Impulsive Pressurization of Neuronal Cells for Traumatic Brain Injury Study
Summary
A novel impulsive cell pressurization experiment has been developed using a Kolsky bar device to investigate the molecular/cellular mechanisms of blast-induced traumatic brain injury.
Abstract
A novel impulsive cell pressurization experiment has been developed using a Kolsky bar device to investigate blast-induced traumatic brain injury (TBI). We demonstrate in this video article how blast TBI-relevant impulsive pressurization is applied to the neuronal cells in vitro. This is achieved by using well-controlled pressure pulse created by a specialized Kolsky bar device, with complete pressure history within the cell pressurization chamber recorded. Pressurized neuronal cells are inspected immediately after pressurization, or further incubated to examine the long-term effects of impulsive pressurization on neurite/axonal outgrowth, neuronal gene expression, apoptosis, etc. We observed that impulsive pressurization at about 2 MPa induces distinct neurite loss relative to unpressurized cells. Our technique provides a novel method to investigate the molecular/cellular mechanisms of blast TBI, via impulsive pressurization of brain cells at well-controlled pressure magnitude and duration.
Protocol
1. Neuronal Cell Culture
- Brain cells including neuronal cells, astrocytes, and their co-culture may be used as a cell model. As a feasibility demonstration, impulsive pressurization of cell-line neuronal cells is presented.
- SH-SY5Y human neuroblastoma cells (ATCC, CRL-2266) are cultured on 18 mm diameter glass coverslips. Cells are seeded at a density of 3×103 cells/cm2 using the growth media composed of DMEM supplemented with 10% fetal bovine serum and 1% penicillin-streptomycin. Cell cultured glass slides are kept in a 5% CO2 humidified incubator at 37°C.
- To obtain the neuronal cell differentiation of SH-SY5Y cells, cells are treated with media further supplemented with 10 μM retinoic acid (RA) for 7 days with media changed every two days. On day 7, cells are ready to be pressurized.
2. Pressurization Equipment: Kolsky Bar
- The Kolsky bar, developed by Kolsky1 in 1949, has been used to measure the mechanical property of a material at a very high loading rate. The apparatus consists of two bars with a sample placed in contact between the bars. A stress wave, created on the incident bar, propagates to the sample where the wave splits into a reflected and transmitted wave. The stress in the sample is proportional to the transmitted wave. In this study, we utilize a cell pressurization chamber to be placed between the two bars of the Kolsky set-up.
- The two aluminum alloy bars (each 6-m long) are suspended by aligned brass bearings, and an in vitro cell pressurization chamber is sandwiched between the bars. The upstream bar is the incident bar with a 130 lb mass clamped onto one end. A friction clamp is placed at a position that will give the desired pulse duration. By engaging the clamp and tightening a scissors jack between the mass and a loading support, the clamp-mass section of the incident bar is pre-compressed to the desired level. Forcing a notched bolt that locks the clamp to a sudden break with a hydraulic system releases the pre-stored compression rapidly and thus generates a pressure pulse. This propagates through the incident bar, drives the test chamber piston, and pressurizes the fluid and cells within the chamber impulsively. The chamber pressurization in turn initiates a pressure pulse propagating in the transmitted bar downstream. The strains associated with the pressure pulses in the bars are measured with compound high resistance strain gauges excited to 45 volts. The gauge signals are recorded with a digital oscilloscope at 1 MHz frequency as a loading history.
- The in vitro cell pressurization chamber is composed of a piston-cylinder. The cylinder has 2.6 cm inner diameter and 3.8 cm outer diameter, and has a small hole tapped at the base of the cavity. The hole serves as an air and excess fluid vent during cell sample installation. The piston is 7.5 cm long and is made from the same bar material. The piston is wrapped with two to three layers of plumber’s (Teflon) tape that serve as low friction seal. The end cap is 32 mm long and has two small stainless steel screws that secure the glass coverslips (on which cells are cultured) during the loading.
3. Impulsive Pressurization of Neuronal Cells
- All pressurization chamber parts are sterilized using the autoclave and are kept under UV light. The assembly of the chamber is performed inside the cell culture hood. The vent screw in the chamber is loosely engaged in the air vent at the base of the cavity. Pre-warmed (37°C) fresh growth media is pipetted into the chamber without making bubbles. Then, a cell-cultured glass slide is picked up, and is placed on the cap of the piston (cells facing outside). The small holding screws are tightened down onto the slide to hold it in place. The piston with a cell-cultured glass slide is inserted a little into the cavity of the chamber, and the assembly is tilted with the vent being at the highest point. The vent screw is removed and the piston is pushed into the cavity first forcing out air bubbles, then the excess fluid. Either a reference mark or a jig is used as a guide to ensure the same culture media volume for every test. The axial dimension of the media in the chamber is about 6 mm. Sanitize the vent screw and replace it to make the chamber water tight. The chamber should not leak under this static load, if it does, the Teflon wrap needs to be replaced or reinforced.
- At this point, the Kolsky bar system is reset. The heavy mass is moved back to the original position and a new locking bolt replaces the used (broken) one. Engage the new locking bolt with the hydraulics to about 200 psi. Use the scissors jack to compress the pre-loading section of the incident bar up to a pressure little higher than the desired value, and then back it off to engage the full friction of the jack’s screw. The data acquisition is now armed.
- The assembled cell pressurization chamber is mounted into the system. It is placed in a V block supported by small lab scissors jack and aligned with the two bars. Grease each interface with a light grease layer and rub the butting surfaces together to eliminate any air gaps in between.
- The test is now ready to proceed. The clamp locking bolt is forced to break by quickly pumping the hydraulic clamp driver. The clamp will separate and the data acquisition should display the results. The cell pressurization history is determined from the measurements of the transmitted bar gauge. If the transmitted bar used is long enough, this should only be from the first disturbance to the point where the measurements show a negative pressure. The duration of the transmitted pulse may be shorter than the incident pulse if a bubble is trapped. The magnitude may not reach that of the incident pulse, but it should have a sufficiently long plateau before unloading. Otherwise, either a large air bubble in the sealed chamber or a misalignment between the assembly and a bar might have occurred.
- The cell pressurization chamber is then removed and disassembled inside the cell culture hood. The vent screw is removed and the piston is pulled free of the chamber. The cell-cultured glass slide is taken from the end of the piston.
4. Assessing Pressurized Cell Behavior
- After pressurization, the cells may be inspected immediately or further incubated for later examination. With proper aseptic operation processes, longer-term post-incubation is possible.
- Pressurized cells can be examined by all molecular and cellular biology techniques. Specifically for neuronal cell pressurization, to investigate the cellular and molecular physiology in TBI conditions, assays assessing pressure-induced changes in neurite outgrowth, microtubule cytoskeletal change, neuronal gene expression, apoptosis, etc., can be performed. As control cell samples, cells that are cultured the same, kept inside the pressurization chamber for the same time period, but not pressurized are used (so called, chamber control).
- For assessing the changes in neurite outgrowth, pressurized and chamber control cells are examined by optical microscope immediately after pressurization and 1 and 24 h of post-incubation. An example of neuronal cell images are shown in the ‘Representative Results’ section (Figure 2). The neurite length change can be quantified by actin immunofluorescent staining and image analysis. Cells are fixed with a 4% w/v paraformaldehyde solution, rinsed with a 0.05% v/v Tween-20 wash buffer, and permeabilized with a 0.1% v/v Triton X-100 solution. After blocking with a 1% w/v bovine serum albumin solution, cells are incubated with tetramethylrhodamine isothiocyanate (TRITC)-conjugated phalloidin. Immunofluorescent images of the cells are taken using a fluorescent microscope and the lengths of neurites for pressurized and control cells are quantified using the ImageJ software (NIH).
- The neurites can be identified as axons or dendrites. To assess the morphological changes in axons or dendrites, the experiments described above can be repeated by using antibodies detecting each structure, i.e., neurofilament (NF) antibody for axons and microtubule-associated protein (MAP2) antibody for dendrites.
- Microtubules are one of the key cytoskeletal components of neurons, and the damage to microtubules has been used as a marker of neuronal injury.2 Microtubule can be visualized immunofluorescently using the β-tubulin antibody. Cells are fixed after 0 and 24 h of post-pressurization, stained with β-tubulin antibody, and observed by the fluorescent microscope.
- To assess the effect of impulsive pressurization on neuronal and apoptotic gene expression, total RNA is extracted from pressurized and control cells. Gene expression can be examined by performing quantitative RT-PCR or real-time RT-PCR, similar to our published protocols.3
5. Representative Results:
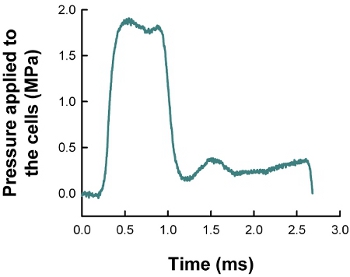
Figure 1. An example of pressure profile applied to the cells within the pressurization chamber. The Kolsky bar apparatus successfully generates 2 MPa level, single-pulse type impulsive pressurization with a duration of about 0.7 ms.
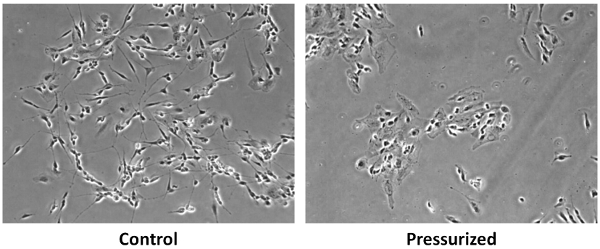
Figure 2. An example of neuronal cell response to impulsive pressurization. SH-SY5Y cells exposed to impulsive pressurization at 2 MPa show distinct neurite/axon breakdown relative to chamber control cells.
6. Difficulties and Solutions
- Chamber sealing is one of the major obstacles to be overcome. It is found that wide disks with o-rings have a problem of gouging and increased friction. In order to remove the friction effects, as well as prevent gouging, a simple method of piston taping with Teflon plumber’s tape is used. This solves the problems and produces desired pressure level and duration.
- Pressurized neuronal cell behavior, especially when assessing secondary TBI mechanism or longer term effects, should be examined after a long post-incubation time. The cell-containing chamber is exposed to potentially unsterile conditions while moving to the Kolsky bar, pressurizing, and moving back to the cell culture hood. With pre-sterilization of the chamber parts and proper operation in assembly/disassembly of the cell-cultured slide and chamber inside the cell culture hood, longer-term post-incubation is possible up to several weeks.
- The reproducibility of the data is an important parameter in cellular mechanical stimulation experiments. For our device, the reproducibility in neuronal cell response is determined how desired impulsive pressurization profile, in both pressure level and duration, is repeatedly obtained. Since we record the obtained pressure profile for each pressurization, the cell response data from unwanted pressure profile can be manually excluded afterwards.
- The entire pressurization steps, including chamber assembly, transfer and mount in the Kolsky bar, pressurization, transfer-back, and chamber disassembly, take less than 10 min. The next cell cultured slide can be pressurized immediately after the previous pressurization. Thus, sufficient number of pressurization experiments can be completed efficiently. Our set-up utilizes 18 mm diameter glass coverslips. One pressurization experiment does not provide enough proteins for western immunoblotting due to substrate size (and also partly because some of the pressurized cells are dead). About three repeated pressurization experiments provide protein amount required for immunoblotting. Again, the reproducibility is checked each time with the pressure profile.
Discussion
Many in vitro experimental techniques have been attempted for studying brain cell damage in TBI conditions. These include neuron/astrocyte stretching, flow-induced shear stress, weight drop, stylus laceration, laser transection, lithotripsy, etc.4,5 It has been assumed that short-duration overpressure is a dominating physical factor for TBI.6,7 Especially, the shockwave of the blast TBI has a pulse duration of an order of milliseconds.4 Based on this assumption and observation, barometer chamber capable of transient pressurization has been developed and used for assessing brain cell behavior under TBI conditions. For example, in the fluid percussion barotrauma chamber a pressure pulse with 20-30 ms long duration and a peak pressure of up to 0.5 MPa was used to examine the human glial cell behavior under TBI.6 Recently, VandeVord et al.7 used metal ball striking barometer chamber to examine the astrocyte cell behavior under TBI but the metal ball striking produced multiple pressure pulses. In this study, we have developed a novel cell pressurization device that generates single-pulse type impulsive pressurization. The device was developed based on the Kolsky bar set-up, which has been used as a material testing apparatus in our8 and other’s studies.1,9 The Kolsky bar’s material testing capability at very high loading rate has been modified to be able to apply single-pulse type overpressure to the cultured cells within the pressurization chamber (Figure 1). We could successfully control the magnitude and duration of the impulsive overpressure. As a representative cell response, we demonstrated that at 2 MPa pressure SH-SY5Y neuronal cells display significant neurite and axonal loss as compared to unpressurized chamber control cells.
In conclusion, we developed a novel impulsive cell pressurization apparatus using a specially designed Kolsky bar device and demonstrated its potential usage in investigating neuronal cell response in TBI conditions. We also note that this technique is very versatile in that any type of cells can be exposed to impulsive pressures at various level and duration. Therefore, the device can be used to investigate the impulsive pressure-induced cellular mechanotransduction behavior, not only for injuring the cells but also for positively stimulating the cells in their function and fate determination.
Declarações
The authors have nothing to disclose.
Acknowledgements
The authors would like to thank the funding sources: Army-UNL Center for Trauma Mechanics (DoD/ARO, #W911NF-11-1-0033, PI: Dr. Namas Chandra), UNL Layman Award (26-1110-0033-001, PI: Lim).
Materials
- SH-SY5Y (ATCC, CRL-2266) human neuroblastoma cells
- 18 mm diameter glass coverslips
- Cell culture media: DMEM, 10% fetal bovine serum, 1% penicillin-streptomycin
- Retinoic acid (10 μM) for inducing neurogenesis
- Kolsky bar impulsive pressurization device
- Piston-cylinder cell pressurization chamber
- Stainless steel screws for securing cell cultured coverslip on the piston
- Grease for interfaces between cell chamber and Kolsky bar
- General molecular biology supplies for assessing cell response to pressurization (fixative, antibody, fluorescent dye, PCR supplies, etc.)
Referências
- Kolsky, H. An investigation of mechanical properties of materials at very high rates of loading. Prec. Phys. Soc. London. 62, 676-700 (1949).
- Tang-Schomer, M. D., Patel, A. R., Baas, P. W., Smith, D. H. Mechanical breaking of microtubules in axons during dynamic stretch injury underlies delayed elasticity, microtubule disassembly, and axon degeneration. FASEB. J. 24, 1401-1410 (2010).
- Lim, J. Y., Taylor, A. F., Li, Z., Vogler, E. A., Donahue, H. J. Integrin expression and osteopontin regulation in human fetal osteoblastic cells mediated by substratum surface characteristics. Tissue. Eng. 11, 19-29 (2005).
- Chen, Y. C., Smith, D. H., Meaney, F. D. In vitro approaches for studying blast-induced traumatic brain injury. J. Neurotrauma. 26, 861-876 (2009).
- Ling, G., Bandak, F., Armonda, R., Grant, G., Ecklund, J. Explosive blast neurotrauma. J. Neurotrauma. 26, 815-825 (2009).
- Shepard, S. R., Ghajar, J. B. G., Giannuzzi, R., Kupferman, S., Hariri, R. J. Fluid percussion barotrauma chamber: a new in vitro model for traumatic brain injury. J. Surg. Res. 51, 417-424 (1991).
- VandeVord, P. J., Leung, L. Y., Hardy, W., Mason, M., Yang, K. H., King, I. A. Up-regulation of reactivity and survival genes in astrocytes after exposure to short duration overpressure. Neurosci. Lett. 434, 247-252 (2008).
- Huang, H., Feng, R. A study of the dynamic tribological response of closed fracture surface pairs by Kolsky-bar compression-shear experiment. Int. J. Solids. Struct. 41, 2821-2835 (2004).
- Song, B., Chen, W. Split Hopkinson pressure bar techniques for characterizing soft materials. Lat. Am. J. Solids. Struct. 2, 113-152 (2005).

