Assaying Surface Expression of Chemosensory Receptors in Heterologous Cells
Summary
Here we demonstrate a protocol to carry out live cell staining that can be used to detect odorant receptors on the surface of HEK293T cells conveniently. In addition, it may also be used to assay for surface expression of other chemosensory receptors or GPCRs.
Abstract
The vivid world of odors is recognized by the sense of olfaction. Olfaction in mice is mediated by a repertoire of about 1200 G Protein Coupled Receptors (GPCRs) 1 that are postulated to bind volatile odorant molecules and converting the extracellular signal into an intracellular signal by coupling with G protein Gαolf. Binding of the odorants to the receptors is thought to follow a combinatorial rule, that is, one odorant may bind several receptors and one receptor may bind several odorants to varying degrees 2. Biochemical, signaling and ligand binding studies have been conveniently carried out for most GPCRs using heterologous cells. However use of heterologous cells for study of odorant receptors, was precluded for a long time since on transfection they failed to export to the surface. Saito et al have demonstrated single membrane pass Receptor Transporting Protein (RTP) family chaperones show enhanced expression in the olfactory sensory neurons and act as chaperones to traffic odorant receptors to the surface in heterologous cells, when co transfected together 3. To carry out biochemical assays for receptors using heterologous cells, one must first determine if the receptor shows robust surface expression in the cell line. This can be assayed by overexpressing the receptors with the chaperone RTP1S followed by live cell staining to fluorescently label the extracellular domain or a tag in the extracellular domain exclusively. Here we demonstrate a protocol to carry out live cell staining that can be used to detect odorant receptors on the surface of HEK293T cells conveniently. In addition, it may also be used to assay for surface expression of other chemosensory receptors or GPCRs.
Protocol
1. Procedure:
The procedure comprises of three parts completed over a total time of 3 days: transferring cells, transfection and immunocytochemistry, one step carried out per day. Transfer and transfection of cells over the first two days must be carried out in sterile conditions in a laminar flow chamber.
Day 1: Transfer of HEK293T cells for surface expression assay.
- Transfer medium (M10): Mix minimum essential medium supplemented with 10% (by volume) fetal bovine serum in sterile condition.
- Grow cells to a desired confluency in a 100mm culture plate, depending on the number of plates required to set up. It is important to determine the fraction of cells to be transferred to avoid overgrown or sparse cells: for instance, from a 100% confluent 100 mm dish, one may transfer about 3% to a 35 mm dish to obtain approximately 30% confluency in the latter.
- Prior to transferring cells, in the sterile laminar flow chamber, coat 22 X 22 mm glass cover slips with sterile poly D lysine (1mg/ mL) and place one in each 35 mm cell culture dish, coated side up for plating cells (Figure 1). Allow the solution to dry for 5- 10 minutes. During this interval, the UV source in the laminar chamber may be turned on to sterilize the cover slips, if desired. Poly D lysine helps heterologous cells stick to the cover slips.
- To transfer cells, aspirate out the existing media in the 100mm dish, wash by carefully adding 8 mL sterile PBS, aspirate PBS and trypsinize the cells using 3 mL 0.05% Trypsin-EDTA; when cells detach from the dish, block further enzymatic reaction by adding 5 mL M10. Triturate the cells and transfer requisite volume to a sterile conical tube; centrifuge at 200g for 5 minutes to pellet the cells. Aspirate out supernatant containing Trypsin-EDTA, leaving the pellet of cells intact. To transfer the cells to 35 mm dishes, add 1 mL M10 for each dish and triturate to dissociate the cells. Add 1 mL of the resuspended cells to each dish. Incubate cells overnight at 37°C, 5% CO2.

Figure 1. Coat 22 X 22 mm glass coverslips with poly D lysine and place one coated side up in each 35 mm dish.
Day 2: Transfection of HEK293T cells.
- 18-24 hours after transferring, cells are ready for transfection of the receptor to be assayed. Use receptor and RTP1S cloned in mammalian expression vector pCI, prepared from bacterial cultures using Qiagen Miniprep Kit following protocol described before4. Use receptors that are N terminally tagged with 20 amino acid long rhodopsin epitope to facilitate immunostaining.
- Use 1000ng of receptor construct, 250 ng of RTP1S construct and 50 ng of green fluorescence protein each transfection. Perform transfection using Lipofectamine 2000, as per manufacturers manual.
Day 3: Immunocytochemistry to visualize cell surface stained receptors.
- Prior to starting, prepare staining and washing solutions and cool them to 4°C. Staining medium: Minimum Essential Medium, 45 mL; Fetal Bovine Serum, 5 mL; HEPES (1M), 500 μL (final concentration 10mM); Sodium Azide (1.5M), 500 μL (final concentration 15mM). Wash solution: HBSS, 500 mL; HEPES (1M), 5 mL (final concentration 10mM); Sodium Azide (1.5M), 5 mL (final concentration 15mM). Both solutions may be stored at 4°C for reuse. Prepare antibody solutions by diluting required antibodies in the staining medium 100 fold.
- Start immunochemistry for surface staining for odorant receptors about 24 hours post transfection. To perform live cell staining, the experimental set up must be cooled to 4°C: fill a large contained with ice and pat the surface of ice to make it as even as possible, place a tray on the ice and spray it with 70% ethanol. Cut a piece of parafilm (smaller than the length of the tray) and affix it on the ethanol sprayed tray, to obtain a smooth surface. Transfer the glass cover slips containing transfected cells on the parafilm, one at a time, cell side up, using tweezers. Layer each cover slip with 100 μL cold primary staining solution, extruding carefully from a corner of the slip (Figure 2). After layering all the cover slips with antibody solution, cover the tray to prevent drying up of the solution in air. Carry out primary incubation for 45-60 minutes.
- After primary incubation, quickly transfer cover slips to the original 35mm culture dishes, containing the media. Aspirate the media and add 2 mL chilled wash solution gently from the edge of the dish to prevent losing cells from the cover slip. Aspirate the wash solution and repeat twice to complete three washes. At the end of the third wash, do not aspirate the wash solution out.
- Prepare secondary antibody solution in the staining media and transfer the cover slips from the wash solution to a newly affixed parafilm layer in the same tray. Again gently extrude 100 μL of antibody solution to layer each cover slip, from the corner like the primary incubation. Carry out secondary incubation for 30- 45 minutes and transfer back to the wash solution in the original 35 mm dishes. Once more, wash the cover slips three times as described.
- Aspirate wash solution from last wash and add 2 mL 1% chilled paraformaldehyde. Fix cells in chilled 1% paraformaldehyde solution, on ice for 15 minutes.
- Mount the cover slips on clean microscope glass slides using mounting reagent Mowiol, with the cell side down and cautiously exclude all air bubbles (Figure 3). Once Mowiol air dries, wash cover slips gently by adding distilled water on the surface and aspirating immediately.

Figure 2. Layer each coverslip with 100 μL cold primary staining solution.
Figure 3. Mount the coverslips with the cell side down on clean glass microscope slides using Mowiol mounting reagent.
2. Representative Results:
Live cell staining enables one to visualize proteins on the surface of cells, on transfection of receptors with chaperones (in this case odorant receptor Olfr62, with Receptor Transporting Protein RTP1S) (Figure 4). Cell surface staining of receptors is characterized by punctate pattern of staining (Figure 4B, inset). Staining carried out inappropriately may lead to higher noise, making it difficult for the observer to distinguish the real surface staining from noise.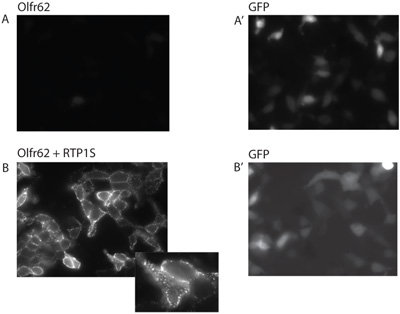
Figure 4. Transfection of receptors with chaperones allows for the visualization of proteins on the surface of cells using live cell staining. Transfection of the odorant receptor Olfr62 alone (A) and transfection of Olfr62 with RTP1S chaperone (B). GFP expression levels serve as a transfection control (A’ and B’).
Discussion
Each step must be carried out carefully to ensure prominent and distinctive surface staining. The entire staining process must be carried out in cold (on ice), and the tray on which cover slips are laid must be cooled prior to use to ensure cells stay alive. Moreover fixation is done at the end of the surface staining process. This is in sharp contrast with internal staining where fixation precedes staining to permeabilize the cell membrane to primary and secondary antibodies. Time of exposure of cover slips to air must be minimized to prevent the cells from drying up; therefore layering of cover slips with antibody solutions, transferring cover slips to wash solution, exchange of wash solution between washes must be done quickly and one at a time when handling multiple cover slips.
Depending on the epitope tag and antibodies one uses, one may have to adjust incubation time, number of washes, and fold of antibody dilution. Background noise can be avoided by diluting the antibodies more or increasing the number of washes, or shortening incubation time.
Declarações
The authors have nothing to disclose.
Acknowledgements
We thank the members of Matsunami laboratory for critical reading of this manuscript.
Materials
| Material Name | Tipo | Company | Catalogue Number | Comment |
|---|---|---|---|---|
| Cover slip 22 X 22 mm (#1) | Thermo Scientific | 72200-11 | ||
| Fetal bovine serum | Gibco | 16000 | ||
| Lipofectamine 2000 | Invitrogen | 11668-019 | ||
| Minimal essential medium | Sigma | M4655 | With L glutamate, Earle’s salt and bicarbonate | |
| Mowiol | Calbiochem | 475904 | ||
| Paraformaldehyde | EMS | 19208 | ||
| Hanks Balanced Salt Solution (HBSS) 1X | Gibco | 14025 | With calcium chloride and magnesium chloride | |
| HEPES Buffer Solution | Gibco | 15630 | ||
| Phosphate Buffer Saline (PBS) | Cellgro | 21-040-CV | Without calcium and magnesium | |
| Poly D Lysine Hydrobromide | Sigma | P7280 | ||
| Sodium Azide | EM Science | SX0299-1 | ||
| Tissue culture dish 35 X 10 mm | Falcon | 353801 | ||
| Trypsin-EDTA | Gibco | 25300 | 0.05% |
Referências
- Buck, L., Axel, R. A novel multigene family may encode odorant receptors: a molecular basis for odor recognition. Cell. 65, 175-187 (1991).
- Malnic, B., Hirono, J., Sato, T., Buck, L. B. Combinatorial receptor codes for odors. Cell. 96, 713-723 (1999).
- Saito, H., Kubota, M., Roberts, R. W., Chi, Q., Matsunami, H. RTP family members induce functional expression of mammalian odorant receptors. Cell. 119, 679-691 (2004).
- Zhuang, H., Matsunami, H. Evaluating cell-surface expression and measuring activation of mammalian odorant receptors in heterologous cells. Nat Protoc. 3, 1402-1413 (2008).

