Silk Film Culture System for in vitro Analysis and Biomaterial Design
Summary
Silk films are a novel class of biomaterials readily customizable for an array of biomedical applications. The presented silk film culture system is highly adaptable to a variety of in vitro analyses. This system represents a biomaterial design platform offering in vitro optimization before direct translation to in vivo models.
Abstract
Silk films are promising protein-based biomaterials that can be fabricated with high fidelity and economically within a research laboratory environment 1,2 . These materials are desirable because they possess highly controllable dimensional and material characteristics, are biocompatible and promote cell adhesion, can be modified through topographic patterning or by chemically altering the surface, and can be used as a depot for biologically active molecules for drug delivery related applications 3-8 . In addition, silk films are relatively straightforward to custom design, can be designed to dissolve within minutes or degrade over years in vitro or in vivo, and are produce with the added benefit of being transparent in nature and therefore highly suitable for imaging applications 9-13. The culture system methodology presented here represents a scalable approach for rapid assessments of cell-silk film surface interactions. Of particular interest is the use of surface patterned silk films to study differences in cell proliferation and responses of cells for alignment 12,14 . The seeded cultures were cultured on both micro-patterned and flat silk film substrates, and then assessed through time-lapse phase-contrast imaging, scanning electron microscopy, and biochemical assessment of metabolic activity and nucleic acid content. In summary, the silk film in vitro culture system offers a customizable experimental setup suitable to the study of cell-surface interactions on a biomaterial substrate, which can then be optimized and then translated to in vivo models. Observations using the culture system presented here are currently being used to aid in applications ranging from basic cell interactions to medical device design, and thus are relevant to a broad range of biomedical fields.
Protocol
1. Fabrication of Silicone Rubber Molds
- Produce or purchase a desired topographic surface for casting. For this publication a standard 100 mm etched silicon wafer will be described (Figure 1).
- Weigh out polydimethylsiloxane (PDMS) potting (component A) and catalyst (component B) solution in a 1:9 ratio (9 g potting and 1 g catalyst) as provided in the purchased kit.
- Mix solutions thoroughly to initiate curing process.
- Place silicon wafer surface within a casting dish.
- Weigh out 4.5 g of PDMS solution onto silicon wafer.
- Spread PDMS solution as to cover a 100 mm diameter area of the wafer surface.
- Tilt wafer to spread PDMS solution evenly.
- Cover wafer with 100 mm diameter petri dish lid.
- Place casting setup into 60 °C oven over night, making certain that curing is taking place on a flat surface.
- Place cured PDMS/silicon wafer into 70% ethanol bath before removal.
- Begin removing PDMS from wafer by using razor blade to lift edge (entire circumference) first.
- Gently pull PDMS off using forceps within a 70% ethanol bath being careful not to tear silicone rubber casting.
- Punch out individual PDMS molds using 14 mm hole punch. This diameter is designed to fit into a 24-well plate setup.
2. Production of Silk Solution
- Bring 2 L of distilled water (dH2O) to a boil within a glass beaker 7 .
- Cut 5 g of Bombyx mori silkworm cocoons into thirds.
- Dispose of extensively contaminated cocoons as indicated by excessive insect particulates coating the inner cocoon surface.
- Measure 4.24 g of sodium carbonate.
- Add sodium carbonate slowly to boiling dH2O volume to prevent boiling over of water, and allow complete dissolution before continuing.
- Add cocoon pieces to boiling dH2O for 40 min., and use a Teflon coated stir bar to stir cocoons during boiling process.
- After boiling, carefully drain dH2O into sink and ring out the silk extract by hand to remove excess water.
- Wash silk extract three times for 20 min. each in 1 L of dH2O in a lab beaker, and use a stir bar to circulate volume within beaker.
- After washing, ring out the silk extract by hand and place silk fiber extract inside a chemical hood to allow for drying for a 12 hr. period.
- Next day, weigh the dried silk fibers, which is typically ~3.5 g: ___________-g
- Prepare 9.3 M LiBr solution for a 20% w/v solution of silk. Utilize following equations to calculate necessary weight and volumes:
- (Silk extract weight from step 10) x (4) = ___________-mL of total 9.3 M LiBr solution
- [(807.705) x (LiBr volume from part 11.a)]/1000 = ___________ g of LiBr to add to dH2O
- Add measured LiBr weight into a glass beaker of the following dH2O volume:
- (0.8) x (calculated volume from step 11.a) = ___________ mL of dH2O
- Pour this solution into an appropriate sized graduated cylinder, and bring solution up to final volume as calculated in part 11.a.
- Place the silk extract into beaker and pour LiBr solution over the silk fibers making sure the silk fibers are immersed within LiBr solution using a lab spatula.
- Place the dissolved silk into 60 °C oven for 4 hr:
Start time: ___________
End time: ___________ - Using an appropriate sized syringe draw up 12 mL of the silk solution. Place an 18G needle on end of the syringe, and then inject the solution into a dialysis casette (3,500 MW coutoff, Slide-A-Lyzer, Thermo Scientific). After filling the cassette, draw the remaining air out of the cassette with the emptied syringe.
- Place filled dialysis cassette within 1 L of dH2O.
- Change dH2O volume after 1 hr, 4 hr, 8 hr and then every 12 hr 3x for a total of 6 changes:
- Begin: ___________
- 1hr: ___________
- 4hr: ___________
- 8 hr: ___________
- 12 hr: ___________
- 12 hr: ___________
- 12 hr: ___________
- End: ___________
- After dialysis, slowly collect the silk solution from cassettes with syringe. Place solution into 10,000 g rated centrifuge tubes.
- Centrifuge the solution twice at 10,000 g at 4 °C for 20-min each. After each centrifugation place supernatant into a new tube.
- Store thr silk solution in 4 °C refrigerator.
- Pipette 2 samples of 0.5 mL silk solution into small weigh dish, and place weigh dishes inside a 60 °C dry oven for 12 hours or until the silk solution dries.
- Weigh the remaining solid silk film from both samples to measure silk solution percent weight to volume protein concentration:
- Weight of combined 2 silk film samples:___________ mg
- (Weight from 23.a) x (100) = ___________% silk
3. Preparation of Silk Films and Culture System Setup
- Prepare PDMS casting surfaces by cleaning with clear tape to remove dust.
- Clean PDMS substrates by soaking in 70% EtOH and wash three times with dH2O.
- Place 14 mm PDMS molds onto holder plate, which is typically a 24 well plate lid.
- To produce a 50 μm thick film, spread 70 μls of 8% silk solution on PDMS molds using a liquid spreading tool that is typically a 1 mL syringe tip 2.
- Allow the silk films to dry uncovered on a clean bench running an air flow pressure of 150 Pa for a period of 90 min or until films are dry.
- Once films are dry place entire set of films, including PDMS molds, into a water-annealing chamber for >4 hrs to produce a water insoluble film. This is typically an empty desiccator chamber with water in the bottom of the basin pulled at a 25 kPa vacuum 15 .
- Remove silk films from water-annealing chamber and place onto a clean bench.
- Prepare a 70% EtOH bath within a 35 mm Petri dish, and place control substrates (i.e. glass or plastic surfaces) and stainless steel rings within wells for at least 10 min to sterilize.
- Remove silk films from PDMS molds using forceps, dip them into 70% EtOH, and place sample into 24-well plate prefilled with 1 mL of 70% EtOH making sure patterned side is facing up to allow for cell adhesion.
- After placing each silk film sample into a corresponding well place stainless steel ring weights (11.65 mm inner diameter, 15.45 mm outer diameter, and 1.18 mm thickness) on top.
- Allow samples with rings to incubate for 10 minutes to ensure sterility.
- Remove EtOH and wash each sample 3x with 1 mL of PBS. Let each wash sit for 5 min to allow for complete diffusion.
- Remove PBS using aspirating glass pipette, while making sure to remove majority of bubbles beneath silk film samples.
- Prepare cell line for seeding. As an example, trypsinize human corneal-limbal epithelial (HCLE) cell line with 0.25% trypsin and ethylenediaminetetraacetic acid (EDTA) solution for 7-min. Deactivate trypsin using 1:1 volume of Dulbecco’s modified Eagle medium (DMEM) passage media with 10% FBS added. Centrifuge trypsinized HCLE cells, add 8-mL of keratinocyte-serum free media (K-SFM) to cell pellet, gently agitate to disperse HCLEs, and generate cell count.
- Sample 500 μL of HCLE suspension per well typically using a 10,000 cells/cm2 density.
- Place cultures within incubator at 37 °C and 5% CO2 and run desired experimental protocol.
4. Representative Results

Figure 1. Flow chart illustrating summarized 10-step process of silk film production and culture system preparation.
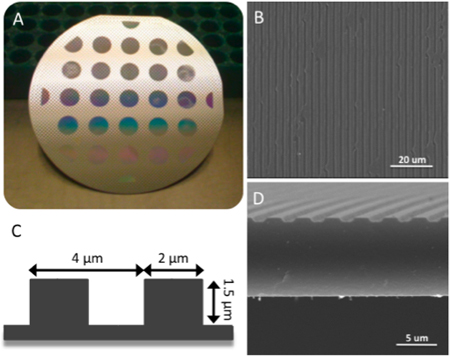
Figure 2. (A) 21-dye array of patterned line surface topographies produced upon a 90 mm diameter silicon wafer employing standard photolithographic and dry etching techniques. (B) Silk films retain original parallel line patterned features after casting on PDMS molding surfaces. (C) Schematic demonstrating feature size design chosen to promote cellular alignment. (D) Cross-section of silk film illustrating retained parallel lined patterned surface.
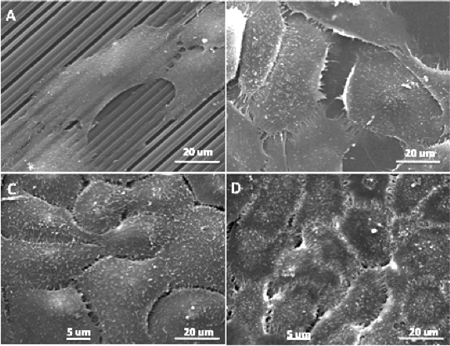
Figure 3. Scanning electron micrographs of HCLE cell line adhering to (A) patterned and (B) flat silk films at day 2 in culture. HCLE cultures continue to proliferate to confluence on (C) patterned and (D) flat surfaces by day 8 in culture.
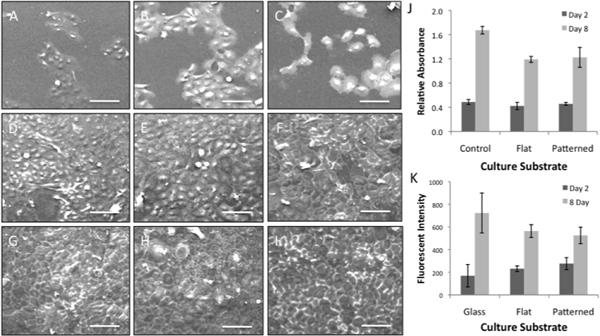
Figure 4. (A, D, G) Patterned and (B, E, H) flat silk films culture HCLE cells comparatively to (C, F, I) glass control substrates at (A-C) day 1, (D-F) day 4, and (G-I) day 8 in culture. (J) CyQuant nucleic acid content and (K) (3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) metabolic activity assay data demonstrating HCLE viability on both patterned and flat silk film substrates when compared to glass control surfaces over time (scale bars = 100 μm).
Video 1. Time lapse phase-contrast imaging of HCLE cells migrating over a patterned silk film surface during an 18 hr. time period. Cells were seeded at 10k/cm2 density and cultured for 2 hr. before imaging. Click here to view movie.
Video 2. Time lapse phase-contrast imaging of HCLE cells migrating over a flat TCP control surface during an 18 hr. time period. Cells were seeded at 10k/cm2 density and cultured for 2 hr. before imaging. Click here to view movie.
Discussion
The use of regenerated silk films as a substrate for cell culture has gained in popularity over the past two decades due to extensive characterization of the material properties of this protein and increased understanding of its biomaterial utility 3,8 . The culture system described here represents a novel in vitro testing system for assessing cell surface interactions on patterned silk film biomaterial substrates 7 . The system allows for in depth analysis of cellular interactions over time that can be easily adopted for high-throughput data collection. This is largely enabled because silk films possess a number of tunable biomaterial properties that can be modified to directly affect cell function 8,9,12, including: control of surface micro/nano-surface topography 7; various surface chemistries through covalent modification or adsorption of biologically active molecules 13; robust mechanical properties 15,16; control of material hydrophilicity/hydrophobicity 16 ; bulk loading of biological compounds for release 4,8,17 ; and controlled dissolution/enzymatic degradation rates through control of the secondary structure (beta sheet content) 11,18,19 .
The transparency of silk films is achieved through annealing the films for a period of time under vacuum in the presence of water vapor 7,15. This processing approach allows for the formation of β-sheet secondary structure, fostering the insolubility of the material in water while allowing minimal diffraction of light 15. This transparency of films is key in enabling direct live-cell imaging that can be employed under a number of imaging modalities (i.e. wide-field and fluorescence) using any number of microscope systems 12,20. In addition to live-cell imaging, silk films can be easily removed from the culture system to allow for additional fixation and analysis. Thus, the large variety of direct experimental assessments that can be performed on this system are applicable to a wide variety of cell/tissue sources for many technical fields 3,8,9,12,13,21. The time-lapse imaging results illustrate how real-time culture data can be collected, and as an example were utilized to illustrate how surface topography affects cell interactions. The representative results demonstrate how silk film biomaterials can be utilized to support HCLE culture growth, and are amendable to a number of standard cellular proliferation and metabolic assays (Fig. 4). In addition, the cultures are fixed and processed for scanning electron imaging or other protocols (Fig. 3).
Silk film substrates are produced in the lab with high fidelity, consistency, and with relatively low cost (Fig. 1). This enables reproducibility in both culture system setup and experimental outcomes. It has been demonstrated that water-anneal processing produces a stable silk film material within culture that has defined degradation rates pending the concentration of proteases in solution 2,15,22. As a result these materials may be used for extended periods of time for long-term cell culture, or remain implanted for months or years depending on the physiological location 8. In addition, recent work has shown that both the protein structure and material properties of water-annealed silk films are consistent from batch to batch allowing for reproducible culture results as shown through various mechanical and biophysical testing methods 15,16. In addition, the material’s surface has shown great fidelity amongst film batches as indicated by SEM, atomic force micrscopy (AFM), and cell culture studies {Lawrence:2008wr, Omenetto:2008tc, Bray:2011kq}7,23,24. Material stability and consistency is an important factor to how the cell will sense the culture substrate through the various mechanotransduction pathways, and ultimately produce a desired/undesired cellular response 25,26.
Historical standards for culture substrates, such as tissue culture treated plastic or glass, provide adequate substrates for cell attachment. However, these materials are not amendable for further utility in vivo. It can be envisioned that a silk film biomaterial could be customized in vitro, and once experimental expectations have been achieved the customized film can be directly translated to an in vivo model. Such paired design between in vitro and in vivo experimentation offers a great advantage for such implantable silk biomaterials over other substrates that are routinely used in vitro.
Declarações
The authors have nothing to disclose.
Acknowledgements
Funding from NIH K08EY015829, R21EY019561, R24EY015656, P41 EB002520, and R01 EY020856 Research to Prevent Blindness Career Development Award, and Tri-Institutional Stem Cell Initiative. HCLE cell line provided courtesy of Dr. Ilene Gipson. The authors would like to thank Dr. Aihong Liu at Weill Cornell Medical College for her technical assistance and guidance with cell culture, Anthony Labissiere at the Hospital for Special Surgeries for her technical assistance with SEM imaging, the Tissue Engineering Resource Center (TERC) at Tufts University for technical support with material development, and the Cornell Center for NanoScale Science and Technology Facility (CNF) for assistance in silicon wafer manufacturing.
Materials
| Material Name | Company | Catalogue Number |
| Silk cocoons | Tajima Shoji Co., LTD. | NA |
| PDMS monomer and cross-linker | Momentive | RTV615A 01P |
| Sodium Carbonate | Sigma | S2127 |
| Lithium Bromide | Sigma | 213225 |
| Slide-A-Lyzer | Thermo Scientific | 66110 |
| 1 mL Syringe | Becton-Dickenson | 309602 |
| Stainless steel washer | Superior Washer | 81610 |
| 24-well plate | VWR | 353047 |
Referências
- Rockwood, D., Preda, R. C., Yucel, T., Wang, X., Lovett, M. L., Kaplan, D. L. Materials Fabrication from Bombyx mori Silk Fibroin. Nature protocols. , (2011).
- Lawrence, B., Omenetto, F., Chui, K., Kaplan, D. Processing methods to control silk fibroin film biomaterial features. Journal of materials science. 43, 6967-6985 (2008).
- Altman, G., Diaz, F., Jakuba, C., Calabro, T., Horan, R., Chen, J. Silk-based biomaterials. Biomaterials. 24, 401-416 (2003).
- Hofmann, S., Wong, P. o., Foo, C., Rossetti, F., Textor, M., Vunjak-Novakovic, G., Kaplan, D. Silk fibroin as an organic polymer for controlled drug delivery. Journal of Controlled Release. 111, 219-227 (2006).
- Demura, M., Asakura, T. Immobilization of glucose oxidase with Bombyx mori silk fibroin by only stretching treatment and its application to glucose sensor. Biotechnology and bioengineering. 33, 598-603 (1989).
- Cebe, P., Kaplan, D. Mechanism of enzymatic degradation of beta-sheet crystals. Biomaterials. , (2010).
- Lawrence, B., Cronin-Golomb, M., Georgakoudi, I., Kaplan, D., Omenetto, F. Bioactive silk protein biomaterial systems for optical devices. Biomacromolecules. 9, 1214-1220 (2008).
- Meinel, L., Hofmann, S., Karageorgiou, V., Kirker-Head, C., McCool, J., Gronowicz, G. The inflammatory responses to silk films in vitro and in vivo. Biomaterials. 26, 147-155 (2005).
- Vepari, C., Kaplan, D. Silk as a biomaterial. Progress in Polymer Science. 32, 8-9 (2007).
- Li, M., Ogiso, M., Minoura, N. Enzymatic degradation behavior of porous silk fibroin sheets. Biomaterials. 24, 357-365 (2003).
- Arai, T., Freddi, G., Innocenti, R., Tsukada, M. Biodegradation of Bombyx mori silk fibroin fibers and films. Journal of Applied Polymer Science. 91, 2383-2390 (2004).
- Lawrence, B., Marchant, J., Pindrus, M., Omenetto, F., Kaplan, D. Silk film biomaterials for cornea tissue engineering. Biomaterials. 30, 1299-1308 (2009).
- Ma, X., Cao, C., Zhu, H. The biocompatibility of silk fibroin films containing sulfonated silk fibroin. Journal of Biomedical Materials Research Part B: Applied Biomaterials. 78, 89-96 (2006).
- Patel, A., Thakar, R., Chown, M., Ayala, P. Biophysical mechanisms of single-cell interactions with microtopographical cues. Biomedical. , (2010).
- Jin, H., Park, J., Karageorgiou, V., Kim, U., Valluzzi, R., Cebe, P. Water-Stable Silk Films with Reduced β-Sheet Content. Advanced Functional Materials. 15, 1241-1247 (2005).
- Lawrence, B., Wharram, S., Kluge, J., Leisk, G., Omenetto, F., Rosenblatt, M. Effect of Hydration on Silk Film Material Properties. Macromolecular Bioscience. 10, 393-403 (2010).
- Zhang, Y. Natural silk fibroin as a support for enzyme immobilization. Biotechnology Advances. 16, 961-971 (1998).
- Cebe, P., Kaplan, D. Mechanism of enzymatic degradation of beta-sheet crystals. Biomaterials. , (2010).
- Shaw, J. Fractionation of the fibroin of Bombyx mori with trypsin. Biochemical Journal. 93, 45 (1964).
- Rice, W., Firdous, S., Gupta, S., Hunter, M., Foo, C., Wang, Y. Non-invasive characterization of structure and morphology of silk fibroin biomaterials using non-linear microscopy. Biomaterials. 29, 2015-2024 (2008).
- Chirila, T., Barnard, Z., Harkin, D., Schwab, I., Hirst, L. Bombyx mori silk fibroin membranes as potential substrata for epithelial constructs used in the management of ocular surface disorders. Tissue Engineering Part A. 14, 1203-1211 (2008).
- Hu, X., Shmelev, K., Sun, L., Gil, E. -. S., Park, S. -. H., Cebe, P. Regulation of Silk Material Structure by Temperature-Controlled Water Vapor Annealing. Biomacromolecules. 12, 1686-1696 (2011).
- Omenetto, F., Kaplan, D. A new route for silk. Nature Photonics. 2, 641-643 (2008).
- Bray, L. J., George, K. A., Ainscough, S. L., Hutmacher, D. W., Chirila, T. V., Harkin, D. G. Human corneal epithelial equivalents constructed on Bombyx mori silk fibroin membranes. Biomaterials. 32, 5086-5091 (2011).
- Wang, N., Tytell, J. D., Ingber, D. E. Mechanotransduction at a distance: mechanically coupling the extracellular matrix with the nucleus. Nat. Rev. Mol. Cell Biol. 10, 75-82 (2009).
- Jaalouk, D. E., Lammerding, J. Mechanotransduction gone awry. Nat. Rev. Mol. Cell Biol. 10, 63-73 (2009).

