Orthotopic Xenografting of Human Luciferase-Tagged Malignant Peripheral Nerve Sheath Tumor Cells for in vivo Testing of Candidate Therapeutic Agents
Summary
A method for reliably grafting luciferase-tagged human malignant peripheral nerve sheath tumor cells into the sciatic nerve of immunodeficient mice is described. The use of bioluminescence imaging to demonstrate proper establishment of tumor grafts and criteria for random segregation of animals into study groups are also discussed.
Abstract
Although in vitro screens are essential for the initial identification of candidate therapeutic agents, a rigorous assessment of the drug’s ability to inhibit tumor growth must be performed in a suitable animal model. The type of animal model that is best for this purpose is a topic of intense discussion. Some evidence indicates that preclinical trials examining drug effects on tumors arising in transgenic mice are more predictive of clinical outcome1and so candidate therapeutic agents are often tested in these models. Unfortunately, transgenic models are not available for many tumor types. Further, transgenic models often have other limitations such as concerns as to how well the mouse tumor models its human counterpart, incomplete penetrance of the tumor phenotype and an inability to predict when tumors will develop.
Consequently, many investigators use xenograft models (human tumor cells grafted into immunodeficient mice) for preclinical trials if appropriate transgenic tumor models are not available. Even if transgenic models are available, they are often partnered with xenograft models as the latter facilitate rapid determination of therapeutic ranges. Further, this partnership allows a comparison of the effectiveness of the agent in transgenic tumors and genuine human tumor cells. Historically, xenografting has often been performed by injecting tumor cells subcutaneously (ectopic xenografts). This technique is rapid and reproducible, relatively inexpensive and allows continuous quantitation of tumor growth during the therapeutic period2. However, the subcutaneous space is not the normal microenvironment for most neoplasms and so results obtained with ectopic xenografting can be misleading due to factors such as an absence of organ-specific expression of host tissue and tumor genes. It has thus been strongly recommended that ectopic grafting studies be replaced or complemented by studies in which human tumor cells are grafted into their tissue of origin (orthotopic xenografting)2. Unfortunately, implementation of this recommendation is often thwarted by the fact that orthotopic xenografting methodologies have not yet been developed for many tumor types.
Malignant peripheral nerve sheath tumors (MPNSTs) are highly aggressive sarcomas that occur sporadically or in association with neurofibromatosis type 13and most commonly arise in the sciatic nerve4. Here we describe a technically straightforward method in which firefly luciferase-tagged human MPNST cells are orthopically xenografted into the sciatic nerve of immunodeficient mice. Our approach to assessing the success of the grafting procedure in individual animals and subsequent non-biased randomization into study groups is also discussed.
Protocol
1. Initial Preparation of Non-nude Immunodeficient Mice (not Necessary for Nude Strains):
- All procedures were reviewed and approved in advance by the UAB Institutional Animal Care and Use Committee and conducted by properly trained personnel. We have successfully used three strains of immunodeficient mice for these experiments: a) Foxchase outbred SCID mice (CB17/lcr-Prkdcscid/lcrCrl mice); b) NIH III mice (NIHS-LystbgFoxn1nuBrkxid mice); and c) NOD-SCIDγ mice (NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ mice). In our hands, the growth of orthotopic xenografts is virtually identical in NIH III and NOD-SCIDγ mice, both of which carry mutations affecting the function of T cells, B cells and NK cells; for this protocol, the number of days required for graft growth, the time at which therapy is initiated and the times post-grafting when imaging is performed are those we have empirically determined using NOD-SCIDγ mice. Graft growth in Foxchase outbred SCID mice, which have defects in T- and B-cell function, typically takes longer.
- To facilitate grafting of large numbers of non-nude immunodeficient mice, we remove hair from the surgical site on the afternoon prior to grafting. Mice are anesthetized using an isoflurane induction chamber attached to a vaporizer with scavenge for the waste gas. To maintain body temperature, animals are handled on a heating pad throughout this process. Clippers are used to remove hair from the mid-back down to the knee of the hind leg on the side which is to be grafted. Any remaining hair is carefully removed from the site in ~5 minutes using a chemical depilatory agent (e.g. Nair, in particular a product with aloe vera for sensitive skin). Removal of the depilatory product is recommended as it can lead to irritation of the skin, eyes, and other body surfaces to which it might spread. We do not graft both sciatic nerves in these experiments as this may bilaterally impair hindleg function, resulting in the loss of mice from the study groups.
- Mice are allowed to fully recover on a heating pad in an isolation cage without bedding; this both ensures that the mice are not injured by other, more fully alert animals and prevents them from accidentally inhaling bedding. When mice are fully mobile and show no evidence of grogginess, they can be reintroduced into a cage with other mice.
2. Preparation of MPNST Cells for Injection on the Day of Grafting
- In this protocol, the specific numbers of cells grafted and the times required for tumor growth post-grafting are those we have empirically determined for ST88-14 cells, a commonly used MPNST line that was derived from an NF1-associated tumor5. The cells we use for grafting have been transduced with a lentiviral vector containing a CMV-luciferase-IRES-puromycin cassette and clones stably expressing firefly luciferase identified by bioluminescence imaging6. We have also performed grafting experiments with MPNST cells stably transfected with plasmids expressing firefly luciferase under the control of the CMV immediate early promoter. We do not recommend this as we have found that these plasmid vectors are prone to undergo extinction of expression following grafting.
- ST88-14 cells are grown in Dulbecco’s minimal essential medium supplemented with 10% fetal calf serum, 10 μg/mL streptomycin and 10 IU/mL penicillin (DMEM10); 5 μg/ml puromycin is also included to maintain selection for the lentiviral vector. We have maintained these cells in our laboratory for 50 passages and have not observed any variation in cell growth in any of these passages. 9 x 105 ST88-14 cells are plated in a T175 flask and grown for 3 days, at which point the flask is approximately 80% confluent. To graft 35 mice, a single 80% confluent T175 flask is required; at this density, our average yield for ST88-14 cells is 7.3 x 106 cells per T175 flask. It is critically important that the cells not be allowed to grow to confluence prior to harvesting for grafting. We have found that grafting cells harvested from confluent flasks results in a higher degree of graft failure and often prolongs the in vivo course of graft growth.
- ST88-14 cells are rinsed once with room temperature Hanks’ balanced salt solution. Cells are removed from the flask using Cell Stripper cell dissociation solution for 30 seconds to 1 minute at room temperature. Five milliliters of DMEM10 (Note: puromycin is not included in this medium) per each milliliter of Cell Stripper used are then added to the cells.
- Cells are counted using a hemocytometer. 5 x 103 ST88-14 cells suspended in 3 μL will be injected per mouse. A quantity of cells sufficient to graft the entire cohort, with allowance for pipetting error (e.g., for 35 mice, we typically transfer a number of cells sufficient to graft 40 mice), is transferred into a sterile 1.5 mL tube. The cells are centrifuged at 3000 rpm for 5 minutes. The supernatant is carefully removed and the cell pellet resuspended in DMEM10 (Note: puromycin is not included in this medium) to a final concentration of 5 x 103 cells per 3 μL. Keep the cells on ice.
3. Grafting Protocol
- Mice are anesthetized in the induction chamber of an isoflurane vaporizer until they no longer withdraw their hindlimb to a toe pinch stimulus. Mice are placed on their side and injected subcutaneously with 0.05 mg/kg of buprenorphine hydrocholoride. Anesthesia is then maintained via continuous administration of isoflurane delivered via nose-cone. Please note that a euthanized mice was used to demonstrate this procedure for the video; consequently, the isoflurane tubing used to deliver isoflurane is not visible in the video.
- To maintain body temperature, animals are handled on a heating pad throughout the grafting process. To prevent corneal desiccation, a small drop of ophthalmic ointment (Puralube veterinary ointment) is administered to each eye. As it will be necessary for each mouse to be uniquely identified throughout the trial, an ear tag with a unique identifier number is clipped to the right ear of each mouse.
- Place animal on stomach, spreading hind legs. Cover the mouse with a sterile drape, exposing the surgical window (flank) where grafting will occur. The sterile drape should allow visualization of the head, both to ensure that the nose is still inside the cone and to allow monitoring of respiration and the animal’s color. Apply betadine to the surgical site with a cotton tipped applicator, starting in the center of the flank and gradually spiraling outward to the edges of the surgical window. 70% ethanol is then applied to the surgical site in the same manner. Consecutive applications of betadine followed by ethanol are repeated twice more.
- Remove cells from ice and either gently vortex or flick the tube to resuspend settled cells. Using a pipette, remove 3.2 μL of the cell suspension and eject it onto a piece of Parafilm. By removing more than is needed and placing the suspension on Parafilm, we can ensure that there are no bubbles in the suspension and that we have a full 3 μL for the injection. Pull as much of the cell suspension possible into a 10 μL Hamilton syringe equipped with a 33 gauge needle. Flick bubbles from the syringe and expel the excess cell suspension to exactly 3 μL. Hold the cells and the cell-containing syringe on ice until ready to use; to ensure that the needle remains sterile, do not allow contact with the ice or the bucket. A piece of saran wrap can be placed on top of the ice to the syringe from directly contacting the ice.
- Using a No. 4 scalpel handle equipped with a No. 22 scalpel blade, make an incision through the skin of the flank just below and parallel to the femur; make sure that the incision does not extend beyond the limits of the surgically scrubbed field. Open the skin incision with a surgical clamp or manually to expose the underlying muscle. A fascial plane will be visible running along the length of the leg; the sciatic nerve lies beneath this and between the two muscles connected by the fascia. Using a pair of sharp-tipped scissors, blunt dissect through this fascia to expose the sciatic nerve, which should be apparent as a white linear structure about the thickness of a thick thread.
- Using a pair of sharp-tipped curved forceps, carefully dissect under the nerve, loosening it from the underlying muscle; this both elevates and isolates the nerve. Leave the forceps under the nerve to maintain this position. Carefully insert the needle of the Hamilton syringe into the nerve, trying to maintain the angle of injection as parallel with the nerve as possible so the needle does not puncture through the underside of the nerve. The needle should be inserted towards the end of the nerve distal to the spine-we have found that injection of tumor cells into the nerve towards the spinal cord establishes the grafted tumor cells in closer proximity to the spinal cord. When this occurs, the tumor cells often invade the spinal cord, resulting in premature loss of the animal from the study group due to compromise of spinal cord function.
- Once the needle is properly positioned in the nerve, relax the tension produced in the nerve by the forceps and slowly inject cells from the syringe into the nerve over 45-60 seconds. It is essential that this be done slowly as rapidly expulsion of the syringe contents will result in a “back-wash” of tumor cells around the injection site and their loss. After all cells have been successfully injected, slowly withdraw the needle to minimize loss of injected material.
- Remove the forceps and carefully place nerve back into its original location under the muscle. Close the surgical incision with Vetbond surgical glue. Hold incision together with forceps for approximately 20 seconds to allow the glue to dry.
- The mouse is removed from the isoflurane nose-cone and placed alone in a bedding-free cage to recover. This cage should be kept on the top of a heating pad until the animal has fully recovered. Part of the recovery cage should not be on a heating pad, this allows the animal to move away from the heat if they get too warm. Following grafting, the animals should be assessed regularly for chewing at the site, lameness or anorexia; these signs could indicate the animal is still in pain and that proper analgesics should be given. After gaining experience with this procedure, we have found that 30-40 mice can readily be grafted in a single day.
4. Assessment of Graft Success and Randomization Into Study Groups
- Bioluminescence imaging is used to assess the success of the grafting procedure. We use an IVIS-100 system (originally from Xenogen, which is now known as Calipers Inc.) to detect light emission from tumor-expressed firefly luciferase that is produced as a result of the chemical reaction of luciferase with its substrate, D-Luciferin. Analyses of these images determine whether specific grafted mice are suitable for entry into the therapeutic trial cohorts. In preliminary experiments, we have quantified the bioluminescence signals detectable, sacrificed grafted mice, harvested their tumors and correlated tumor weights with the bioluminescence signals from region of interest analyses using the manufacturer’s Living Image Software. These studies demonstrated that there was a linear correlation between tumor graft weights and bioluminescence light emission, indicating that bioluminescence imaging is an appropriate surrogate means of assessing xenograft growth throughout the course of preclinical trials. Typically we image 5 mice at the same time. Additional details on bioluminescence imaging were previously published7.
- To assess graft success, bioluminescence imaging is performed 1 and 3 days post-grafting. Images are collected from mice oriented in the same position 10 min after intraperitoneal injection of 2.5 mg D-Luciferin. During imaging, mice are maintained under isoflurane anesthesia and their body temperature kept at 37°C by a heating pad present in the Xenogen imager. Image acquisition times are in the range of 2 sec to 10 min; the data acquisition software insures that no pixels are saturated during image collection. Light emission from tumor regions (relative photon counts/sec) is measured using proprietary software from Xenogen. Light emission intensity is represented by pseudocolor scaling of the bioluminescence that is overlayed on black and white photographs of the mice that are collected at the same time.
- To be eligible for inclusion in a preclinical trial cohort, mice must have a detectable bioluminescence signal both 1 and 3 days post-grafting and the intensity of the bioluminescence signal must increase between days 1 and 3. Animals that have a decreased or static signal are excluded. In addition, signals detected in mice grafted on the same day should be within 1 order of magnitude of each other; mice with bioluminescence signals that are one order of magnitude lower or higher than those detected in animals grafted on the same day are also excluded.
- Once mice have been identified that meet these criteria, they must be randomized into treatment groups. To achieve this, luciferase signals detected in grafted mice 3 days post-grafting are first ordered from highest to lowest intensity. Mice are then randomized into groups by assigning them in order of intensity to the treatment groups, reversing this order and then repeating the process until all mice are assigned to groups. For example, if there are 4 treatment groups (e.g., placebo and three concentrations of test drug) mice are assigned to the groups in the order 1, 2, 3, 4, 4, 3, 2, 1, 1, 2, 3, 4, 4, 3, 2, 1..etc. Before initiating treatment, the bioluminescence signals detected in each member of the assigned group are averaged to make sure that the average starting signals within all groups are as close as possible. Administration of the candidate therapeutic agent is begun on day 3.
- To assess the effect the therapeutic agent has on graft growth during the course of treatment, bioluminescence imaging is performed once a week after beginning therapy (days 10, 17, 24, 30 post-grafting). For non-nude immunodeficient mice, it is critically important that animals again have new fur growth removed with Nair prior to performing each imaging session. This is because hair blocks bioluminescent signals, with coat color determining how much of the signal is lost. For instance, white fur such as is found on Swiss Webster mice produces an 18% reduction in bioluminescent signal intensity8, while black fur can decrease signals as much as 10-fold9. We would also note that it cannot be assumed that uniform fur regrowth will occur in all treatment groups and “normalize” signal reductions among the treatment groups. This is because the therapeutic agent itself may affect hair growth. For instance, we have noted that tamoxifen treatment inhibits hair regrowth, thereby minimizing the perceived effect of this agent on tumor growth when compared to placebo-treated mice.
- With ST88-14 MPNST cells, mice can be carried for 30 days post-grafting. At this time, tumors typically have grown to the maximal size allowed by institutional animal care and use committees and must be sacrificed. Following the final imaging session, mice are killed and specimens necessary for further analysis of treatment outcomes (e.g., blood for determination of circulating levels of the therapeutic agent, tumor tissue for weight determination, examination of histology, determination of Ki67 and TUNEL labeling indices and assessment of biochemical parameters) collected. Precisely what specimens are collected will depend on the needs of the experimental design.
- Mice with tumors should be monitored daily and terminated if they become ill (lack normal grooming and avoidance behaviors), are unable to eat or drink, or if the tumor becomes overly large as to hinder normal body movement. Tumor burden should not be allowed to exceed 10% of the animal’s normal body weight. Tumor weight as a percentage of body weight is calculated by comparing the weight of the tumor bearing animals to the weight of age/sex matched control animals. Cachexia should not be allowed to become clinically significant. Weight loss in tumor-bearing animals should not exceed 20% of normal body weight.
5. Representative Results:
Figure 1 illustrates the typical progressive increase in bioluminescence observed 1 to 18 days post-grafting in a properly established orthotopic xenograft in a nude (NIH III) mouse (A-C). Quantification of the bioluminescence signals observed 1-24 days after grafting shows that, although these signals progressively increase, tumor growth markedly accelerates in the later stages of the study period (Figure 1D). To rigorously demonstrate that tumor growth has occurred in grafted mice, we routinely collect the sciatic nerve and, if it appears that tumor has ruptured the epineurium and invaded adjacent tissue, surrounding soft tissue and skeletal muscle. Given the aggressive growth of MPNSTs, it is not surprising that we often find that the grafted tumor cells have breached the normal barriers of the nerve and invaded adjacent tissues (Figure 1E). We also often find that grafted MPNST cells migrate aggressively into nerve proximal and distal to the graft site.
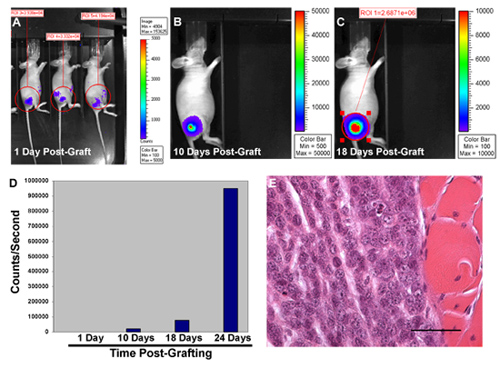
Figure 1. Bioluminescence imaging of NIH III mouse orthotopically grafted with luciferase-tagged MPNST cells 1 day post-grafting (A). Note that the signals detected within the region of interest (ROI) in different mice are all within one order of magnitude of each other. Reimaging 10 days (B) and 18 days (C) post-grafting shows that bioluminescent signals progressively increase at the graft site in individual mice. (D) Graph illustrating the progressive increase in the relative photon counts/second detected over the graft site 1-24 days post-grafting. (E) Photomicrograph of the graft site from this mouse demonstrating tumor growth and focal invasion into adjacent skeletal muscle.
Discussion
The detailed orthotopic xenografting method presented here is one that we developed using ST88-14 MPNST cells, a line which is widely used in studies of these NF1-associated peripheral nerve sheath tumors. However, this methodology is easily adaptable for preclinical studies with other MPNST cell lines. For instance, we have also performed orthotopic xenografting with STS-26T10cells, a line derived from a sporadically occurring MPNST and T265-2c11cells, which are derived from a NF1-associated MPNST, with success similar to that we observed with ST88-14 cells.
Having said that, we would note that the exact conditions we describe here for orthotopic xenografting of ST88-14 cells cannot be directly adopted for the grafting of other MPNST lines. Instead, key parameters of the methodology must be empirically determined for each cell line. These parameters include the number of MPNST cells initially grafted, the time allowed for graft development and, to a lesser extent, the volume in which the tumor cells are injected. To establish these parameters for a new line, we graft groups of mice (5 mice per group) with 103 to 5 x 106 tumor cells, checking two concentrations of cells for each order of magnitude (i.e., 103 cells, 5 x 103 cells, 104 cells, 5 x 104 cells, etc.). Larger numbers of tumor cells (>106) must be injected in a larger volume; we have found that up to 5 mL can be injected without loss of cells from the grafted nerve. We have also found that no more than 5 x 106 tumor cells per 5 mL volume can be injected as denser suspensions become increasingly prone to shear which kills the tumor cells. We follow these mice until at least three animals within each group have palpable tumors, at which point we terminate that group and examine the nerve histologically to confirm graft growth. Obviously, the time required to reach this point will differ, depending on the number of cells injected; in general, we are seeking a concentration of cells that will reach maximal allowable tumor growth within 30-60 days. More rapid growth can make it difficult to achieve effective therapeutic concentrations prior to termination of the experiment and/or prevent the collection of sufficient bioluminescence imaging datapoints over the course of the experiments. A time course longer than 60 days makes adjusting experimental parameters unwieldy and unnecessarily prolongs the duration of the planned experiments.
Finally, we would also note that we have used this orthotopic xenografting methodology with ST88-14 cells grafted into NIH III mice to demonstrate the therapeutic effectiveness of tamoxifen6. A detailed description of the methods we routinely use to assess the effects of candidate therapeutic agents on orthotopic xenografts can be found in that manuscript. It has also been demonstrated that neurofibroma cells can be successfully grafted into peripheral nerve12, suggesting that, with modifications, the procedures outlined in this protocol can be used to perform preclinical trials with candidate therapeutic agents directed against neurofibromas.
Declarações
The authors have nothing to disclose.
Acknowledgements
This work was supported by the National Institute of Neurological Diseases and Stroke (R01 NS048353 to S.L.C.), the National Cancer Institute (R01 CA122804 to S.L.C., R01 CA134773 to Kevin A. Roth and S.L.C. and CA13148-35 to K.R.Z.) and the Department of Defense (X81XWH-09-1-0086 to S.L.C.). Funds supporting the operation of the UAB Comprehensive Cancer Center Small Animal Imaging Shared facility were provided by a NCI Core Support grant (P30 CA13148-35; E. Partridge, P.I.). We thank the Alabama Neuroscience Blueprint Core Center (P30 NS57098) and the UAB Neuroscience Core Center (P30 NS47466) for their assistance. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the Department of Defense.
Materials
| Material Name | Tipo | Company | Catalogue Number | Comment |
|---|---|---|---|---|
| Foxchase outbred SCID mice | Charles River | #236 | ||
| NIH III mice | Taconic | #NIHBNX | ||
| NOD-SCIDγ mice | Jackson Laboratories | #005557 | ||
| Cell Stripper | Fisher Scientific | 25-056-cl | ||
| Vetbond surgical glue | 3M | 1469SB | ||
| Hamilton syringe | Hamilton Company | #80030 | 10 μL volume | |
| Hamilton syringe needles | Hamilton Company | #7803-05 | 33 Ga., 0.5 inch, PT4 (custom made) | |
| Ear tags | National Band and Ear Tag Co. | 1005-1 | ||
| Ophthalmic ointment | Dechra | NDC 17033-211-38 |
Referências
- Rosenberg, M. P., Bortner, D. Why transgenic and knockout animal models should be used (for drug efficacy studies in cancer). Cancer Met. Rev. 17, 295-299 (1999).
- Killion, J. J., Radinsky, R., Fidler, I. J. Orthotopic models are necessary to predict therapy of transplantable tumors in mice. Cancer Met. Rev. 17, 279-284 (1999).
- Carroll, S. L., Ratner, N. How does the Schwann cell lineage form tumors in NF1?. Glia. 56, 1590-1605 (2008).
- Woodruff, J. M., Kourea, H. P., Louis, D. N., Scheithauer, B. W., Kleihues, P., Cavenee, W. K. . Pathology and Genetics of Tumours of the Nervous System. , 172-174 (2000).
- DeClue, J. E. Epidermal growth factor receptor expression in neurofibromatosis type 1-related tumors and NF1 animal models. J. Clin. Invest. 105, 1233-1241 (2000).
- Byer, S. J. Tamoxifen inhibits malignant peripheral nerve sheath tumor growth via an estrogen receptor-independent mechanism. Neuro-Oncology. , (2010).
- Zinn, K. R. Noninvasive bioluminescence imaging in small animals. ILAR J. 49, 103-115 (2008).
- Wu, J. C., Sundaresan, G., Iyer, M., Gambhir, S. S. Noninvasive optical imaging of firefly luciferase reporter gene expression in skeletal muscles of living mice. Mol. Therapy. 4, 297-306 (2001).
- Luker, G. D., Leib, D. A. Luciferase real-time bioluminescence imaging for the study of viral pathogenesis. Methods Mol. Biol. 292, 285-296 (2005).
- Dahlberg, W. K., Little, J. B., Fletcher, J. A., Suit, H. D., Okunieff, P. Radiosensitivity in vitro of human soft tissue sarcoma cell lines and skin fibroblasts derived from the same patients. Int. J. Radiat. Biol. 63, 191-198 (1993).
- Badache, A., DeVries, G. H. Neurofibrosarcoma-derived Schwann cells overexpress platelet-derived growth factor (PDGF) receptors and are induced to proliferate by PDGF. BB. J. Cell. Physiol. 177, 334-342 (1998).
- Muir, D., Neubauer, D., Lim, I. T., Yachnis, A. T., Wallace, M. R. Tumorigenic properties of neurofibromin-deficient neurofibroma Schwann cells. Am. J. Pathol. 158, 501-513 (2001).

