Non-contact, Label-free Monitoring of Cells and Extracellular Matrix using Raman Spectroscopy
Summary
Raman spectroscopy is a suitable technique for the non-contact, label-free analysis of living cells, tissue-engineered constructs and native tissues. Source-specific spectral fingerprints can be generated and analyzed using multivariate analysis.
Abstract
Non-destructive, non-contact and label-free technologies to monitor cell and tissue cultures are needed in the field of biomedical research.1-5 However, currently available routine methods require processing steps and alter sample integrity. Raman spectroscopy is a fast method that enables the measurement of biological samples without the need for further processing steps. This laser-based technology detects the inelastic scattering of monochromatic light.6 As every chemical vibration is assigned to a specific Raman band (wavenumber in cm-1), each biological sample features a typical spectral pattern due to their inherent biochemical composition.7-9 Within Raman spectra, the peak intensities correlate with the amount of the present molecular bonds.1 Similarities and differences of the spectral data sets can be detected by employing a multivariate analysis (e.g. principal component analysis (PCA)).10
Here, we perform Raman spectroscopy of living cells and native tissues. Cells are either seeded on glass bottom dishes or kept in suspension under normal cell culture conditions (37 °C, 5% CO2) before measurement. Native tissues are dissected and stored in phosphate buffered saline (PBS) at 4 °C prior measurements. Depending on our experimental set up, we then either focused on the cell nucleus or extracellular matrix (ECM) proteins such as elastin and collagen. For all studies, a minimum of 30 cells or 30 random points of interest within the ECM are measured. Data processing steps included background subtraction and normalization.
Protocol
1. Preparation of the Biological Sample
- Preparation of living cells
- Preparation of adherent cells
- Seed in vitro-cultured or freshly isolated cells on a glass bottom dish (Greiner BioOne/Germany) and incubate them at 37 °C and 5% CO2 until cell attachment is completed.
- Remove culture medium and wash gently three times with PBS prior measurement. Keep the cells covered with either PBS or cell culture medium throughout the whole measurement procedure.
- Preparation of cells in suspension
- Detach in vitro-cultured cells according to common protocols (e.g. trypsin-EDTA, cell scraping), centrifuge the cells and re-suspend the obtained cell pellet in PBS or cell culture medium.
- Transfer 100 μl of the cell suspension (concentration: max. 100,000 cells/ml) to a glass bottom dish.
- Preparation of adherent cells
- Preparation of native tissues
- After harvesting the tissues, transfer them to sterile, ice-cold PBS and keep them no longer than 12 hours at 4 °C or on ice prior measurement. Previous experiments have shown that a prolonged storage time (cold ischemia time > 12 hours at 4 °C) resulted in spectral changes due to natural degradation processes.
- Measurements must be performed with the tissue covered in PBS or medium to avoid damage of the ECM proteins and cells due to tissue drying.
2. Raman Spectrometer
Our customized Raman spectrometer combines a standard fluorescence microscope (Olympus IX 71) with a Raman spectrometer that allows the direct comparison of bright-field and fluorescence images with the Raman spectra. The basic set up consists of a 784 nm diode laser (Toptica photonics AG/Germany), a notch filter for the separation of the Raman-scattered light from the excitation light, a microscope and a spectrograph (Kaiser Optical Systems Inc., Ann Arbor/USA) with a charge coupled device (CCD camera) optimized for the detection of spectral information (F-view from Soft Imaging Systems/Germany).
3. Control of Laser Function
- Start the software Andor Solis (Andor/United Kingdom) and set the temperature of the CCD camera to -60 °C to minimize the noise caused by thermally induced currents in the camera.
- Place a silicon wafer on the microscope stage for the calibration procedure.
- Turn the laser on and set the power to 85 mW.
- Use the software Cell B (Olympus/Germany) to focus the laser onto the wafer until XY appears.
- Measure the silicon wafer with a single integration time of 1 sec using a 60x air objective.
- Change the unit of the x-axis from pixel number to Raman shift (cm-1) in the Andor Solis software.
- Vary the laser focus of the silicon peak at 520 cm-1 in the collected spectrum in order to find the maximum possible intensity for this Raman band. The minimum amount of counts must be higher than 11,000 to have a successful calibration.
4. Raman Spectroscopic Measurements
All measurements are performed at room temperature.
- Basic settings
- Use a 60x water immersion objective (Olympus/Germany) with a numerical aperture of 1.2 to collect the spectrum of the samples.
- Change the acquisition settings to 10 integrations/ 10 seconds for a total of 100 seconds per measurements.
- Measurement of adherent cells
- Take the glass bottom dish with the cells and place it on the microscope stage.
- In order to obtain a better signal and ensure reproducibility, focus the laser on the cell nucleus, turn the microscope light off and start collecting the spectrum.
- Measure a reference spectrum of the background every 10 spectra by moving the laser focus beside the cell. It is important to consider that when changing the focus a new background must be collected for each focus depth.
- Measurement of cells in suspension
- Transfer 100 μl of the cell suspension into the glass bottom dish and place it onto the microscope stage.
- Focus the laser on the center of the cell, turn the microscope light off and start collecting the spectrum.
- Measure a reference spectrum of the background every 10 spectra by moving the laser focus beside the cell. When changing the focus, a new background must be collected for the new focus depth.
- Measurement of native tissues
- Take the sample and place it into a glass bottom dish. The region of interest (ROI) should be oriented facing the bottom of the dish.
- Fill the dish with enough PBS to cover the sample.
- Place a cover glass over the sample to avoid any movement of the sample during measurements.
- Set the laser focus into the structure of interest (depth resolution is laser- and tissue-dependent) and start collecting the spectra.
- Collect a reference spectrum of the background every 10 spectra by moving the laser focus out of the whole tissue area. When changing the focus, a new background must be collected for the new focus depth.
- Measurement of immunofluorescence (IF)-labeled cryosections
- Section fresh, snap-frozen tissue samples using a standard cryotom and mount them on silica-coated cover glasses.
- Stain the cryosections following a routine protocol for IF, employing only a short fixation step (max. 10 minutes with 4% paraformaldehyde) and using appropriate antibodies for the detection of the protein of interest.
- Perform Raman measurements focusing in the area where fluorescence occurs.
- Elastin degradation experiments
- Place the ventricularis of the dissected porcine aortic valve leaflets (elastin-rich, blood inflow-side layer of the heart valve leaflet) facing to the bottom of the glass bottom dish.
- Measure the native tissue as a ‘non-incubated control’ at 30 random points across the whole tissue surface focusing in the fibrillar structures.
- Divide the sample into 3 sections and place them into separate 2.5 ml Eppendorf tubes filled with 2 ml of an elastase solution (5 U/ ml, Worthington/Germany).
- Incubate the tissue for either 15 or 30 minutes at 37 °C.
- After incubation for either 15 or 30 minutes, remove the tissues from the Eppendorf tube and wash carefully with PBS in order to completely stop the enzymatic reaction.
- Measure each sample at 30 random points, focusing in the fibrillar structures.
5. Data Processing and Analysis
- Raman spectra processing
The pre-treatment of the generated spectra was performed using OPUS software (Bruker Optik GmbH/Germany).- In order to reduce interfering signals from the glass and medium as well as to avoid variations caused by changes in the focus during measurements, subtract the corresponding background spectrum from the collected spectra.
- Reduce the spectra to the wavenumber region between 400-1800 cm-1, which offers the highest amount of information.
- If needed, normalize the spectra to the maximum peak. Normalization factors out the intensity fluctuations and systematic failures, simplifying the detection of structural changes in the sample spectra.
- Perform a baseline correction to increase the comparability between different experiments.
- Raman spectra analysis
The Raman spectra were analyzed using PCA with The Unscrambler (CAMO/ Norway) software. This multivariate analysis detects differences and similarities within the spectral data sets. Every spectrum is plotted as a single point in a multidimensional space based on the collected counts for every Raman shift. Each principle component (PC) describes a certain quantity of the total information contained in the original data. The first PC is the one that contains the highest source of variation. Each following PC contains, in order, less information than the previous one. Every variable has a score and a loading on each PC. By plotting PCs (=scores), important sample correlations can be exposed. The loadings describe the contribution of each analyzed variable to the PCA.- Label each group of measurements by creating row ranges for every sample group.
- Use the following basic settings for the PCA: cross validation, the NIPALS algorithm, no rotation and start the analysis. These settings are spectra dependent.
- Perform the PCA.
6. Representative Results
Raman spectra generated from adherent cells often reveal a low signal-to-noise ratio and a low overall signal intensity (Fig. 1).11 Due to the fact that the laser focus has to be set near the glass bottom, the influence of the interfering glass signal is rather high, causing masking of the actual sample signal. Consequently, the sample signal might be minimized or even eliminated during a subsequent background subtraction. Thus, we prefer to use cells in suspension for our Raman spectroscopic analysis, as they allow the detection of more detailed spectral information. However, the spectra of adherent and suspension cells exhibit the same main peaks differing only in their intensities.
For the characterization of different cell types within a suspension, no pre-treatment is required. The mean Raman spectra and standard deviations of human fibroblasts, mesenchymal stem cells (MSCs), chondrocytes and keratinocytes measured in suspension are depicted in Figure 2. All Raman spectra are similarly structured, with peaks originating from typical biomolecules such as proteins, nucleic acids and lipids (see Table 1).12 For these cell types, the spectral region between 600 and 1800 cm-1 contains most relevant spectral information, by which clear differences are detectable between the different cell types (Fig. 2A). Exemplary, we highlighted one spectral region (1280-1350 cm-1) displaying clear structural differences, which is assignable to molecular vibrations of collagen and lipids. In contrast, morphological analyses are not suitable for the identification and distinction of most cells (Fig. 2B-I). While the difference between chondrocytes and skin cells is observable (Fig. 2D,H versus B,F and E,I), fibroblasts and MSCs are difficult to separate using solely bright-field micrsocopy (Fig. 2B,F versus C,G).13
Raman spectroscopic analysis of native tissue, particularly of ECM proteins, requires that a ROI can be visualized by bright-field imaging in order to be able to focus on the respective structure. For the assignment of a protein to a specific fingerprint spectrum, we generated Raman spectra of commercially available pure proteins and immunohistologically stained cryosections. Here, we identified the fingerprint spectra of elastic fibers within native tissues comparing lyophilized elastin and immunofluorescence-stained cryosections employing an antibody against elastin. However, since elastin features a high autofluorescence, which is reflected in the Raman spectra, the data analysis is challenging (Fig. 3A). To reduce the systematic failure due to sample-specific properties such as autofluorescence, an appropriate processing of the data sets is crucial. In our data analyses, we used normalization to eliminate the significantly higher signal intensity of the pure elastin protein, and thus, we were able to generate comparable Raman spectra (Fig. 3B). Elastin is one of the most stable ECM proteins in the body and is therefore very difficult to degrade.14 In our experimental set up, we induced elastin degradation in healthy porcine aortic valve leaflets by performing an enzymatic digestion. Applying the multivariate analysis PCA, we identified significant differences between the Raman spectra of enzymatically-treated samples and native controls (Fig. 3C). These spectral differences were observed in the loading spectrum at 861, 1003 and 1664 cm-1. Expected structural changes in the elastin-containing fibers due to extended exposure times to elastase were shown by HART’s staining (Fig. 4), which were also reflected in more distinct separable score clusters (Fig. 3C).
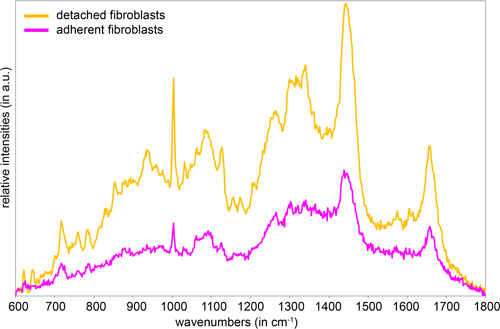
Figure 1. Mean Raman spectra of detached and adherent fibroblasts.
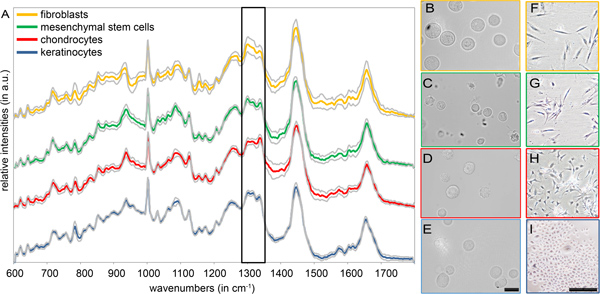
Figure 2. (A) Mean Raman spectra and standard deviations of four different primary isolated cell types (fibroblasts, MSCs, chondrocytes and keratinocytes). The frame highlights the spectral region of 1280 – 1350 cm-1 with structural differences. (B-E) Bright-field images of detached (B) fibroblasts, (C) MSCs, (D) chondrocytes and (E) keratinocytes. Scale bar equals 20 μm. (F-I) Bright-field images of adherent (F) fibroblasts, (G) MSCs, (H) chondrocytes and (I) keratinocytes. Scale bar equals 200 μm.
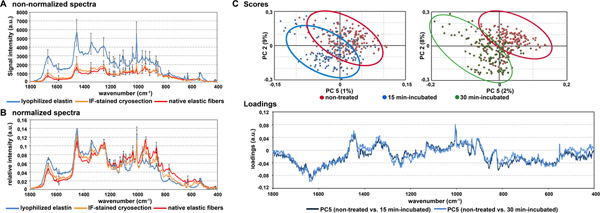
Figure 3. (A) Raman spectra without normalization of lyophilized elastin (blue line), immunofluorescence (IF)-labeled cryosections (orange line) and elastic fibers (red line) measured within native aortic valve leaflets. The high signal intensity of lyophilized elastin is caused by autofluorescence. (B) Raman spectra after normalization in order to eliminate systemic failures. (C) Scores and loadings of the comparison between non-treated control (red) and enzymatically-degraded (blue and green) elastic fibers within the native tissue.
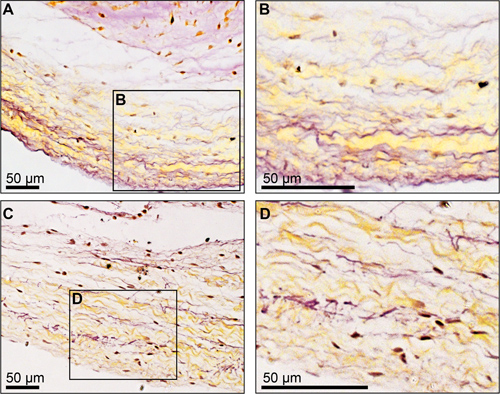
Figure 4. HART’s-stained porcine aortic valve leaflets. Elastic fibers are visualized in black. (A) and (B) show non-treated control and (C) and (D) depict tissue samples that were exposed to the elastin-degrading enzyme elastase for 30 minute.
| Wavenumber in cm-1 | Assignment12 | |
| 717-719 | C-N | Phospholipids |
| 785-788 | DNA/RNA bases, O-P-O backbone |
DNA/RNA |
| 1003- 1005 | Phenylalanine | Protein |
| 1220-1280 | Amide III | Protein |
| 1445-1447 | CH2 | Protein/Lipid |
| 1655-1680 | Amide I C=C | Protein Lipid |
Table 1. Raman bands that are detected within spectra of all cell types (fibroblasts, MSCs, chondrocytes and keratinocytes).
Discussion
Raman spectroscopy is a suitable tool to analyze biological samples, such as in vitro-cultured cells and ECM proteins as well as cells within native tissues.11,15,16 Here, we demonstrated that this non-contact, label-free technique allows the discrimination of different cell types and the detection of ECM protein degradation solely based on the intrinsic biomolecular composition of these biological samples.
The major advantage of Raman spectroscopy is the ability to non-invasively quantify the biochemical fingerprint of a sample by its resulting Raman spectra. In contrast to infrared spectroscopy, which yields similar information, Raman spectra can be collected from aqueous samples, as the Raman scatter from water is weak. In addition, Raman spectroscopy is solely based on the detection of backscattering of monochromatic light; therefore, no sample processing is required prior measurement. These attributes make Raman spectroscopy a promising alternative for potential in vivo imaging applications. In this regards, coherent anti-stokes Raman spectroscopy (CARS) is a very interesting technique since it enables faster and more sensitive acquisition of data based on the same vibrational signals utilized in our experiments.15,16 Other alternative methods including multiphoton-induced autofluorescence and second harmonic generation imaging have been previously proven to be suitable for monitoring biological samples non-or minimal invasively.17 However, these imaging modalities are associated with very high costs and are limited to autofluorescence-generating molecules. In addition, Raman spectrometer is easy to combine with conventional optical microscopes. These characteristics make Raman spectroscopy a valuable tool to study biological samples in physiological environments.
One of the current limitations of our Raman spectroscopy set up is the relatively small laser focus (250 nm full width half maximum (FWHM) lateral and 700 nm FWHM axial) that is created by a high numerical aperture objective (NA=1.2). Although a high numerical aperture allows to cover a good amount of the emitted Raman light yielding in a high signal-to-noise ratio, the high NA produces only a small collection focus within the sample that is typically much smaller than a cell. In order to compare Raman spectra of different cells, the collection of a representative spectrum is essential, which is difficult to obtain with a small focus area. To address this issue, we are working on a process to automate the signal collection at different points within the cell (= Raman spectroscopic mapping), resulting in a spectral averaging and yielding to a representative spectrum. Additionally, this technique will provide an overview of the distribution of specific Raman bands, for instance of the protein distribution within a cell.
Biological samples are highly complex and consist of a heterogeneous mixture of biomolecules that contribute to the collected Raman spectra. Therefore, the spectral pattern is highly complex and the monitoring of a single type of a molecule within a Raman spectrum is difficult to accomplish with the overlapping of different molecule signals. Additionally, intrinsic fluorescence of the sample may mask valuable information of weaker Raman signals. Interestingly, in some of our previous studies, we identified autofluorescence in the Raman spectra as the main differentiating factor between cell types (MSCs and fibroblasts) using an appropriate analysis tool.13 We also identified that changes in the overall Raman signal intensity can serve as an indicator for the state of collagen and collagen fibers within the ECM of aortic valve leaflets.9 However, when analyzing the state of elastin in these tissues, we were not able to detect similar results. As mentioned in the results section, we were only able to detect alterations of specific Raman bands in the elastase-treated samples when compared to the native controls. We did not see a decrease of the overall Raman signal in the enzymatically-treated samples as expected. These observations resulted in a score plot that did not reveal a clear cluster formation as seen in the previous study.9 In contrast, the influence of the enzymatic treatment was detectable within the PCA results. We assume that these discrepancies between the two ECM proteins, elastin and collagen, are based on morphological differences and different enzymatic degradation processes: within the aortic valve leaflet, the collagen-rich zone (fibrosa) is a continuous layer that becomes loosened due to the enzymatic treatment, whereby the elastin containing zone (ventricularis) has a network configuration that appears fragmented after exposure to elastase (Fig. 4). Single spot measurements were therefore not appropriate to detect such small ruptures within the elastin network. Here, a Raman mapping of the tissue would help to identify network breakdowns.
A further challenge in Raman spectroscopy of biological samples is to reduce the measurement times. One solution is to increase the laser power, which is suitable as long as the biological samples are not affected by photo-damage. All of our current experiments are proof-of-principle studies focusing on basic research; however, our overall goal is to implement Raman spectroscopy for clinical applications including regenerative medicine (e.g. quality control of tissue-engineered products), pre-transplantation graft monitoring and cancer diagnostics.
Declarações
The authors have nothing to disclose.
Acknowledgements
We thank Steffen Koch for his technical support and Shannon Lee Layland (both Fraunhofer IGB Stuttgart) for his helpful suggestions on the manuscript. This work was financially supported by the Attract program of the Fraunhofer-Gesellschaft and the BMBF (both to K.S.-L.).
Materials
| Name of the reagent | Company | Catalogue number | Comments |
| elastase | Worthington | LS006363 | |
| anti-elastin antibody | Sigma | HPA018111 | 1:75; citrate buffer |
| PBS | Lonza | 17-512F | |
| glass bottom dishes | Greiner BioOne | 627860 | |
| The Unscrambler | CAMO | ||
| Opus | Bruker |
Referências
- Chan, J. W., Lieu, D. K. Label-free biochemical characterization of stem cells using vibrational spectroscopy. J. Biophotonics. 2, 656-668 (2009).
- Downes, A., Mouras, R., Elfick, A. Optical spectroscopy for noninvasive monitoring of stem cell differentiation. J. Biomed. Biotechnol. 2010, 101864-101864 (2010).
- Gentleman, E. Comparative materials differences revealed in engineered bone as a function of cell-specific differentiation. Nat. Mater. 8, 763-770 (2009).
- Notingher, I., Hench, L. L. Raman microspectroscopy: a noninvasive tool for studies of individual living cells in vitro. Expert. Rev. Med. Devices. 3, 215-234 (2006).
- Schenke-Layland, K. Optimized preservation of extracellular matrix in cardiac tissues: implications for long-term graft durability. Ann. Thorac. Surg. 83, 1641-1650 (2007).
- Raman, C. V., Krishnan, K. S. A new type of secondary radiation. Nature. 122, 12 (1928).
- Frushour, B. G., Koenig, J. L. Raman scattering of collagen, gelatin, and elastin. Biopolymers. 14, 379-391 (1975).
- Pudlas, M., Koch, S., Bolwien, C., Walles, H. Raman spectroscopy as a tool for quality and sterility analysis for tissue engineering applications like cartilage transplants. Int. J. Artif. Organs. 33, 228-237 (2010).
- Votteler, M. Raman spectroscopy for the non-contact and non-destructive monitoring of collagen damage within tissues. J. Biophotonics. , (2011).
- Wold, S., Esbensen, K., Geladi, P. Principal component analysis. Chemometrics and Intelligent Laboratory Systems. 2, 37-52 (1987).
- Pudlas, M. Raman Spectroscopy: A Noninvasive Analysis Tool For The Discrimination of Human Skin Cells. Tissue Eng. Part C Methods. 10, (2011).
- Movasaghi, Z., Rehman, S., Rehman, I. U. . Raman Spectroscopy of Biological Tissues. Applied Spectroscopy Reviews. 42, 493-541 (1080).
- Pudlas, M. Non-contact discrimination of human bone marrow-derived mesenchymal stem cells and fibroblasts using Raman spectroscopy. Medical Laser Application. 26, 119-125 (2011).
- Mecham, R. P. Methods in elastic tissue biology: elastin isolation and purification. Methods. 45, 32-41 (2008).
- Downes, A., Mouras, R., Bagnaninchi, P., Elfick, A. Raman spectroscopy and CARS microscopy of stem cells and their derivatives. Journal of Raman Spectroscopy. 42, 1864-1870 (2011).
- Krafft, C., Dietzek, B., Popp, J. Raman and CARS microspectroscopy of cells and tissues. Analyst. 134, 1046-1057 (2009).
- Schenke-Layland, K. Non-invasive multiphoton imaging of extracellular matrix structures. J. Biophotonics. 1, 451-462 (2008).

