CD45R (B220) | eBioscience | 15-0452-81 | ||
| PE-α-mouse-IgG1 | BD Pharmigen | A85-1 |
Referências
- Bartholdy, B., Matthias, P. Transcriptional control of B cell development and function. Gene. 327, 1-23 (2004).
- Henderson, A., Calame, K. Transcriptional regulation during B cell development. Annu Rev Immunol. 16, 163-200 (1998).
- Graf, T. Differentiation plasticity of hematopoietic cells. Blood. 99, 3089-30101 (2002).
- Xiao, C., Rajewsky, K. MicroRNA control in the immune system: basic principles. Cell. 136, 26-36 (2009).
- Waterston, R. H. Initial sequencing and comparative analysis of the mouse genome. Nature. 420, 520-562 (2002).
- Griffiths, A. J. F., Miller, J. H., Suzuki, D. T., Lewontin, R. C., Gelbart, W. M. . Introduction to Genetic Analysis. , (2000).
- Gondo, Y. Next-generation gene targeting in the mouse for functional genomics. BMB Rep. 42, 315-3123 (2009).
- Plachy, J. Proviruses selected for high and stable expression of transduced genes accumulate in broadly transcribed genome areas. J Virol. 84, 4204-4211 (2010).
- Felice, B. Transcription factor binding sites are genetic determinants of retroviral integration in the human genome. PLoS One. 4, e4571-e4571 (2009).
- Karavanas, G. Cell targeting by murine retroviral vectors. Crit Rev Oncol Hematol. 28, 7-30 (1998).
- Noelle, R. J. A 39-kDa protein on activated helper T cells binds CD40 and transduces the signal for cognate activation of B cells. Proc Natl Acad Sci U S A. 89, 6550-6554 (1992).
- Muramatsu, M. Class switch recombination and hypermutation require activation-induced cytidine deaminase (AID), a potential RNA editing enzyme. Cell. 102, 553-563 (2000).
- Yelle, J., Dion, M., Hamelin, C. Efficient transfection of mammalian cells with viral DNA in optimal culture conditions. J Virol Methods. 7, 321-326 (1983).
- Chen, C., Okayama, H. High-efficiency transformation of mammalian cells by plasmid DNA. Mol Cell Biol. 7, 2745-2752 (1987).
- Fagarasan, S. In situ class switching and differentiation to IgA-producing cells in the gut lamina propria. Nature. 413, 639-6343 (2001).
- Basu, U. The AID antibody diversification enzyme is regulated by protein kinase A phosphorylation. Nature. 438, 508-5011 (2005).
- McBride, K. M. Regulation of class switch recombination and somatic mutation by AID phosphorylation. J Exp Med. 205, 2585-2594 (2008).
- Barreto, V. M. AID from bony fish catalyzes class switch recombination. J Exp Med. 202, 733-738 (2005).
- Delphin, S., Stavnezer, J. Regulation of antibody class switching to IgE: characterization of an IL-4-responsive region in the immunoglobulin heavy-chain germline epsilon promoter. Ann N Y Acad Sci. 764, 123-135 (1995).
- Castigli, E. CD40 expression and function in murine B cell ontogeny. Int Immunol. 8, 405-411 (1996).
- Ballantyne, J. Efficient recombination of a switch substrate retrovector in CD40- activated B lymphocytes: implications for the control of CH gene switch recombination. J Immunol. 161, 1336-1347 (1998).

