Isolation and Culture of Human Fungiform Taste Papillae Cells
Summary
We aimed to develop a reproducible protocol for isolating and maintaining long-term cultures of human fungiform taste papillae cells. Cells from human fungiform papillae obtained by biopsy were successfully maintained in culture for more than eight passages (12 months) without loss of viability.
Abstract
Taste cells are highly specialized, with unique histological, molecular and physiological characteristics that permit detection of a wide range of simple stimuli and complex chemical molecules contained in foods. In human, individual fungiform papillae contain from zero to as many as 20 taste buds. There is no established protocol for culturing human taste cells, although the ability to maintain taste papillae cells in culture for multiple cell cycles would be of considerable utility for characterizing the molecular, regenerative, and functional properties of these unique sensory cells. Earlier studies of taste cells have been done using freshly isolated cells in primary culture, explant cultures from rodents, or semi-intact taste buds in tissue slices1,2,3,4. Although each of these preparations has advantages, the development of long-term cultures would have provided significant benefits, particularly for studies of taste cell proliferation and differentiation. Several groups, including ours, have been interested in the development and establishment of taste cell culture models. Most attempts to culture taste cells have reported limited viability, with cells typically not lasting beyond 3-5 d5,6,7,8. We recently reported on a successful method for the extended culture of rodent taste cells9. We here report for the first time the establishment of an in vitro culture system for isolated human fungiform taste papillae cells. Cells from human fungiform papillae obtained by biopsy were successfully maintained in culture for more than eight passages (12 months) without loss of viability. Cells displayed many molecular and physiological features characteristic of mature taste cells. Gustducin and phospholipase C β2, (PLC-β2) mRNA were detected in many cells by reverse transcriptase-polymerase chain reaction and confirmed by sequencing. Immunocytochemistry analysis demonstrated the presence of gustducin and PLC-β2 expression in cultured taste cells. Cultured human fungiform cells also exhibited increases in intracellular calcium in response to appropriate concentrations of several taste stimuli indicating that taste receptors and at least some of the signalling pathways were present. These results sufficient indicate that taste cells from adult humans can be generated and maintained for at least eight passages. Many of the cells retain physiological and biochemical characteristics of acutely isolated cells from the adult taste epithelium to support their use as a model taste system. This system will enable further studies of the processes involved in proliferation, differentiation and function of mammalian taste receptor cells in an in vitro preparation.
Human fungiform taste papillae used for establishing human fungiform cell culture were donated for research following proper informed consent under research protocols that were reviewed and approved by the IRB committee. The protocol (#0934) was approved by Schulman Associates Institutional Review Board Inc., Cincinnati, OH. Written protocol below is based on published parameters reported by Ozdener et al. 201110.
Protocol
1. Obtaining Human Fungiform Taste Papillae
- Remove four to eight human fungiform taste papillae from the dorsal surface of the anterior portion of the tongue using curved spring microscissors.
- Place immediately into an isolation solution (26 mM NaHCO3, 2.5 mM NaH2PO4, 20 mM glucose, 65 mM NaCl, 20 mM KCl, and 1 mM EDTA dissolved in nuclease-free water and filter sterilized).
2. Human Fungiform Taste Papillae Digestion
Human fungiform taste cell papillae can be dissociated using two different enzymatic digestion protocols with slight differences.
- Incubate fungiform papillae in isolation solution with pronase (1 mg/ml, Sigma Aldrich) and elastase (1 mg/ml Sigma Aldrich) at room temperature for 30-45 min (protocol 1).
- Incubate fungiform papillae with Collagenase type I (550 U/mL, from Worthington) + elastase (10 U/mL, from Worthington) + Trypsin inhibitor (0.9 mg/mL, from Worthington) in 2 ml calcium free Ringer solution in 35 °C water bath with circulation for 30 minutes and gentle oxygenation with 95% O2 / 5% CO2. (Alternative method for digestion, protocol 2)
- After incubation, wash papillae with ringer and triturate the papillae with fire polished glass Pasteur pipette for 10 times.
- Centrifuge it for 3 minutes at 2500 rpm at RT and remove isolation solution and add 1 ml of taste cell culture medium.
- Transfer digested fungiform papillae into glass dish.
- Dissect fungiform papillae gently with surgical razor.
- Add 250 μl of dissected papillae into cloning cylinder onto rat tail collagen type-1 coated coverslip.
- Add 1 ml of taste cell culture medium into each well.
3. Culturing of Human Fungiform Taste Papillae Cells
- Incubate plate at 36 °C in a humidified incubator containing 5% CO2.
- Place in an incubator undisturbed for 2 days prior to the first change of complete medium. Taste cells will eventually bind to the coated coverslip, although it may not be clearly visible in 1 to 3 days.
- Remove cloning cylinder from plate and medium completely and add 1 ml of taste cell medium into each well.
- Replace 1/3 of medium every 6-7 days. Do not change medium more often and/or completely.
- Check cell growth under microscope every other day. Most of the new cells grow underneath cell clusters. Cell clusters usually detach after 2-3 weeks in culture leaving the newly generated cells.
- Once 40-50% of the cloning cylinder is covered in expanding taste cells, trypsinize cells using 0.25% w/v trypsin/EDTA for 2-3 minutes at 36 °C.
- Transfer cells from wells into 15 ml tubes, add 3 volumes of taste cell culture medium followed by centrifugation at 3000 rpm for 5 minutes at room temperature.
- Remove supernatant and resuspend cells with 1 ml of taste cell medium.
4. Propagation of Human Fungiform Taste Papillae Cells
- Transfer cells into T25 plate and add 4 ml of taste cell medium (passage 0). Maintain cells at 36 °C in a humidified incubator containing 5% CO2.
- Replace 1/3 of medium every 6-7 days until cultured taste cells have reached 100% confluence. At this time, to enable taste cells to harvest, wash cells once with sterile PBS then trypsinize cells using 0.25% w/v trypsin/EDTA for 2-3 minutes at 36 °C.
- After centrifugation as described above, resuspend in complete taste cell medium and transfer cells to fresh T-75 flasks (passage 1).
- Replace 1/3 of medium every 6-7 days. Do not change medium more often and/or completely.
- Repeat step 4.3 and 4.4 when cells have reached close to 100% confluence. Split cells to no more than 1:4 dilution in a T75 flask for maintaining adequate growth of the cells over time.
5. Freezing and Thawing Cultured Human Fungiform Taste Papillae Cells
- To freeze stocks of primary taste cells, after trypsinization (step 4.2), add 3 ml complete taste cell medium and transfer cells to sterile 15 ml conical centrifuge tubes. Centrifuge at 2500 rpm for 5 min at room temperature.
- Carefully remove the supernatant and gently resuspend cells with appropriate volume of freezing medium containing 95% Fetal Bovine serum (FBS) and 5% DMSO.
- Transfer cells to labeled, sterile cryovials, cap tightly, and place in a freezing container containing isopropanol. Place into a -80 °C freezer for at least one day prior to transferring indefinitely to liquid nitrogen.
- To thaw a vial of frozen primary human fungiform taste papillae cells, place at 37 °C water bath until just thawed (approximately 1 minute), while working in a laminar-flow cell culture hood.
- Transfer cells and freezing medium to a sterile 15 ml conical centrifuge tube and add 5 ml of taste cell culture medium.
- Centrifuge cells at 2500 rpm for 5 min at room temperature. Carefully discard supernatant, gently resuspend cells with complete taste cell culture medium, and transfer to a sterile T-25 tissue culture flask.
- Continue to culture cells according to Step 3 to 4.
6. Representative Results
Attempts to grow taste biopsies in culture were successful 90% of the time. A few days after biopsy and dissociation, cells typically began to grow and migrate outwards from the pieces of dissected fungiform tissue that remained after the dissociation procedure. Although individual cells were visible 24-48 hr after plating (Figure 1A), cells grew for up to 2-3 weeks under attached cell clusters, occasionally generating daughter cells (Figure 1B). From biopsying 4-8 human fungiform papillae, initial isolates of primary human fungiform taste papillae cells reach approximately 2000 – 8000 cells in 4 weeks in culture. Expansion in T25 cell culture flasks (passage 0) for additional 3-4 weeks will result in having human fungiform taste papillae cells in culture adapted (Figure 1C). Primary human fungiform taste papillae cells can be further expanded in culture to yield upwards of at least a million cells by passage-1 in 3-4 weeks (Figure 1D). Primary taste cells will continue to grow for more than 8 passages.
The morphology of the cultured human fungiform taste papillae cells in culture was similar to cultured chemosensory cells described previously11,12.. Most cultured human fungiform taste papillae cells maintained their original compact appearance of cell bodies with or without one or more processes up to 15-30 days. After they reached confluence most of the cultured cells had a polarized-elongated appearance. Mature taste cells consist of several different histological subtypes and exhibit taste cell specific features. Here we show that cells within these cultures display many molecular and physiological features characteristic of mature taste cells. Gustducin and phospholipase C – β2, (PLC-β2) mRNA were detected by reverse transcriptase-polymerase chain reaction and products confirmed by sequencing (Figure 2). Expression of gustducin and PLC-β2 was detected immunocytochemically indicating the presence of type II-like cells (Figure 3 B and D respectively). The prevalence and relative expression patterns of gustducin and PLC-β2 reflecting cell signaling pathways and cell types does not precisely reflect that seen in vivo. The factors governing the relative distribution of different cell types within a taste bud are unknown, and may not be replicated in the culture conditions. Therefore, both the similarities and differences between the cultured cells and their in vivo counterparts provide an opportunity to examine the regulation of these characteristics at a molecular level, under conditions which can be manipulated and monitored.
An important criterion for a model system is the presence of relevant functional properties. Cultured cells also exhibited robust increases in intracellular calcium in response to appropriate concentrations of several taste stimuli (Figure 4). In these studies, sweet and bitter stimuli elicit an increase in intracellular calcium that may correspond to a depolarization. These taste cell-like properties of the cultured cells lend support to the assertion that the model system we describe here will be a valuable asset for further studies of the transduction pathways and regenerative capacity of human taste cells.
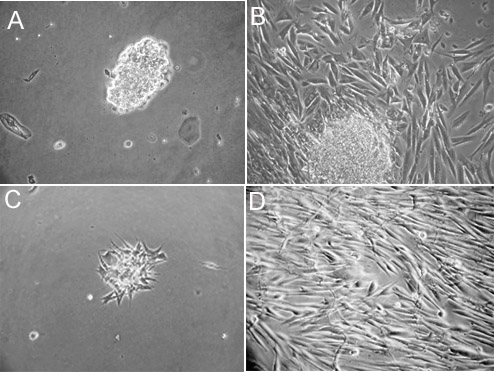
Figure 1. Attachment and morphology of cultured human fungiform taste papillae cells. Primary human fungiform taste papillae cell cultures grown on collagen type-1 coated plates were imaged after 2 days (A). Human fungiform taste papillae cells grew for up to 4 weeks under attached cell clusters, which seemed to give rise to daughter cells. The cells typically grew to confluence within four weeks (B), and (C and D) represents day 2 and 4 weeks after harvesting and reseeding respectively. Cultured cells continued to grow for at least 8 months. Scale bars = 50 nm. Part of Figure 1 was also presented in Ozdener et. al.10.
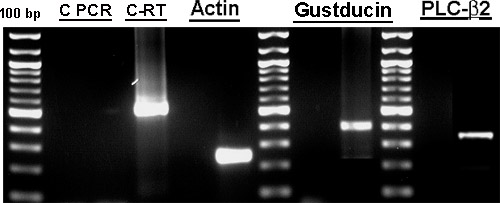
Figure 2. RT-PCR results demonstrated the presence of specific taste cell biomarker mRNAs (gustducin and PLC-β2,). Total RNA from cultured human fungiform cells was reverse transcribed using the Superscript First Strand Synthesis System for RT-PCR (Invitrogen) and used for PCR by amplifying with specific primers designed for detection of gustducin and PLC-β2. Primers (Table 1) were chosen to span one or more introns. Each mRNA was detected in cultured human fungiform taste papillae cells from passage 4 or 5 with amplification products of the expected size, confirmed by sequencing. Specific mRNA was not detected in control experiments without reverse transcriptase indicating no genomic DNA contamination. PCR products were separated on 2% agarose gels and the amplified products on the gel were excised with a razor. DNA was extracted from the gel using QIAquick Gel Extraction Kit (Qiagen). PCR products were sequenced at the University of Pennsylvania Sequencing facilities. C-PCR and C-RT are control experiments for PCR and Reverse Transcriptase, respectively. Part of Figure 2 was also presented in Ozdener et. al.10.
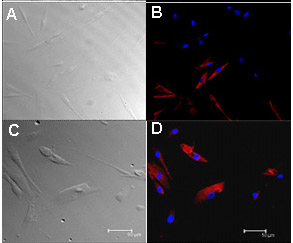
Figure 3. Immunostaining of cultured human fungiform taste papillae cells passage 4 or 5 showed presence of taste cell biomarkers. Cultured human fungiform taste papillae cells (5 days old) were fixed with 4% paraformaldehyde, and incubated with primary antibodies (Table 2) overnight at 4 °C. Images were acquired with a Leica TCS-SP2 confocal laser scanning microscope. Approximately, 60% of cultured fungiform taste papillae cells were labeled with gustducin (B) and 20-30 % with PLC-β2 (D). Transmission images of corresponding cells are shown (A and C). For controls, immunostaining with antibody specific immunoglobulin demonstrated the absence of nonspecific immuno reactivity (data not shown). Scale bars = 50 nm. Part of Figure 3 was also presented in Ozdener et. al.10.
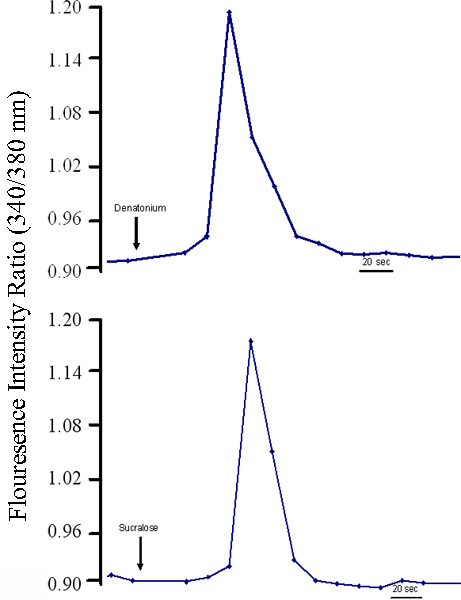
Figure 4. Cultured human fungiform taste papillae cells from passage 4 or 5 respond to different stimuli. Cultured human fungiform taste papillae cells (4-5 days old) were loaded with 1mM Fura-2 AM (Molecular Probes) and 10 mg/ml Pluronic F127 (Molecular Probes). Changes in intracellular calcium levels ([Ca2+]i) in cultured human fungiform taste papillae cells were measured using standard manual imaging techniques. The cells were visualized using an inverted fluorescence microscope at excitation wavelengths of 340 nm and 380 nm and an emission wavelength set by a band pass filter centered at 510 nm. Stimuli were dissolved in Bath solution and then pH and osmolarity readjusted if needed. Cultured human fungiform taste papillae cells responded to sweet and bitter stimuli. Graphs illustrate representative responses of [Ca+2]i levels in individual cells during exposure to (A) Denatonium (2 mM), (B) Sucralose (1 mM). Each trace represents single individual cells.
| Gene | Sequence | PCR condition | Expected size (bp) |
Reference | |
| β-Actin | GGACTTCGAGCAAGAGATGG AGCACTGTGTTGGCGTACAG |
7 min 45 sec 45 sec 45 sec 7 min |
95 94 53 72 72 |
234 | NM_001101.3 |
| Gustducin | TCTGGGTATGTGCCAAATGA GGCCCAGTGTATTCTGGAAA |
7 min 45 sec 45 sec 45 sec 7 min |
95 94 53 72 72 |
386 | NM_001102386 |
| PLC-β2 | GTCACCTGAAGGCATGGTCT TTAAAGGCGCTTTCTGCAAT |
3 min 30 sec 30 sec 60 sec 7 min |
95 94 53 72 72 |
333 | NM_004573 |
Table 1. Primers and conditions used for detecting taste specific molecules.
| Primary Antibody | Source | Host | Dilution | Secondary Antibody | Source | Host | Dilution |
| Gustducin | SantaCruz | Rabbit | 1:500 | Anti-rabbit IgG Alexa 633 | Molecular Probes | Goat | 1:500 |
| PLC-β2 | SantaCruz | Rabbit | 1:500 | Anti-rabbit IgG Alexa 633 | Molecular Probes | Goat | 1:500 |
Table 2. Antibodies used for detecting expression of specific molecules.
Discussion
We have maintained cells obtained from human fungiform taste papillae for over 8 passages, spanning a period of 12 months. These cultures generate new cells, many of which mature in vitro to the stage where they express several markers of mature taste bud cell types, in addition to key molecular and physiological properties of taste receptor cells. The presence of gustducin- and PLC-β2 immunoreactivity in subsets of cultured cells is consistent with this conclusion. Most importantly, some cells responded to applications of sweet and bitter taste stimuli with transient increases in intracellular calcium, which is a feature of dissociated taste cells5, cells in taste bud slices3 and cultured human and rat taste cells19,10. This indicates not only that some of the cells in culture expressed molecular receptors for sweet and bitter, but that they had enough of the G-protein coupled transduction cascade to generate calcium responses.
Long term primary cell cultures often can not maintain the original tissue and cell structure and cellular factors which may play a role in the physiological function of cells. By successful long term establishment of human fungiform taste papillae cell culture, great progress has been made yet, a number of limitations must be mentioned. Foremost among these is that each batch of primary cultured cells can vary due to differences in the initial population of cells used to start the culture. Primary cell cultures often miss the original tissue organization and structure and growth factors that play a role in the physiological function of cells. Cells are often not always and entirely in contact with other cells, as cultures are never grown to 100% confluence. In general, primary cells are also subject to dedifferentiation, and chromosomal instability, characterized by losses or gains of chromosomes during cell replication in continuous cell lines, may be another problem13,14,15. The comparison of cultured human fungiform cells with cultured nontaste cells of tongue needs to be explored. These questions will require the use of many experimental approaches and model systems before our understanding is complete.
In conclusion, this protocol described here allows maintaining human fungiform taste papillae cells in vitro for at least 8 passages (12 months) without losing relevant cell-physiological and molecular characteristics. The further development of this long term primary culture will provide a model system for studies of proliferation and differentiation, stimulus specificity, cross-talk and adaptation properties of human taste receptor cells, as well as tissue regeneration. In addition, the effects of conditions that impair taste cell function such as infection, medications, radiation, toxic or chemical exposures can be examined at molecular and physiological levels.
Declarações
The authors have nothing to disclose.
Acknowledgements
We thank Aimee Myers and Esi Quayson for technical skills and help. This work was supported in part by NSF 0216310 and Givaudan Inc grant (NER).
Materials
| Name of the reagent | Company |
| MCDB 123 | Sigma-Aldrich |
| Iscove’s DMEM Medium | MediaTech |
| Elastase | Sigma-Aldrich |
| Pronase E | Sigma-Aldrich |
| Elastase | Worthington |
| Soy bean trypsin inhibitor | Worthington |
| Collagenase type 1 | Worthington |
| Rat tail collagen type 1 | BD Sciences |
| Fura-2 AM | Molecular Probes |
| Superscript First Strand Synthesis System for RT-PCR | Invitrogen |
| Leica TCS SP2 Spectral Confocal Microscope | Leica Microsystems Inc. |
| Discovery-1 imaging station and Metamorph software | Molecular Devices |
| Small fine-tip forceps and extra fine spring scissors | Fine Science Tools |
Referências
- Mbiene, J. P., Maccallum, D. K., Mistretta, C. M. Organ cultures of embryonic rat tongue support tongue and gustatory papilla morphogenesis in vitro without intact sensory ganglia. J. Comp. Neurol. 377, 324-340 (1997).
- Miyamoto, T., Fujiyama, R., Okada, Y., Sato, T. Strain difference in amiloride-sensitivity of salt-induced responses in mouse non-dissociated taste cells. Neurosci. Lett. 277, 13-16 (1999).
- Caicedo, A., Jafri, M. S., Roper, S. D. In situ Ca2+ imaging reveals neurotransmitter receptors for glutamate in taste receptor cells. J. Neurosci. 20, 7978-7985 (2000).
- Qin, Y. M., Shi, J. Q., Zhang, G. H., Deng, S. P., Wang, T. H. A reliable method to obtain cells of taste buds from fungiform papillae of mice. Acta Histochem. 112, 107-112 (2010).
- Spielman, A. I., Mody, I., Brand, J. G., Whitney, G., MacDonald, J. F., Salter, M. W. A method for isolating and patch-clamping single mammalian taste receptor cells. Brain Res. 503, 326-329 (1989).
- Kishi, M., Emori, Y., Tsukamoto, Y., Abe, K. Primary culture of rat taste bud cells that retain molecular markers for taste buds and permit functional expression of foreign genes. Neurociência. 106, 217-225 (2001).
- Ruiz, C. J., Stone, L. M., McPheeters, M., Ogura, T., Bottger, B., Lasher, R. S., Finger, T. E., Kinnamon, S. C. Maintenance of rat taste buds in primary culture. Chem. Senses. 26, 861-873 (2001).
- Stone, L. M., Tan, S. S., Tam, P. P., Finger, T. E. Analysis of cell lineage relationships in taste buds. J. Neurosci. 22, 4522-4529 (2002).
- Ozdener, H., Yee, K. K., Cao, J., Brand, J. G., Teeter, J. H., Rawson, N. E. Characterization and long-term maintenance of rat taste cells in culture. Chem. Senses. 31, 279-290 (2006).
- Ozdener, M. H., Brand, J. G., Spielman, A. I., Lischka, F. W., Teeter, J. H., Breslin, P. A., Rawson, N. E. Characterization of Human Fungiform Papillae Cells in Culture. Chem. Senses. 36, 601-612 (2011).
- Gomez, G., Rawson, N. E., Hahn, C. G., Michaels, R., Restrepo, D. Characteristics of odorant elicited calcium changes in cultured human olfactory neurons. J. Neurosci. Res. 62, 737-749 (2000).
- Wolozin, B., Sunderland, T., Zheng, B. B., Resau, J., Dufy, B., Barker, J., Swerdlow, R., Coon, H. Continuous culture of neuronal cells from adult human olfactory epithelium. J. Mol. Neurosci. 3, 137-146 (1992).
- Wick, N., Saharinen, P., Saharinen, J., Gurnhofer, E., Steiner, C. W., Raab, I., Stokic, D., Giovanoli, P., Buchsbaum, S., Burchard, A., Thurner, S., Alitalo, K., Kerjaschki, D. Transcriptomal comparison of human dermal lymphatic endothelial cells ex vivo and in vitro. Physiol. Genomics. 17, 179-192 (2007).
- Fu, L., Zhu, L., Huang, Y., Lee, T. D., Forman, S. J., Shih, C. C. Derivation of neural stem cells from mesenchymal stemcells: evidence for a bipotential stem cell population. Stem Cells Dev. 17, 1109-1121 (2008).
- Sandow, S. L., Grayson, T. H. Limits of isolation and culture: intact vascular endothelium and BKCa. Am. J. Physiol. Heart Circ. Physiol. 297, H1-H7 (2009).

