Elastomeric PGS Scaffolds in Arterial Tissue Engineering
Summary
Elastomeric PGS scaffolds with vascular smooth muscle cells cultured in a pulsatile flow bioreactor may lead to promising small-diameter arterial constructs with native ECM production in a relatively short culture period.
Abstract
Cardiovascular disease is one of the leading cause of mortality in the US and especially, coronary artery disease increases with an aging population and increasing obesity1. Currently, bypass surgery using autologous vessels, allografts, and synthetic grafts are known as a commonly used for arterial substitutes2. However, these grafts have limited applications when an inner diameter of arteries is less than 6 mm due to low availability, thrombotic complications, compliance mismatch, and late intimal hyperplasia3,4. To overcome these limitations, tissue engineering has been successfully applied as a promising alternative to develop small-diameter arterial constructs that are nonthrombogenic, robust, and compliant. Several previous studies have developed small-diameter arterial constructs with tri-lamellar structure, excellent mechanical properties and burst pressure comparable to native arteries5,6. While high tensile strength and burst pressure by increasing collagen production from a rigid material or cell sheet scaffold, these constructs still had low elastin production and compliance, which is a major problem to cause graft failure after implantation. Considering these issues, we hypothesized that an elastometric biomaterial combined with mechanical conditioning would provide elasticity and conduct mechanical signals more efficiently to vascular cells, which increase extracellular matrix production and support cellular orientation.
The objective of this report is to introduce a fabrication technique of porous tubular scaffolds and a dynamic mechanical conditioning for applying them to arterial tissue engineering. We used a biodegradable elastomer, poly (glycerol sebacate) (PGS)7 for fabricating porous tubular scaffolds from the salt fusion method. Adult primary baboon smooth muscle cells (SMCs) were seeded on the lumen of scaffolds, which cultured in our designed pulsatile flow bioreactor for 3 weeks. PGS scaffolds had consistent thickness and randomly distributed macro- and micro-pores. Mechanical conditioning from pulsatile flow bioreactor supported SMC orientation and enhanced ECM production in scaffolds. These results suggest that elastomeric scaffolds and mechanical conditioning of bioreactor culture may be a promising method for arterial tissue engineering.
Protocol
1. Tubular Scaffold Fabrication
- Prepare glass tubing (length = 70 mm, outer diameter = 9.0 mm, wall thickness = 1.0 mm; Small Parts, Miramar, FL) as a mold for scaffold.
- Prepare 1.0 wt /vol % hyaluronic acid (HA) (Sigma-Aldrich, St. Louis, MO) by dissolving 100 mg of HA into 10 ml of deionized water. Mix using a rotator overnight until there are no air bubbles in solution.
- Pour 1.0 % HA solution into the top of glass tubing. Let it flow slowly down along the inner wall of the mold. Flip over the tubing when the solution reached the bottom of the mold using the rotator and repeat this step until the inner wall of the mold is evenly coated by the solution.
- After coating, dry all glass tubings into the vacuum oven at 37°C for 24 h.
- Grind and sieve salt particles (size = 25-32 μm) used as porogens.
- Assemble a glass tubing (coated with 1.0 % HA solution inside), mandrel (stainless steel encased by PTFE tubing), heat-shrinkable (HS) sleeve, and PTFE ring as described previously8.
- Add salt particles of the desired size to the glass mold using a spatula through the silicon rubber funnel. Tap the mold gently to ensure even distribution of salt particles. Scrape off the excess salt particles across the top of the mold using the side of spatula.
- Turn on the hybridization incubator for 30 min before transferring the salt-packed mold. Turn off the hybridization incubator and Put the salt-packed mold into the hybridization incubator for salt fusion.
- Remove the molds from the hybridization incubator and dry them into the vacuum oven at 37°C for 24 h.
- Remove the salt-packed molds from the vacuum oven and allow them to cool at room temperature. Remove the stainless steel mandrel by pushing it down with holding the PTFE ring. If the mandrel is too tight to the mold, use the needle pliers to pull it out. Remove the PTFE ring from the bottom of the mold.
- Put the molds into the oven at 120 °C for 5 min to shrink HS sleeve. Check if the HS sleeve is shrunk. Remove the HS sleeve from the salt-packed molds and allow them to cool at room temperature. Keep the molds into the dessicator until they are used.
- Prepare the PGS solution by dissolving 3 g of PGS into 15 ml of tetrahydrofuran (THF) (Sigma-Aldrich, St. Louis, MO) to form a 20 wt/vol % solution.
- Add the PGS solution to the lumen of salt-packed molds by dropping it using a spoid in the hume hood. Lean the glass mold about 45° and drop the PGS solution into inner lumen with rotating the mold slowly. Check if the PGS solution flows down along the wall of mold. If dry spot is observed, add more solution.
- After adding the PGS solution, place the molds in the hume hood for at least 30 min for evaporating THF.
- Put the molds into the vacuum oven for pre-determined curing time (ex. 24/ 36/ 48 h) at 100 mTorr and 150°C for curing PGS.
- After curing, take out the molds and place them at room temperature to cool down. Dip each mold into the deionized water with vertical position slowly. Don’t dip them into the water fast because air bubbles might cause tear the scaffold.
- Transfer the molds to the water bath carefully and place them at an angle for 1 h using silicon tube to dissolve HA easily. Check if HA is dissolved from the mold. If no HA is released from the mold, push the one end of the scaffold slowly using spatula to release it from the mold. Repeat this step at the other end of the scaffold and shake the mold back and forth slowly in the water bath. Check if the scaffold is moved inside the mold.
- Transfer the scaffold to another water bath with stirrer carefully. Don’t grab the scaffold firmly, which might crack the scaffold. Place the scaffold in the water bath for at least 3 days with changing water to leach out salt particles.
- After leaching out salt particles, transfer each scaffold to the 15 ml centrifuge tube filled with the deionized water and place them in the dry-ice box for 1 h to freeze. Put centrifuge tubes into the lyophilizer (all tube cabs must be opened) and lyophilize them for 2 days.
- Put all scaffolds into the dessicator before until they are used.
2. Scaffold Preparation for Cell Seeding
- Take out scaffolds from the dessicator and cut them as 25-30mm of length.
- Prepare two silicone rubber stoppers (diameter = 20 mm, wall thickness = 6.35 mm, middle hole diameter = 3 mm) and connect two PTFE tubings (outer diameter = 3.97 mm, inner diameter = 2.38 mm, wall thickness = 0.79 mm, length = 60 mm and 40 mm) through the middle holes of each stopper.
- Cut a HS ring as a 1.5 mm of length and put it on top of one end of the scaffold. Push one PTFE tubing (connected silicone rubber stopper) into the one end of scaffold as small overlapping portion as possible.
- Put the scaffold and PTFE tubing into the oven at 120 °C for 10 min to shrink HS ring. Check if HS ring is shrunk and scaffolds are connected to PTFE tubing firmly.
- Take out the scaffold and PTFE tubing and place them at room temperature to cool down. Put the scaffold-PTFE tubing assembly into the polycarbonate tube (outer diameter = 15.5 mm, inner diameter = 9 mm, length = 50 mm) as a bioreactor chamber.
- Connect another end of PTFE tubing to the scaffold as steps 2.3) and 2.4).
- Adjust two silicone rubber stoppers to attach their inner surfaces to both ends of the polycarbonate tube. Attach both outer surfaces of silicone rubber stoppers to two aluminum alloy plates (top and bottom; width = 20 mm, length = 70 mm, thickness = 6.35 mm). Put two threaded rods into the side hole of plate and fix the polycarbonate tube (included the scaffold inside) to plates by tightening thumb nut screw on the plate.
- Measure the seedable length of each scaffold (a length between two HS rings) and calculate inner surface area (π x inner diameter x seedable length) of each scaffold for cell seeding.
- Wrap one bioreactor chamber unit with aluminum foil and sterilize it by autoclaving at 120 °C for 30 min. Sterilize each part (medium reservoir, pump tubing, gas exchanger, manifolds, and needle valve) of the bioreactor by autoclaving at 120 °C for 30 min and assemble them inside the cell culture hood.
- Pre-treat and rinse scaffolds with a series of perfusions (70, 50, and 25 % ethanol for 1 h, phosphate-buffered saline (PBS; Mediatech, Herndon, VA) for 2 h, and SMC culture medium for 24 h by using a peristaltic pump at 1.0 ml/min in the flow circuit.
3. Cell Seeding and Culture
- Isolate primary arterial smooth muscle cells (SMCs) from the carotid arteries of juvenile male baboons (Papio Anubis) and characterize them in two-dimensional (2D) culture before passaging and seeding as described previously9.
- Expand SMCs using the MCDB 131 medium (Mediatech, Herndon, VA) with 10% fetal bovine serum (Lonza, Walkersville, MD), 1 % L-glutamine (Mediatech), 50 μg/ml ascorbic acid (Fisher Scientific, Fair Lawn, NJ), and an antibiotic-antimycotic solution (10,000 IU/ml penicillin, 10,000 μg/ml streptomycin, and 25 μg/ml amphotericin B; Mediatech).
- Detach each bioreactor chamber from the flow circuit in the bioreactor. Seed SMCs (passage 4-6) to the scaffold with a density of 2×106 cells/cm2 by injecting suspension with 5 ml syringe into the lumen of scaffolds after cutting off both abluminal (inlet) and luminal (outlet) flows.
- Put all bioreactor chambers into the hybridization incubator at 37 °C and rotate them at 2 rpm for 4 h to allow the cells to uniformly spread into the scaffold.
- Take out bioreactor chambers from the hybridization incubator reattach them to flow circuit of the bioreactor in the hood. Transfer all bioreactor system into cell culture incubator.
- Connect the pump tubing (inner diameter = 1.6 mm) to the cartilage of peristaltic pump and load fresh culture medium in the reservoir. Start the through-wall perfusion (luminal to abluminal) at 1.0 ml/min for 15 min followed by luminal flow only.
- Increase the flow rate and pressure gradually from 1.0 ml/min (luminal shear stress: 1.1 dynes/cm2) and 20 mmHg on day 1 to 14.2 ml/min (15.3 dynes/cm2) and 120 mmHg on day 21, respectively. Change the pump tubing (inner diameter = 3.2 mm) at day 4 and the medium once every week.
4. Tissue Harvesting and Sample Preparation for Analysis
- After 21-day culture, stop the pump and clamp tubings (inlet and outlet) of the medium reservoir. Detach the pump tubing from the cartilage of peristaltic pump and transfer bioreactor system into the hood.
- Clamp inlet and outlet tubings of each bioreactor chamber and detach it from the flow loop. Open the inlet tubing and prepare a petri-dish at the outlet tubing to collect culture medium inside the polycarbonate tube. Open the outlet tubing slowly and remove the medium from the chamber.
- Detach abluminal gauge needle and luminal silicone tubing from the chamber and detach aluminum plates by releasing thumb screws. Remove the polycarbonate tube and detach one end of the tissue construct from the PTFE tubing.
- Prepare a petri-dish filled with 10 ml of PBS and detach other end of the construct from the PTFE tubing. Put construct into the PBS and rinse three times.
- Cut the construct with a razor blade as 5-mm of length. Freeze it at -80 °C freezer for biochemical assay or transfer it to 15 ml centrifuge tube filled with 5 ml of fresh medium for mechanical testing or snap freeze into the Tissue-Tek optimal cutting temperature compound (Sakura Finetek Inc., Torrance, CA) for histology/immunofluorescence staining.
5. Representative Results:
The tubular PGS scaffolds were fabricated using biodegradable elastomer by salt fusion method (Fig. 1A). Each bioreactor chamber provided scaffolds with both luminal and abluminal flows and could be detached as a separate unit from a main flow loop (Fig. 1B). Bioreactor system was designed for culturing four scaffolds at a time by controlling and monitoring flow as well as pressure (Fig. 1C & D).
Schematic of bioreactor culture was shown in Fig. 2. After seeding SMCs, each bioreactor chamber was rotated at 37 °C for 4 h to distribute cells uniformly in the lumen of scaffold. And then, pulsatile flow was applied to scaffolds until day 14 with gradually increasing flow rate (Fig. 2A) and pressure (Fig. 2B). After day 14, flow and pressure kept constant until the end of culture (day 21).
Surface morphology of the PGS scaffold was examined by scanning electron microscopy (SEM). Scanning electron micrographs showed that scaffold had consistent wall thicknesses (539 ± 18 μm) (Fig. 3A) and randomly distributed macro- and micro-pores on the luminal surface (Fig. 3B). Morphometric parameters of the scaffold was measured from microcomputed tomography (micro-CT) and imaging analysis. Average pore size is 23.3 ± 3.9 μm and pore interconnectivity is 99.4 ± 0.62 %, which means that all pores are fully interconnected in scaffold. Porosity measured by ethanol displacement is 75.6 ± 2.7 %.
Cellular morphology of the PGS construct was examined by SEM (Fig. 4). Multilayered SMCs completely covered the luminal surface and they were perpendicularly oriented to flow direction. These results show that mechanical conditioning from pulsatile flow bioreactor supports SMC orientation in the scaffold.
The presence of extracellular matrix (ECM) and elastic fibers were examined by H&E staining and elastin autofluorescence (Fig. 5). H&E staining demonstrated that cells and ECM proteins completely covered the lumen of the PGS construct. Elastin autofluorescence also showed circumferentially organized elastic fibers at the luminal surface of the construct. Production of ECM proteins in PGS constructs were measured from biochemical assays. The insoluble elastin and collagen contents were 20.2 ± 9.1 μg/mg of tissue and 6.3 ± 1.9 μg/mg of tissue, respectively.
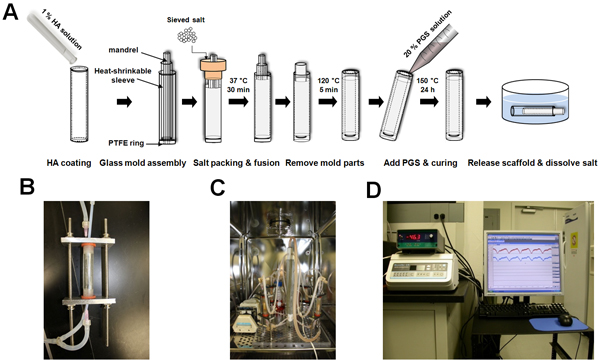
Figure 1. Scaffold fabrication and bioreactor system. (A) Schematic of tubular scaffold fabrication. (B) Bioreactor chamber. Scaffolds were connected to PTFE tubing, placed into the polycarbonate tube, and fixed by silicone rubber stoppers and aluminum alloy plates. Each chamber has two flow paths: luminal (by silicone tubing) and abluminal (by gauge needle) flows. (C) Bioreactor system placed inside the incubator. It includes medium reservoir, peristaltic pump module, gas exchanger, pressure transducers, two manifolds (top and bottom), and needle valve. (D) Bioreactor system placed outside the incubator. It includes pressure monitor, flow control unit, data acquisition system, and computer.
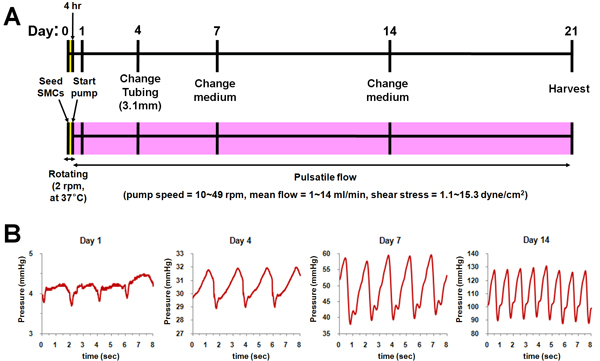
Figure 2. Schematic of the bioreactor culture. (A) Culture protocol. (B) Applied pressure profiles at each time point (Day 1, 4, 7, and 14).
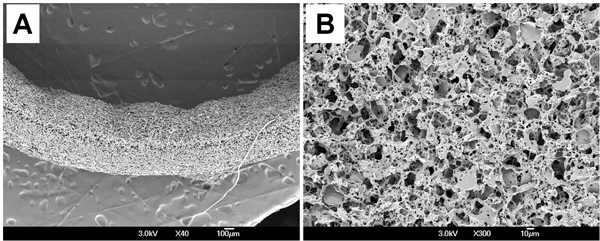
Figure 3. Surface morphology of the PGS scaffold. (A) Cross-section. (B) Lumen.
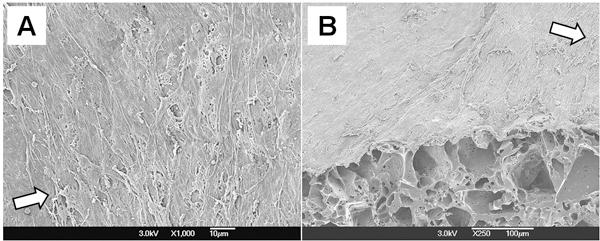
Figure 4. Cellular morphology of the PGS construct. (A) Lumen. (B) Cross-section of 45° cut. Arrows in both figures represent the flow direction.
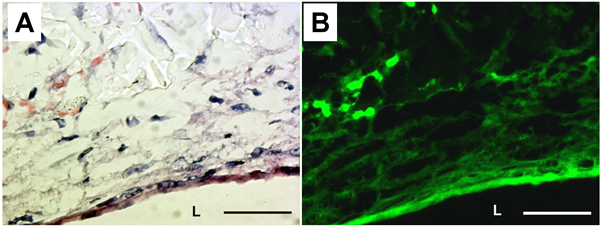
Figure 5. Histology and elastin autofluorescence of the PGS construct. (A) H&E staining. (B) Corresponding elastin autofluorescence. L: lumen. Magnification: 40X. Scale bar: 50 μm.
Discussion
The fabrication technique using a biodegradable elastomer described here has several features. (1) We used hyaluronic acid (HA) as a mold release. Since HA is water soluble, scaffold was easily released from the glass mold after soaking it into water. In this report, we used 1.0 wt/vol % of HA solution because low concentration (< 0.5 wt/vol %) of solution is not viscous and flows down so fast when we pouring it on top of glass tubing. To coat HA solution uniformly, we flipped over the glass tubing when the solution flew down the bottom of the tubing and repeated this step. This HA coating is a critical to our fabrication procedure for releasing final scaffolds. (2) We used heat-shrinkable (HS) sleeve for retaining salts in the glass tubing. Since salts were densely packed in the space between the inner wall of glass tubing and the HS sleeve, HS sleeve retained salts after removing mandrel and PTFE ring in the bottom of the tubing. We could remove HS sleeve easily by putting the mold into an oven at 120 °C for 5 min, and then get tubular salt templates. (3) We used the salt fusion method. It is well known that salt fusion method can improve pore interconnectivity and mechanical properties by varying fusion time10. Furthermore, since we used PGS, macro-pores were produced by the salt particles during leaching process, while the micro-pores were likely generated by glycerol vapor formed during PGS curing as we described previously11. Thus, this method has a potential to fabricate porous tubular scaffolds with different macro- and micro-structures by varying salt particles as well as PGS curing condition.
The mechanical conditioning from the bioreactor has provided pulsatile flow perfusion (maximum mean flow = 14 ml/min, maximum shear stress = 15.3 dyne/cm2, frequency = 0.5 – 1.7 Hz) and physiologically relevant pressure with the PGS scaffold, which led to SMC growth and orientation (Fig. 4). These results are consistent with previous studies reporting that cyclic stretch at this frequency and shear stress increases SMC proliferation12, and ECM protein production13,14. In addition to SMC growth and orientation, PGS construct supported ECM protein production, especially circumferentially organized elastic fibers (Fig. 5) within 3-week culture in the bioreactor. Some studies using an elastomeric scaffold as a small-diameter arterial construct have demonstrated mechanical strength and burst pressure comparable to native arteries15, and rapid SMC integration in compliant scaffolds using spinner flask16,17, while no elastic fibers were found in these constructs. Our results suggest that cyclic radial distension from the bioreactor improved mechanical signal transduction more effectively to SMCs in PGS scaffold, which likely contributed to elastin synthesis and organization.
Since vascular SMCs were the only cells that produced ECM proteins in our approach, quiescent endothelium and improve mechanical strength are necessary to develop a clinically successful small-diameter arterial constructs. We have reported that endothelial cells co-cultured with SMCs generated a confluent monolayer and supported phenotype protein expression under our culture conditions and mechanical conditioning9. Therefore, based on our approach described here, modification of co-culture experiment conditions would be a next step to improve the functions of resultant constructs and generate nonthrombogenic, robust, and compliant arterial construct similar to native arteries.
Declarações
The authors have nothing to disclose.
Acknowledgements
The author thanks Dr. Jin Gao for PGS synthesis, Dr. Peter Crapo for insightful discussion for bioreactor setup, Drs. Mohamed Ezzelarab and Wei Wu for explanting baboon carotid arteries. This study was supported by a grant from the National Institutes of Health (R01 HL089658).
Materials
| Name of the reagent | Company | Catalogue number |
|---|---|---|
| Hyaluronic acid sodium salt | Sigma-Aldrich | H7630 |
| Tetrahydrofuran | Sigma-Aldrich | 401757 |
| MCDB 131 | Mediatech | 15-100-CV |
| Fetal bovine serum | Lonza | BW14-502F |
| L-glutamine | Mediatech | 25-005-CV |
| Ascorbic acid | Fisher Scientific | A62-500 |
| Antibiotic-antimycotic solution | Mediatech | 30-004-CI |
| Phosphate Buffered Saline (PBS) | Mediatech | 21-031-CV |
| Tissue-Tek optimal cutting temperature compound, 4583 | Sakura Finetek | 25608-930 |
Referências
- The American Heart Association Statistics Committee Stroke Statistics Subcommittee. American Heart Association: Heart disease and stroke statistics-2009 update. Circulation. 119, 480-486 (2009).
- Isenberg, B. C., Williams, C., Tranquillo, R. T. Small-diameter artificial arteries engineered in vitro. Circ Res. 98, 25-35 (2006).
- Conte, M. S. The ideal small arterial substitute: a search for the Holy Grail. FASEB J. 12, 43-45 (1998).
- Wang, X., Lin, P., Yao, Q., Chen, C. Development of small-diameter vascular grafts. World J Surg. 31, 682-689 (2007).
- L’Heureux, N., Paquet, S., Labbe, R., Germain, L., Auger, F. A. A completely biological tissue-engineered human blood vessel. FASEB J. 12, 47-56 (1998).
- Niklason, L. E., Gao, J., Abbott, W. M., Hirschi, K. K., Houser, S., Marini, R., Langer, R. Functional arteries grown in vitro. Science. 284, 489-493 (1999).
- Wang, Y., Ameer, G. A., Sheppard, B. J., Langer, R. A tough biodegradable elastomer. Nat Biotechnol. 20, 602-606 (2002).
- Crapo, P. M., Gao, J., Wang, Y. Seamless tubular poly(glycerol sebacate) scaffolds: high-yield fabrication and potential applications. J Biomed Mater Res A. 86, 354-363 (2008).
- Gao, J., Ensley, A. E., Nerem, R. M., Wang, Y. Poly(glycerol sebacate) supports the proliferation and phenotypic protein expression of primary baboon vascular cells. J Biomed Mater Res A. 83, 1070-1075 (2007).
- Murphy, W. L., Dennis, R. G., Kileny, J. L., Mooney, D. J. Salt fusion: an approach to improve pore interconnectivity within tissue engineering scaffolds. Tissue Eng. 8, 43-52 (2002).
- Gao, J., Crapo, P. M., Wang, Y. Macroporous elastomeric scaffolds with extensive micropores for soft tissue engineering. Tissue Eng. 12, 917-925 (2006).
- Wilson, E., Mai, Q., Sudhir, K., Weiss, R. H., Ives, H. E. Mechanical strain induces growth of vascular smooth muscle cells via autocrine action of PDGF. J Cell Biol. 123, 741-747 (1993).
- Leung, D. Y., Glagov, S., Mathews, M. B. Cyclic stretching stimulates synthesis of matrix components by arterial smooth muscle cells in vitro. Science. 191, 475-477 (1976).
- Kim, B. S., Nikolovski, J., Bonadio, J., Mooney, D. J. Cyclic mechanical strain regulates the development of engineered smooth muscle tissue. Nat Biotechnol. 17, 979-983 (1999).
- Yang, J., Motlagh, D., Webb, A. R., Ameer, G. A. Novel biphasic elastomeric scaffold for small-diameter blood vessel tissue engineering. Tissue Eng. 11, 1876-1886 (2005).
- Stankus, J. J., Soletti, L., Fujimoto, K., Hong, Y., Vorp, D. A., Wagner, W. R. Fabrication of cell microintegrated blood vessel constructs through electrohydrodynamic atomization. Biomaterials. 28, 2738-2746 (2007).
- Nieponice, A., Soletti, L., Guan, J., Deasy, B. M., Huard, J., Wagner, W. R., Vorp, D. A. Development of a tissue-engineered vascular graft combining a biodegradable scaffold, muscle-derived stem cells and a rotational vacuum seeding technique. Biomaterials. 29, 825-833 (2008).

