Preparation of Synaptoneurosomes from Mouse Cortex using a Discontinuous Percoll-Sucrose Density Gradient
Summary
A method to prepare translationally active, intact synaptoneurosomes (SNs) from mouse brain cortex is described. The method uses a discontinuous Percoll-sucrose density gradient allowing for the quick preparation of active SNs.
Abstract
Synaptoneurosomes (SNs) are obtained after homogenization and fractionation of mouse brain cortex. They are resealed vesicles or isolated terminals that break away from axon terminals when the cortical tissue is homogenized. The SNs retain pre- and postsynaptic characteristics, which makes them useful in the study of synaptic transmission. They retain the molecular machinery used in neuronal signaling and are capable of uptake, storage, and release of neurotransmitters.
The production and isolation of active SNs can be problematic using medias like Ficoll, which can be cytotoxic and require extended centrifugation due to high density, and filtration and centrifugation methods, which can result in low activity due to mechanical damage of the SNs. However, the use of discontinuous Percoll-sucrose density gradients to isolate SNs provides a rapid method to produce good yields of translationally active SNs. The Percoll-sucrose gradient method is quick and gentle as it employs isotonic conditions, has fewer and shorter centrifugation spins and avoids centrifugation steps that pellet SNs and cause mechanical damage.
Protocol
1. Preparations1-4
- Prepare 500 mL of gradient medium (GM) buffer by mixing 50 mL of a 2.5 M sucrose slurry, 2.5 mL of a 1M Tris-HCl, pH7.5 stock, and 0.1 mL of a 0.5 m EDTA, pH 8.0 stock with milli-Q water to volume. Filter sterilize the solution, aliquot and store frozen.
- Prepare a 1000x stock of tetrodotoxin (TTX) by making a 1 mM solution in milli-Q H2O. Aliquots of TTX can be stored at -20°C.
- Prepare 10x Stimulation Buffer by making a solution of 100 mM Tris (pH 7.5), 5 mM Na2HPO4, 4 mM KH2PO4, 40 mM NaHCO3, and 800 mM NaCl in milli-Q water. Stock can be stored at 4°C.
- Pre-cool centrifuges to 4°C.
- Prepare a stock solution of Isosmotic Percoll (SIP) by adding 9 volumes of Percoll (18 mL) to 1 volume of 2.5 M sucrose (2 mL) in milli-Q water and mix well.
- Prepare the 3, 10, 15, and 23% gradient layers by adding the respective amounts of SIP to GM buffer and mixing each:
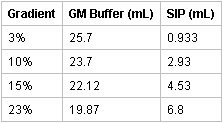
- Pour gradient layers by pipetting 2 ml each of the 23, 15, 10, and 3% isosmotic Percoll solutions into the Beckman centrifuge tubes with caps using a P1000 Gilson Pipetman. The interface between the layers should be clearly discernable with no mixing of the layers. Store the gradients at 4°C for at least 20 min before use. The gradients can be stored for up to 24 hours although same day use is recommended. [Inexperienced lab personnel may practice preparing gradients by adding blue dye to the isomotic Percoll solutions for visualization of the layer interfaces.]
2. Mouse dissection 1
- Ensure that all mouse husbandry and euthanasia procedures are performed in accordance with NIH and University guidelines. Euthanize pups (age P13 to P21) by carbon dioxide asphyxiation or by a method approved of in the animal protocol.
- Pin the mouse to a dissection board and spray the back of the neck and head with ethanol. Using a sharp scissors cut through the spinal cord at the base of the skull, remove the skin from the top of the skull, cut the skull laterally between the parietal bone and the interparietal bone or between the cerebrum and the cortex regions. Then cut the skull from the base to the nose (along the sagital suture), carefully remove the soft parietal bone by pulling each hemisphere to the side and remove the cortex with a spatula. Insert the spatula into the brain above the cerebellum and then scoop out the cortex and place it in ice-cold GM buffer. Proceed immediately to the homogenization step. Do not let the cortices sit in ice-cold buffer for very long as this will adversely affect the formation of SNs.
3. Preparation of homogenate and SNs 1-4
- Rinse the cortices in ice-cold GM buffer and transfer two cortices to a glass Dounce homogenizer containing 5 mL of cold GM buffer.
- Gently homogenized the cortices with 5-10 strokes of the loose pestle (pestle “A”), followed by 5-10 strokes of the tight pestle (pestle “B”). Strokes should be rapid but slow enough not to cause air bubbles. A picture of the homogenate after homogenization is in Scheme 1. The number of strokes will vary dependent on the individual homogenizer and accompanying pestles. When finished the gradients should give a strong SN band, if not, adjust the number of strokes.
- Transfer the homogenate to a 15 mL conical and centrifuge at 1000xg for 10 min at 4°C in a swinging bucket rotor (Allegra 6KR centrifuge) to pellet cellular debris and nuclei. A Picture of the spun homogenate is in Scheme 1.
- Layer 2 mL of the supernatant per Percoll-sucrose gradient, cap the tubes, and centrifuge at 32,500xg for 5 min at 4°C in a fixed-angle rotor (Beckman J2-21 centrifuge, JA-17 rotor) using appropriate adapters (See Reagents and Equipment Table).
- Pipette off and discard the solution above the SN band using a glass Pasteur pipette. Pipette off the SN band at the 15/23% interface, transfer to a conical and store on ice. Each gradient or one cortex will produce 0.9-1.1 mL of SN.
- Adjust the salt concentration of the SNs by adding one-tenth volume of 10x stimulation buffer, add 1000x CaCl2 to a final concentration of 12 nM, and add 1 mM TTX stock to a final concentration of 1 μM to suppress nonspecific excitation. (Addition of CaCl2 is optional if it will interfere with downstream SN applications).
- Determine the protein concentration of the SNs using the Micro BCA Protein Assay Kit. (The Bradford protein assay is not recommended because of inference from the Percoll.)
- SNs can be used directly and quickly for protein translation studies. For other applications SN lysate may be cleaned up or concentrated using the Pierce SDS-PAGE Sample Prep Kit.
4. Representative results:
When mouse cortex was homogenized and separated on discontinuous Percoll-sucrose gradients (Scheme 1), 6 bands or fractions were seen (Figure 1). It was previously shown for rat cerebral cortex3,5 that the constituents of the high molecular weight bands were broken membrane and myelin and that the pelleted material contained mitochondria or organelles (Table 1). The 23%/15% interface (Band 5) contained the enriched SNs.
The band at the 23%/15% interface, containing intact SNs, was removed and examined by electron microscopy (EM) as described previously.2,6 EM showed the presence of intact synaptic vesicles and the preservation of presynaptic and postsynaptic elements (Figure 2). Isolation of pre- and postsynaptic compartments is important for examining signaling and protein synthesis, which occurs in both compartments.7-13 The Percoll-sucrose gradient is a crude purification so we saw minor cellular, nuclear and organelle contamination in the SN fraction but the overall separation was good.
When examined by Western blot, the Percoll fractions contained the expected protein markers with an increase in synaptic purity in the SN band (Figure 3, Table 2). Synaptic and neuronal markers were maintained in the enriched SN band (Band 5) but the abundance of other markers was significantly reduced. In particular, the presence of GFAP, a marker for astrocyte and neoplastic cells of glial origin, and Lamin-B1, a nuclear marker, were negligible, while structural and synaptic markers were maintained or enhanced. In addition, there was retention of markers for organelles, such as the mitochondria and Golgi, which play a role in protein synthesis.
Typical yields for the SN band were 250-500 ng of protein per μL, as determined by BCA protein assay. The activity of the SNs was determined by the amount of de novo protein synthesis present as monitored by [35S]-Met incorporation (Figure 4).2 Basal translational activity was increased 1.56 fold when SNs were stimulated with 50 μM glutamate/10 μM glycine (Glu) or 5.12 fold when activated with the Pin1 inhibitor juglone. Pin1 inhibition is known to result in increased protein synthesis.2 To confirm that the increase in [35S]-Met incorporation was due to de novo protein synthesis, we added 40 μM anisomycin, a protein synthesis inhibitor, and saw a marked decrease in incorporation in the presence and absence of Glu when compared to basal levels. Also, upon the addition of 2% Triton-X 100, which disrupts the integrity of the SNs, basal translation was decreased by 75%.2 In addition, when examining the bands (B4-B6) that showed the most synaptic marker enrichment, the SNs or Band 5 had the greatest activity for de novo protein synthesis (Figure 5). Thus, the discontinuous Percoll-sucrose gradients quickly produce a highly active and enriched SN band that can be used to study protein translation.
The discontinuous Percoll-sucrose gradient procedure is more advantageous than other methods because mechanical damage is caused by harsher conditions. When compared to the filtration method, in which cleared cortical homogenate is passed through a series of filters that decrease in size,3,14-15 the Percoll method forms more SNs with greater activity. As seen in Figure 6, when an aliquot of SNs prepared by the filtration method was passed over a Percoll-sucrose gradient there are far less SNs at the 15/23% interface and a greater amount of broken membranes. When de novo protein synthesis was measured via S35-met incorporation, the SNs prepared by the filtration method were far less active (Figure 7). To maximize translational activity we use mice that are between age P14 and P18. SN can be prepared from adult mice, but we observed significantly increased translational activity with SN prepared from juvenile mice (Figure 8).

Table 1. Constituents of the bands from the discontinuous Percoll-gradient fractionations of homogenized mouse cortex.3,5
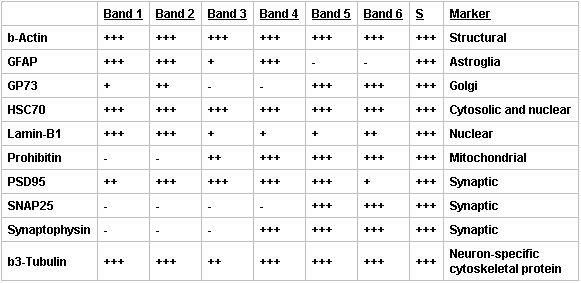
Table 2. Summary of Western bolt analysis of discontinuous Percoll-sucrose gradient band constituents. Relative protein concentrations for each of the band fractions isolated using a discontinuous Percoll-sucrose gradient were determined by Western blot. No protein present (-), 0≥0.2 (+), 0.2≥0.8 (++), or >0.8 (+++) fold greater than protein present in the supernatant (S, centrifuged cortex homogenate), representative of n=3.
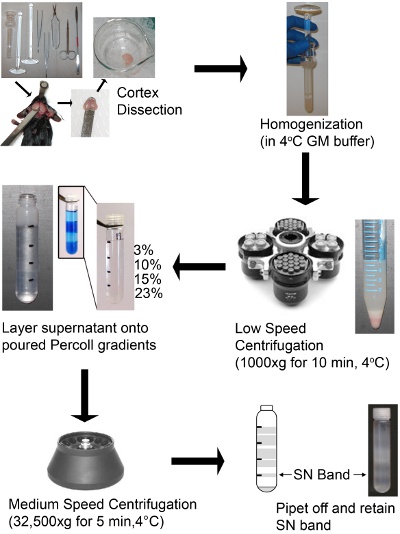
Scheme 1: Schematic of a SN preparation using a discontinuous Percoll-sucrose density gradient. Mouse brain cortices were dissected, homogenized, and centrifuged at low speed to remove non-homogenized tissue, cells, whole organelles such as nuclei and membranes. The discontinuous gradient should have clearly defined layers. The picture inset shows an example of the layers, which have been dyed blue for illustrative purposes only in order to visualize the layers. The supernatant is layered on top of a discontinuous Percoll-sucrose gradient and centrifuged at medium speed. The SN band is then retrieved from the gradient and is ready to use.
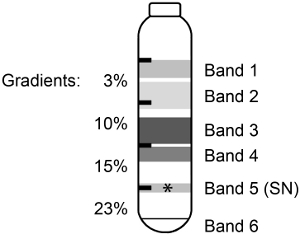
Figure 1. Homogenate fractionation on a discontinuous Percoll-sucrose gradient. Mouse cortex was homogenized and separated on a discontinuous Percoll-sucrose gradient giving rise to 6 bands or fractions. The purified, active SNs (*) were found in Band 5.
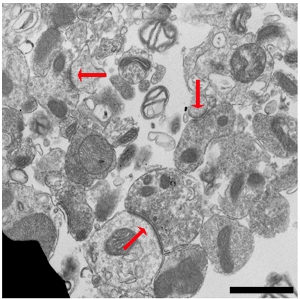
Figure 2. SN preparations give a heterogeneous mix of SNs that retain presynaptic and postsynaptic elements. An electron micrograph of an SN preparation prepared from C57BL/6 mice (age P13 to P21) was performed as previously described and shows SNs with preserved presynaptic and postsynaptic elements.2,6 Red arrows point to post-synaptic densities. Scale bar is 1 μm.
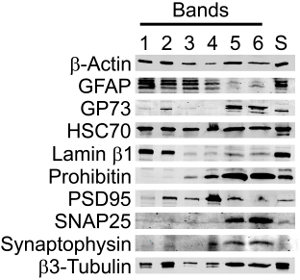
Figure 3. Western blot analyses of SN preparations showed that synaptic and neuronal markers were maintained in the purified SNs (Band 5) but the abundance of other markers was significantly reduced. Immunoblots of supernatant (S, centrifuged cortical homogenate) and Bands 1-6 of a discontinuous Percoll-sucrose gradient were probed for β-Actin (structural, loading control), GFAP (astrocytic), GP73 (Golgi), HSC70 (cytoplasmic and nuclear), Lamin-B1 (nuclear), Prohibitin (mitochondrial), PSD95 (postsynaptic density), SNAP25 (synaptic), Synaptophysin (synaptic) and β3-Tubulin (neuron-specific). The bands were visualized using a Storm 860 Phosphorimager, representative of n=3.
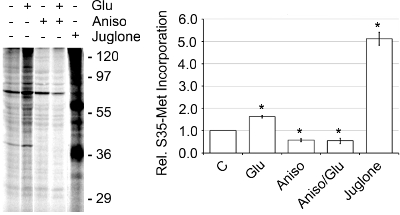
Figure 4. SNs are active and exhibit de novo protein synthesis via [35S]-Met incorporation. SNs were pre-equilibrated to 37°C for 10 min. Then the SNs were untreated or pretreated for 15 min with 40 μM anisomycin or 1 μM juglone at 37°C followed by addition of [35S]-Met, 20 μl Express Pro Label Mix S35 Easy Tag, plus or minus Glu at 37°C for 30 min. The samples were prepared using the Pierce SDS-PAGE Sample Prep Kit, run on a 15% polyacrylamide gel, dried and quantitated using a Molecular Dynamics Storm 860 phosphorimager, representative of n=3, ±SEM.
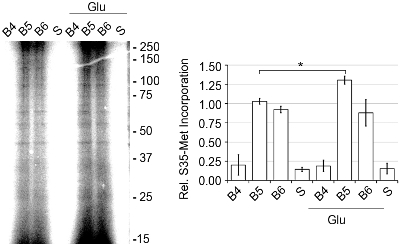
Figure 5. Band 5 from the discontinuous Percoll-sucrose gradient contained the highest levels of de novo protein synthesis. Bands 4, 5 and 6 and the supernatant (S, centrifuged cortical homogenate) were pre-equilibrated to 37°C for 10 min. Then, [35S]-Met, Express Pro Label Mix S35 Easy Tag, was added, plus or minus Glu at 37°C for 30 min. The samples were prepared using the Pierce SDS-PAGE Sample Prep Kit, run on a 15% polyacrylamide gel, dried and quantitated using a Molecular Dynamics Storm 860 phosphorimager, representative of n=3, ±SEM.
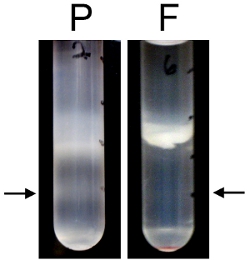
Figure 6. SNs prepared from a filtration method contain more broken membranes and less whole SNs than SNs prepared from the discontinuous Percoll-sucrose gradient method. SNs were prepared by passing homogenized cortex through a series of filters with decreasing pore size as described previously.3,14-15 An aliquot of the SNs prepared by the filtration method was centrifuged over a discontinuous Percoll-sucrose gradient and compared to an equivalent amount of material using the discontinuous Percoll-sucrose gradient method.
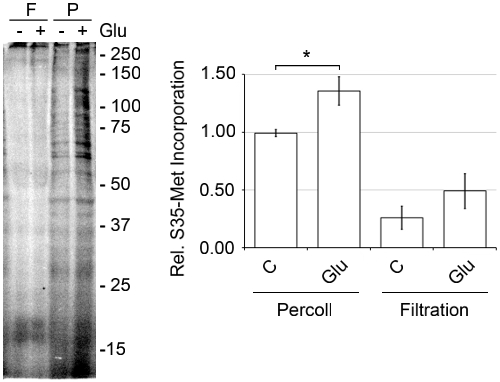
Figure 7. SNs prepared from a filtration method contain less de novo protein synthesis activity than SNs prepared from the discontinuous Percoll-sucrose gradient method. SNs were prepared by passing homogenized cortex through a series of filters with decreasing pore size as described previously,3,14-15 and by the discontinuous Percoll-sucrose gradient methods. SNs from both preparations were pre-equilibrated to 37°C for 10 min. Then, [35S]-Met, Express Pro Label Mix S35 Easy Tag, was added, plus or minus Glu at 37°C for 30 min. The samples were prepared using the Pierce SDS-PAGE Sample Prep Kit, run on a 15% polyacrylamide gel, dried and quantitated using a Molecular Dynamics Storm 860 phosphorimager, representative of n=3, ±SEM.
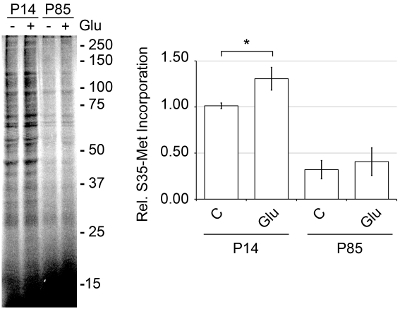
Figure 8. SNs from younger mice (P14) have greater translational activity than older mice (P85). SNs were prepared from different aged mice using the discontinuous Percoll-sucrose gradient method, preequilibrated at 37°C for 10 min, treated or untreated with Glu in the presence of [35S]-Met (Express Pro Label Mix S35 Easy Tag) for 30 min, snap frozen, and later cleaned up with the Pierce SDS-PAGE Sample Prep Kit. The SNs were then run on a 12% SDS-polyacrylamide gel, dried and imaged on a Molecular Dynamics Storm 860 phosphorimager, representative of n=3, ±SEM. SNs from P14 mice were at least 3-fold more active than P85 mice.
Discussion
The discontinuous Percoll-sucrose gradient preparation described herein is a quick, reliable method to isolate active SNs, which can be used in a variety of synaptic transmission experiments. This gradient method, which is based on the method developed by Dunkley et al.,3,4 is a subcellular brain fractionation procedure that isolates both pre- and postsynaptic membrane-derived vesicles that are associated with one another. Without further purification these SNs are compatible with a variety of molecular biology techniques, including immunoprecipitation, western blotting, flow cytometry, monitoring de novo protein synthesis via [35S]-Met incorporation, electron microscopy, and a variety of enzyme activity assays including isomerase and kinase activity assays.
The discontinuous Percoll-sucrose gradient procedure is relatively straight forward; however, it is key to keep solutions and brain material at 4°C throughout the preparation and to pre-chill the gradients. Although the homogenate is kept ice-cold at all times to minimize proteolysis, for better yields with greater activity it is best not to let the cortices sit on ice in GM buffer too long before homogenization. The formation of SNs is best when the cortices are rinsed and then expeditiously homogenized. The homogenate, however, can be kept on ice before the initial centrifugation without adverse affects. Speed is an issue once the cortices have been harvested, so the isolation of SNs should be completed as quickly as possible. Although SNs can retain high activity for 1-2 hours, prolonged periods of time between steps will result in decreased activity. The age of the mice used for the SN preparation is also a key issue. It has been shown previously that there is an age related decline in synaptic plasticity in rodents.16-19 We have seen the same effect in SNs. Although older mice can be used to make translationally active SNs, we have found that younger mice (P14) have greater translational activity then older mice (P85).
There are several variations and combinations of filtration,5,14-15,20-23 centrifugation,24-27 and gradient methods26-29that have been used to prepare SNs and each has its own advantages and disadvantages. Filtration and centrifugation methods can cause mechanical damage to the SNs. The methods that have utilized different pore sized filters resulted in a greater percentage of broken membranes and yield significantly less SNs. We tried the filter method,5,14-15 which passes the homogenate through a series of filters with decreasing pore size and found that the SNs prepared by the filtration method were significantly less active. The centrifugation methods also result in mechanical damage due to the prolonged spins at high speeds which pellet or compact the SNs at the bottom of tubes and require resuspension. Percoll employs milder conditions that avoid filtering or pelleting the SNs, which can crush or lyse the SNs. SN procedures that utilize Ficoll28,30-32 or high molarities of sucrose4,31 also result in damaged or less active SNs, impaired biological function and morphological changes.31-32 Ficoll can cause unwanted cell aggregation at physiological pH and gives osmotic gradients with increasing concentrations.31-32 To keep isoosmotic conditions, the gradients have to be compensated for with a salt gradient.31 SNs prepared with high concentrations of sucrose (1.4-0.8 M) result in smaller and less active SNs.4
The discontinuous Percoll-sucrose method overcomes many of the issues involved with other medias and centrifugation procedures. A major advantage of this method is the use of Percoll, which has a low viscosity, low osmolarity and relatively no toxicity towards cells and their constituents, thus making it better suited for density gradient experiments than alternatives. 4,29 In addition, Percoll can be prepared with sucrose to maintain the osmolarity needed to retain SN integrity.4 Using discontinuous Percoll-sucrose gradients reduces the centrifugation time from 30-45 min to 5 min, thus extending the useful lifespan of the SNs, which have a limited lifetime for activity. Another advantage of the Percoll gradients is the higher yield and activity of SNs when compared to methods that utilize filters to form SNs. We ran the SNs from the filter method over a Percoll-sucrose gradient. We found a barely discernable SN band and an abundance of higher molecular weight broken membranes on the gradient. Also, the translational activity of the SNs prepared by the Percoll method was significantly higher than with the filter method. Overall, Percoll is preferable to other methods because it employs milder conditions that avoid filtering and pelleting the SNs resulting in greater retention of synaptic activity and better vesicle formation. The advantage of our method over Dunkley, et al,3,4 is that a glass, glass Dounce homogenizer was used herein as opposed to the motor driven Teflon, glass homogenizer that damages most postsynaptic terminal attachments and leads to the postsynaptic elements being exposed to the media and very few being enclosed by intact membranes.
Significant work has focused on protein synthesis in the post-synaptic density11-13, but little work has been done to study protein synthesis in the presynaptic compartments. However, there is evidence that protein synthesis occurs in the presynaptic terminals.7-10 As can be seen in the electron micrograph of the SNs, pre- and postsynaptic attachments exists in the SN preparation making them an ideal system for future study of translational activity in both compartments.
Declarações
The authors have nothing to disclose.
Acknowledgements
We would like to thank B. K. August from the University of Wisconsin-Madison Electron Microscope Facility for the electron microscopy. This work was supported by NIH grants R01-DA026067 and P30-HD03352 (to J.S.M.).
Materials
| Name of the reagent | Company | Catalogue number | Comments (optional) |
|---|---|---|---|
| Micro BCA Protein Assay Kit | Pierce | 23235 | |
| CaCl2 | Fisher | C79-500 | |
| CO2 gas | Airgas (UW-MDS) | CD 50 | |
| EDTA | RPI | E57020 | |
| EtOH | Fisher | A407SK-4 | |
| HCl | Fisher | A142-212 | |
| Percoll | GE Healthcare | 17-0891-01 | |
| KH2PO4 | Fisher | P285-500 | |
| PierceSDS-PAGE Sample Prep Kit | Pierce | 89888 | |
| NaCl | RPI | S23020 | |
| NaHCO3 | Fisher | BP328-500 | |
| Na2PHO4 | Fisher | S381-500 | |
| Sucrose | RPI | S24060 | |
| Tris Base | RPI | T60040 | |
| Tetrodotoxin | Sigma | T5651 | |
| Express Pro Label Mix S35 Easy Tag | Perkin Elmer | NEG772 | |
| Equipment | Company | Catalogue number | Comments (optional) |
| Dissection tools | |||
| Dounce homogenizer, 7 mL (comes with two glass pestles labled ” A” and “B”) | Wheaton | ||
| P1000 Gilson Pipetman | Gilson | F123602 | |
| Allegra 6KR Centrifuge | Beckman Coulter | 366830 | |
| GH 3.8 Rotor, Swinging bucket rotor | Beckman Coulter | 360581 | |
| Beckman J2-21 Centrifuge | Beckman | ||
| Beckman tubes with caps | Beckman | 355672 | |
| White walled adapters | Beckman | 342327 | |
| Blue walled adapters | Beckman | ||
| JA-17 Rotor, Fixed-angle rotor | Beckman | 369691 |
Table of antibodies used for western blots:
| Name of Antibody | Company | Catalogue number | Host Species | Dilution Factor |
|---|---|---|---|---|
| β-Actin | Sigma | A5441 | mouse | 1:2000 |
| GFAP | Santa Cruz | sc-65343 | mouse | 1:200 |
| GP73 | Santa Cruz | Sc-134509 | rabbit | 1:200 |
| HSC70 | Santa Cruz | sc-7298 | mouse | 1:200 |
| Laminβ | Santa Cruz | Sc-6261 | goat | 1:200 |
| Prohibitin | Santa Cruz | sc-28259 | rabbit | 1:200 |
| PSD95 | Millipore | MAB1596 | mouse | 5 μg/μL |
| SNAP25 | AbCam | ab5666-100 | rabbit | 1:2000 |
| Synaptophysin | Millipore | MAB368 | mouse | 1:500 |
| β-3-Tubulin | Santa Cruz | sc-80016 | mouse | 1:200 |
Referências
- Westmark, C. J., Malter, J. S. FMRP mediates mGluR5-dependent translation of amyloid precursor protein. PLoS Biol. 5, e52-e52 (2007).
- Westmark, P. R., Westmark, C. J., Wang, S., Levenson, J., O’Riordan, K. J., Burger, C., Malter, J. S. Pin1 and PKMzeta sequentially control dendritic protein synthesis. Sci Signal. 3, ra18-ra18 (2010).
- Dunkley, P. R., Heath, J. W., Harrison, S. M., Jarvie, P. E., Glenfield, P. J., Rostas, J. A. A rapid Percoll gradient procedure for isolation of synaptosomes directly from an S1 fraction: Homogeneity and morphology of subcellular fractions. Brain Res. 441, 59-71 (1988).
- Dunkley, P. R., Jarvie, P. E., Robinson, P. J. A rapid Percoll gradient procedure for preparation of synaptosomes. Nat. Protoc. 3, 1718-1728 (2008).
- Hollingsworth, E. B., McNeal, E. T., Burton, J. L., Williams, R. J., Daly, J. W., Creveling, C. R. Biochemical characterization of a filtered synaptoneurosome preparation from guinea pig cerebral cortex: cyclic adenosine 3′:5′-monophosphate-generating systems, receptors, and enzymes. J. Neurosci. 5, 2240-2253 (1985).
- Yi, H., Leunissen, J., Shi, G., Gutekunst, C., Hersch, S. A novel procedure for pre-embedding double immunogold-silver labeling at the ultrastructural level. J. Histochem. Cytochem. 49, 279-284 (2001).
- Akins, M. R., Berk-Rauch, H. E., Fallon, J. R. Presynaptic translation: stepping out of the postsynaptic shadow. Front. Neural Circuits. 3, (2009).
- Jin, I., Kandel, E. R., Hawkins, R. D. Whereas short-term facilitation is presynaptic, intermediate-term facilitation involves both presynaptic and postsynaptic protein kinases and protein synthesis. Learn. Mem. 18, 96-102 (2011).
- Lyles, V., Zhao, Y., Martin, K. C. Synapse formation and mRNA localization in cultured Aplysia neurons. Neuron. 49, 349-356 (2006).
- Wagatsuma, A., Azami, S., Sakura, M., Hatakeyama, D., Aonuma, H., Ito, E. De Novo synthesis of CREB in a presynaptic neuron is required for synaptic enhancement involved in memory consolidation. J. Neurosci. Res. 84, 954-9560 (2006).
- Sutton, M. A., Schuman, E. M. Local translational control in dendrites and its role in long-term synaptic plasticity. J. Neurobiol. 64, 116-131 (2005).
- Li, K. -. W., Hornshaw, M. P., Van der Schors, R. C., Watson, R., Tate, S., Casetta, B., Jimenez, C. R. Proteomics analysis of rat brain postsynaptic density: implications of the diverse protein functional groups for the integration of synaptic physiology. J. Biol. Chem. 279, 987-1002 (2004).
- Peng, J., Kim, M. J., Cheng, D., Duong, D. M., Gygi, S. P., Sheng, M. Semi-quantitative proteomic analysis of rat forebrain postsynaptic density fractions by mass spectrometry. J. Biol. Chem. 279, 21003-21011 (2004).
- Kim, S. H., Fraser, P. E., Westaway, D., St. George-Hyslop, P. H. Group II metabotropic glutamate receptor stimulation triggers production and release of Alzheimer’s amyloid β42 from isolated intact nerve terminals. J. Neurosci. 30, 3870-3875 (2010).
- Weiler, I. J., Greenough, W. T. Potassium ion stimulation triggers protein translation in synaptoneurosomal poiyribosomes. Mol. Cell. Neurosci. 2, 305-314 (1993).
- Billard, J. M. Ageing, hippocampal synaptic activity and magnesium. Magnes Res. 19, 199-215 (2006).
- Murphy, G. G., Fedorov, N. B., Giese, K. P., Ohno, M., Friedman, E., Chen, R., Silva, A. J. Increased neuronal excitability, synaptic plasticity, and learning in aged Kvbeta1.1 knockout mice. Curr. Biol. 14, 1907-1915 (2004).
- Norris, C. M., Halpain, S., Foster, T. C. Reversal of Age-Related Alterations in Synaptic Plasticity by Blockade of L-Type Ca21 Channels. J. Neurosci. 18, 3171-3179 (1998).
- Sallert, M., Malkki, H., Segersträlea, M., Tairaa, T., Lauri, S. E. Effects of the kainate receptor agonist ATPA on glutamatergic synaptic transmission and plasticity during early postnatal development. Neuropharm. 52, 1354-1365 (2007).
- Quinlan, E. M., Philpot, B. D., Huganir, R. L., Bear, M. F. Rapid, experience-dependent expression of synaptic NMDA receptors in visual cortex in vivo. Nat. Neurosci. 2, 357-35 (1999).
- Scheetz, A. J., Nairn, A. C., Constantine-Patton, M. NMDA receptor-mediated control of proteins synthesis at developing synapses. Nat. Neurosci. 3, 211-216 (2000).
- Villasana, L. E., Klann, E., Tejada-Simon, M. V. Rapid isolation of synaptoneurosomes and postsynaptic densities from adult mouse hippocampus. J. Neurosci. Methods. 158, 30-36 (2006).
- Weiler, I. J., Greenough, W. T. Metabotropic glutamate receptors trigger postsynaptic protein synthesis. Proc. Natl. Acad. Sci. U.S.A. 90, 7168-7171 (1993).
- Gray, E. G., Whittaker, V. P. The isolation of nerve endings from brain: An electron microscopic study of cell fragments derived by homogenization and centrifugation. J. Anat. 96, 79-88 (1962).
- Gylys, K. H., Fein, J. A., Yang, F., Cole, G. M. Enrichment of Presynaptic and Postsynaptic Markers by Size-Based Gating Analysis of Synaptosome Preparations From Rat and Human Cortex. Cytometry A. 60, 90-96 (2004).
- Hajós, F. An improved method for the preparation of synaptosomal fractions in high purity. Brain Res. 93, 485-489 (1975).
- Bai, F., Witzmann, F. A. Synaptosome Proteomics. Subcell. Biochem. 43, 77-98 (2007).
- Rao, A., Steward, O. Evidence that protein constituents of postsynaptic membrane specializations are locally synthesized: analysis of proteins synthesized within synaptosomes. J Neurosci. 11, 2881-2895 (1991).
- Pertoft, H., Laurent, T. C., Catsimpoolas, N. Isopycnic separation of cells and cell organelles by centrifugation and modified colloidal silica gradients. Methods of Cell Separation. , 25-65 (1977).
- Ramarao, C. S., Acharya, S. R., Krishnan, K. S., Kenkare, U. W. High affinity uptake of L-glutamate and γ-aminobutyric acid in Drosophila melanogaster. J. Biosci. 11, 119-135 (1987).
- Munteanu, L. S., Dinu, A. Fractionation of granulocytes from whole human blood by centrifugation. Practical hints. Romanian J. Biophys. 14, 53-58 (2004).
- Vlasselaer, P., Van Palathumpat, V. Cell separation composition, kit and method. US patent. , (1997).

