Use of a Hanging-weight System for Isolated Renal Artery Occlusion
Summary
A precise murine model for acute kidney injury (AKI) due to ischemia is an important tool to investigate acute kidney injury and possibly find therapeutic tools to treat renal injury. The hanging weight system offers a tool for immediate and reliable renal artery occlusion and reperfusion without causing renal congestion.
Abstract
In hospitalized patients, over 50% of cases of acute kidney injury (AKI) are caused by renal ischemia 1-3. A recent study of hospitalized patients revealed that only a mild increase in serum creatinine levels (0.3 to 0.4 mg/dl) is associated with a 70% greater risk of death than in persons without any increase 1. Along these lines, surgical procedures requiring cross-clamping of the aorta and renal vessels are associated with a renal failure rates of up to 30% 4. Similarly, AKI after cardiac surgery occurs in over 10% of patients under normal circumstances and is associated with dramatic increases in mortality. AKI are also common complications after liver transplantation. At least 8-17% of patients end up requiring renal replacement therapy 5. Moreover, delayed graft function due to tubule cell injury during kidney transplantation is frequently related to ischemia-associated AKI 6. Moreover, AKI occurs in approximately 20% of patients suffering from sepsis 6.The occurrence of AKI is associated with dramatic increases of morbidity and mortality 1. Therapeutic approaches are very limited and the majority of interventional trials in AKI have failed in humans. Therefore, additional therapeutic modalities to prevent renal injury from ischemia are urgently needed 3, 7-9.
To elucidate mechanisms of renal injury due to ischemia and possible therapeutic strategies murine models are intensively required 7-13. Mouse models provide the possibility of utilizing different genetic models including gene-targeted mice and tissue specific gene-targeted mice (cre-flox system). However, murine renal ischemia is technically challenging and experimental details significantly influence results. We performed a systematic evaluation of a novel model for isolated renal artery occlusion in mice, which specifically avoids the use of clamping or suturing the renal pedicle 14. This model requires a nephrectomy of the right kidney since ischemia can be only performed in one kidney due to the experimental setting. In fact, by using a hanging-weight system, the renal artery is only instrumented once throughout the surgical procedure. In addition, no venous or urethral obstruction occurs with this technique. We could demonstrate time-dose-dependent and highly reproducible renal injury with ischemia by measuring serum creatinine. Moreover, when comparing this new model with conventional clamping of the whole pedicle, renal protection by ischemic preconditioning is more profound and more reliable. Therefore his new technique might be useful for other researchers who are working in the field of acute kidney injury.
Protocol
General remarks. All operations should be performed under an upright dissecting microscope (Leica) and by using a surgical coagulator. The mice in the experimental groups should be matched in age and weight to ensure comparability of the results. Temperature, blood pressure, anesthesia and fluid administration should be stable and monitored during the experiment.
1. Anesthesia and surgery
- Renal ischemia experiments can either be performed with simultaneously applying ischemia and reperfusion to both kidneys, or after single nephrectomy in combination with ischemia treatment of the remaining kidney. As bilateral ischemia treatment of both kidneys is associated with increased technical difficulties, a more complex experimental setting and longer surgery, we will perform a right nephrectomy followed by ischemia treatment of the remaining left kidney by utilizing a hanging weight system. We utilized this hanging weight system also for coronary artery occlusion in the heart. However, the procedure is different since the renal artery can be isolated from the surrounding tissue whereas the coronary artery lies within the heart muscle.
- Mice (8-14 weeks of age, either sex) will be anesthetized with pentobarbital at a dose of 70mg/kg. We will maintain anesthesia with approx. 10 mg/kg/h sodium pentobarbital. Overdosing can significantly lower blood pressure and thus can alter the results. In our initial studies we also used Ketamine and xylazine. However, these compounds induced a strong bradycardia (150 /min). Beside the observed bradycardia there is also evidence that both compounds are involved in protective mechanisms during conditions of ischemia. Volatile anesthetics cannot be used either due to their capability to induce also renal protection and low blood pressure. Therefore our final choice for anesthesia was pentobarbital. In addition, ketamine and xylazine are primarily excreted by the kidneys. Nephrectomy and ischemia may prolong ketamine anesthesia.
- Once pain reflexes are absent (tested by squeezing the toes with tweezers) mice will be placed on a temperature-controlled heating table (RT, Effenberg, Munich, Germany to maintain body temperature at 37.0°C) in a left lateral decubitus position.
- Attach the lower and upper extremities to the table by using tape.
- For non-survival surgeries, such as shown in the video demonstration, cover the incision area with mineral oil to prevent inhalation of mouse hair by cutting the skin and thus prevent the risk of mouse hair allergies. In case of survival experiments proper aseptic techniques including clipping of hair, disinfection of skin with iodophors (e.g. Betadine), sterile handling of autoclaved instruments and using masks a sterile gloves for the surgeon are required. Mineral oil is not used in survival surgeries. For survival surgeries sterile NaCl is used
- Right flank incision will be performed with a scissor and a coagulation electrode (Erbe, ICC50, Germany) to prevent bleeding of muscle and skin vessels. In contrast to accessing the kidneys via midline laparotomy, this technique does not require access to the peritoneal cavity.
- The renal pedicle including renal artery and renal vein are ligated using a 4/0 silk suture and the right kidney will be cut out with a scissor without interfering with the adrenal vessels.
- The surgical wound is closed using continuous suture (two stitches) of the muscle wall and skin with a 4/0 suture.
- Animals are then placed in a right lateral decubitus position and a left flank incision will be performed.
- The left kidney is carefully removed from the connective tissues, avoiding the adrenal gland and vessels.
- Next, the kidney is turned with its ventral side down into a lucite cup. The lucite cup should be sterilized for survival surgeries. The pole of the kidney is carefully pushed into the lucite cup by holding the surrounding tissue with a forceps. Thereafter the pole is carefully pushed into the lucite cup with a forceps. The kidney will be kept wet and warm with a wet swab soaked with water at 37.0°C.
- Following exposure of the left kidney and placement into a lucite cup the kidney holder is restrained ventrally to have tension on the renal vessels. A sufficient restraining is important for a successful placement of the suture under the renal artery. Thereafter the kidney holder is fixed and the renal artery can be easily identified as it runs on top of the renal vein.
- The renal artery is dissected from adjacent tissues, close to its take-off from the abdominal aorta with fine forceps. The artery is dissected from the underlying vein with a curved forceps at the very top.
- An 8/0 silk suture (Ethicon, Norderstedt, Germany) is placed under the renal artery by using one forceps as a leading guide. This technique allows interruption of only the arterial blood flow to the kidney without compression of the renal vein, as compared to clamping of the renal pedicle.
- The suture is placed over a small pole (can be a prepared safety pin) and a weight of approx. 1g is attached to each end (eppendorf tube). The weight can be adapted to the kidney size by taking out or adding water into the eppendorf tubes. However, the artery should be slightly pulled upwards, so that no blood is running through the artery but without major stretch on the artery. Major stretch stress on the artery can cause vessel wall damage with thickening of the arterial wall and reduced reperfusion.
- While the weights are unsupported, the renal artery is immediately occluded. In contrast, when the weights are supported, blood flow is immediately restored to the organ. Successful occlusion is confirmed by a change of color from red to white.
- Different ischemia times can be performed as required by the experiment. A complete reperfusion will take place depending on the length of the ischemia time. Ischemia times up to 45 minutes allow recoverable kidney injury. In our experimental settings we used 20 up to 30 minutes of ischemia with a medium renal injury which allows us to see improvement as well as impairment of kidney damage depending on the mouse strain and the treatment.
- After complete reperfusion the kidney can be replaced out of the lucite cup. Therefore the kidney holder is unfixed and the tension on the kidney is released. The cranial pole is gently removed out of the cup with a bland forceps and the caudal pole follows immediately.
- The fixation (tapes) of the mouse can be released and the mouse can be placed in a supine position for e.g. FITC-inulin labeled clearance.
- In subsets of experiments, blood pressure and heart rate can be recorded throughout the surgical procedure. Blood pressure measurements are obtained continuously via a carotid artery catheter. In brief, the carotid artery is exposed via blunt dissection of the paratracheal muscles. Following further exposure and careful avoidance of tissue trauma (particular of the vagal nerve), a catheter is inserted into the vessel using two sutures and a small clamp. Blood pressure is measured with a statham element (WK 280, WKK, Kaltbrunn, Switzerland).
- For FITC-labeled inulin clearance a catheter has to be placed into the left jugular vein for infusion of sodium chloride and FITC-labeled inulin. Furthermore a bladder catheter has to be inserted for urine collection in a time dependent fashion as explained below in detail.
2. Measurement of kidney injury
- In general: The following outcome parameters are recommended to determine kidney injury following renal ischemia:
Perform FITC-labeled inulin clearance to assess the glomerular filtration rate (GFR). As an alternative determine creatinine clearance and serum creatinine via metabolic cage investigation 24 hours following renal injury. Perform renal H&E staining and a myeloperoxidase (MPO) ELISA to determine renal damage and inflammation 24 hours following renal ischemia. - Metabolic cage investigations:
- Following ischemia the left kidney is turned back into the retroperitoneal position and the wound is closed as described above.
- At the end of surgery mice receive 0.3 ml normal saline IP and recover for 2 hours under a heating lamp.
- Thereafter mice will be placed into metabolic cages (Tecniplast Deutschland, Hohenpeissenberg, Germany) for determination of renal functional parameters for 2 times of 24 hours.
- The water intake of each mouse has to be determined by weighing the empty and filled bottle with water before supply to the mouse and than 24 hours later by weighing the full bottle again. The mouse weight has to be determined as well.
- After 24 hours approximately 75μl blood are taken from the venous plexus behind the eye via a capillary tube under short isoflorane anesthesia.
- Renal function is assessed by measuring plasma and urine creatinine 24 hours after renal ischemia using a commercially available colorimtric method according to the manufacturer`s protocol (LT-SYS, Labor + Technik, Berlin, Germany). Plasma and urine concentrations of Na+, K+ are determined with a flame-emission photometer (ELEX 6361, Eppendorf AG, Hamburg, Germany). Renal excretory and hemodynamic values are calculated using standard formulas15-17.
- Inulin clearance: FITC-inulin clearance experiments will be performed following renal ischemia.
- The left jugular vein is cannulated for continuous infusion. After dissecting the vein from adjacent tissue with a preparation scissor (Dumont, WPI) place two sutures (4/0) around the vein. Place a clamp on the vein before the proximale suture to stop blood flow. Thereafter close the distal knot and place a weight on one of the distal suture ends to strain tension on the vein. This is very important to get the catheter in the vein. Use a micro-scissor to cut a small opening into the vein. Hold the opening with a fine forceps (Dumont, WPI) and place the catheter into the vein. To advance the catheter take the clamp off. Make sure that there is no tension on the catheter so that you can place it down without holding it. Thereafter close the second proximal suture over the vein and the catheter.
- A catheter is placed in the urinary bladder for timed urine collection. Therefore you cut a short incision in the skin over the bladder. Carefully remove the bladder through the skin hole. Fix the bladder with a wet tissue. Place a prepared suture over the bladder (4/0).
- Cut a short piece of a catheter (1.5cm) and heat on end to get a ring around the catheter which makes it easier to fix it in the bladder.
- Cut a hole into the bladder at its very top. Make the cut between the vessels to prevent bleeding. Insert the big end of the catheter into the bladder and close the suture over the catheter and the bladder.
- All mice receive a bolus of 0.85% sodium chloride solution in an amount equal to 20% of body weight. Continuous infusion is maintained at a rate of 800ul/h/25g body weight and FITC-inulin is added to the infusion for evaluation of whole kidney glomerular filtration rate (GFR). After stabilization of the animals for 20 min, 20 min timed urine collections are performed (four times) for determination of urinary flow rate and FITC-inulin. Blood is obtained in the middle of every period for measurement of FITC-inulin.
- When starting the collection periods for 20 minutes use a timer. Collect the urine by placing the end of the bladder catheter into a small eppendorf tube. Measure the tube weight before and after urine collection so that you can determine the urine amount. Blood samples are taken via retroorbital blood collection form the venous plexus via capillary tubes in the middle of each collection period.
- Blood and the kidney can be collected after each procedure for further analysis e.g. MPO ELISA, cytokine EISA, histology.
3. Representative Results:
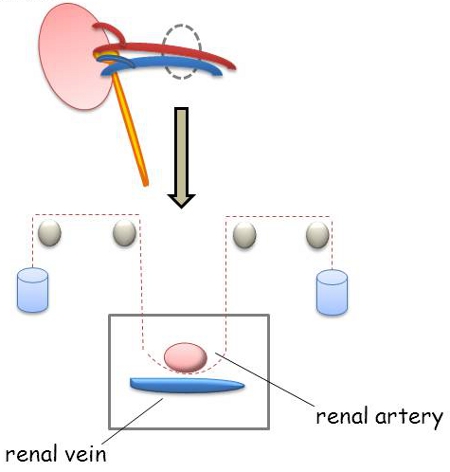
Figure 1. Model of a hanging-weight system for renal ischemia: A hanging weight system has been established to induce renal ischemia of the left kidney. Therefore an 8.0 nylon suture was placed under the renal artery and at the end of each suture a small weight (1g) was attached. This experimental design allows reversible occlusion of the renal artery by applying and releasing the weight load.
Discussion
In summary, the present study describes a novel technique for performing renal ischemia in a murine model using a hanging-weight system for renal artery occlusion. In fact, this study demonstrates highly reproducible injury, thus minimizing the variability associated with clamping of the renal pedicle.
Declarações
The authors have nothing to disclose.
Acknowledgements
The present studies were supported by United States National Institutes of Health grant R01-HL092188, R01-DK083385 and R01HL098294, and Foundation for Anesthesia Education and Research (FAER) Grants to HKE, a FAER grant to JYD and a DFG (Deutsche Forschungsgemeinschaft) Research Fellowship (GR2121/1-1) to AG.
Materials
| Name of the reagent | Company | Catalogue number | Comments |
|---|---|---|---|
| Sodium Pentobarbital | Vortech Pharmaceutical Ls, Ltd | V.P.L. 9372 | 4mg/mL in saline |
| Suture, silk 4.0 | Harvard Apparatus | 517698 | |
| Suture, Prolene 8.0 | Ethicon, USA | M8739 | reusable |
| dissecting microscope | Leica | n/a | consider generous working distance |
| Heating Table | Rt, Effenberger, Germany | n/a | only and single provider |
| Surgical instruments | WPI, Dumont | a fine microvessel scissor |
Referências
- Abuelo, J. G. Normotensive ischemic acute renal failure. N Engl J Med. 357, 797-805 (2007).
- Thadhani, R., Pascual, M., Bonventre, J. V. Acute renal failure. N Engl J Med. 334, 448-460 (1996).
- Lameire, N. The pathophysiology of acute renal failure. Crit Care Clin. 21, 197-210 (2005).
- Gelman, S. The pathophysiology of aortic cross-clamping and unclamping. Anesthesiology. 82, 1026-1060 (1995).
- Yalavarthy, R., Edelstein, C. L., Teitelbaum, I. Acute renal failure and chronic kidney disease following liver transplantation. Hemodial Int. 11, S7-S12 (2007).
- Schrier, R. W., Wang, W. Acute renal failure and sepsis. N Engl J Med. 351, 159-169 (2004).
- Grenz, A., Osswald, H., Eckle, T. The Reno-Vascular A2B Adenosine Receptor Protects the Kidney from Ischemia. PLoS Medicine. 5, e137-e137 (2008).
- Grenz, A., Zhang, H., Hermes, M. Contribution of E-NTPDase1 (CD39) to renal protection from ischemia-reperfusion injury. FASEB J. 21, 2863-2873 (2007).
- Grenz, A., Zhang, H., Eckle, T. Protective role of ecto-5′-nucleotidase (CD73) in renal ischemia. J Am Soc Nephrol. 18, 833-845 (2007).
- Akcay, A., Nguyen, Q., Edelstein, C. L. Mediators of inflammation in acute kidney injury. Mediators Inflamm. , 137072-137072 (2009).
- Faubel, S., Ljubanovic, D., Poole, B. Peripheral CD4 T-cell depletion is not sufficient to prevent ischemic acute renal failure. Transplantation. 80, 643-649 (2005).
- Wei, Q., Bhatt, K., He, H. Z., Mi, Q. S., Haase, V. H., Dong, Z. Targeted deletion of Dicer from proximal tubules protects against renal ischemia-reperfusion injury. J Am Soc Nephrol. 21, 756-761 (2010).
- Joo, J. D., Kim, M., Horst, P. Acute and delayed renal protection against renal ischemia and reperfusion injury with A1 adenosine receptors. Am J Physiol Renal Physiol. 293, F1847-F1857 (2007).
- Grenz, A., Eckle, T., Zhang, H. Use of a hanging-weight system for isolated renal artery occlusion during ischemic preconditioning in mice. Am J Physiol Renal Physiol. 292, F475-F485 (2007).
- Qi, Z., Whitt, I., Mehta, A. Serial determination of glomerular filtration rate in conscious mice using FITC-inulin clearance. Am J Physiol Renal Physiol. 286, 590-596 (2004).
- Lorenz, J. N., Gruenstein, E. A simple, nonradioactive method for evaluating single-nephron filtration rate using FITC-inulin. Am J Physiol. 276, 172-177 (1999).
- Lorenz, J. N. A practical guide to evaluating cardiovascular, renal, and pulmonary function in mice. Am J Physiol Regul Integr Comp Physiol. 282, 1565-1582 (2002).

