A Semi-quantitative Approach to Assess Biofilm Formation Using Wrinkled Colony Development
Summary
We provide a simple, semi-quantitative method to investigate biofilm formation in vitro. This method takes advantage of the Zeiss stemi 2000-C Dissecting Microscope (with camera attachment) to monitor both the timing and pattern of biofilm formation, as assessed by the development of wrinkled colonies.
Abstract
Biofilms, or surface-attached communities of cells encapsulated in an extracellular matrix, represent a common lifestyle for many bacteria. Within a biofilm, bacterial cells often exhibit altered physiology, including enhanced resistance to antibiotics and other environmental stresses 1. Additionally, biofilms can play important roles in host-microbe interactions. Biofilms develop when bacteria transition from individual, planktonic cells to form complex, multi-cellular communities 2. In the laboratory, biofilms are studied by assessing the development of specific biofilm phenotypes. A common biofilm phenotype involves the formation of wrinkled or rugose bacterial colonies on solid agar media 3. Wrinkled colony formation provides a particularly simple and useful means to identify and characterize bacterial strains exhibiting altered biofilm phenotypes, and to investigate environmental conditions that impact biofilm formation. Wrinkled colony formation serves as an indicator of biofilm formation in a variety of bacteria, including both Gram-positive bacteria, such as Bacillus subtilis 4, and Gram-negative bacteria, such as Vibrio cholerae 5, Vibrio parahaemolyticus 6, Pseudomonas aeruginosa 7, and Vibrio fischeri 8.
The marine bacterium V. fischeri has become a model for biofilm formation due to the critical role of biofilms during host colonization: biofilms produced by V. fischeri promote its colonization of the Hawaiian bobtail squid Euprymna scolopes 8-10. Importantly, biofilm phenotypes observed in vitro correlate with the ability of V. fischeri cells to effectively colonize host animals: strains impaired for biofilm formation in vitro possess a colonization defect 9,11, while strains exhibiting increased biofilm phenotypes are enhanced for colonization 8,12. V. fischeri therefore provides a simple model system to assess the mechanisms by which bacteria regulate biofilm formation and how biofilms impact host colonization.
In this report, we describe a semi-quantitative method to assess biofilm formation using V. fischeri as a model system. This method involves the careful spotting of bacterial cultures at defined concentrations and volumes onto solid agar media; a spotted culture is synonymous to a single bacterial colony. This ‘spotted culture’ technique can be utilized to compare gross biofilm phenotypes at single, specified time-points (end-point assays), or to identify and characterize subtle biofilm phenotypes through time-course assays of biofilm development and measurements of the colony diameter, which is influenced by biofilm formation. Thus, this technique provides a semi-quantitative analysis of biofilm formation, permitting evaluation of the timing and patterning of wrinkled colony development and the relative size of the developing structure, characteristics that extend beyond the simple overall morphology.
Protocol
1. Initial Characterization and Considerations
- Biofilm formation is generally influenced by cell density and growth rate. Therefore, it is necessary to determine the growth rate (increase in optical density (OD) over time) and yield (final cell number) of the strain(s) of interest by performing simple growth curve and cell plating assays. Defects in growth, or a lack of correlation between OD and cell number, must be taken into consideration when interpreting results from spotting experiments.
- Include the appropriate positive and negative controls on the same plate when assessing wrinkled colony formation, as minor plate-to-plate variation may impact biofilm development.
- Identify the best conditions for spotting, i.e., those that reveal the most distinct differences between the control and mutant(s) of interest. Grow strains in liquid culture under various conditions, such as different media or temperatures, and to different stages of growth (exponential or stationary phase) prior to spotting. Spot 10 μl of culture at various cell densities onto the appropriate media, and incubate at the desired temperature until colony morphologies become apparent.
- The end-point assay involves assessment of wrinkled colony formation at a predetermined time point after spotting (i.e., 48 hours). This assessment is useful for strains that exhibit (or are proposed to exhibit) severe defects in biofilm formation.
- The time course assay assesses wrinkled colony formation over a period of time (i.e., every hour) post-spotting. This assay permits a semi-quantitative assessment of biofilm formation by allowing the determination of the start of wrinkled colony formation and pattern development over a period of time. The duration of the experiment and number of collection points for a given time course should be determined in preliminary experiments.
- To easily keep track of the time post-spotting, set a timer to count up.
2. Microscopic Assessment of Wrinkled Colony Morphology in V. fischeri
- Inoculate V. fischeri cells into 5 ml of LB-Salt (LBS) medium 13 (1% [w/v] tryptone, 0.5% [w/v] yeast extract, 2% [w/v] sodium chloride, 50 mM Tris-HCl [pH 7.5]) containing any necessary antibiotics and incubate, with shaking, overnight at 28 °C. In the morning, subculture the cells with a 1:100 dilution into 5 ml of fresh LBS medium and incubate under the same conditions until the cells have reached the desired OD600 (e.g., OD600=0.2 or 0.5).
- Pipette 1 ml of culture into a microfuge tube and centrifuge in a microfuge set at maximum speed for one minute. Remove the supernatant by aspiration. Wash the cells to remove residual media and extracellular components by resuspending the pellet in 1 ml of sterile 70% artificial seawater (ASW) (35 mM MgSO4-7H2O, 7 mM CaCl2-2H2O, 210 mM NaCl, 7 mM KCl) and repeating the centrifugation. Resuspend the washed pellet in 1 ml of 70% ASW.
- Make sure that each sample contains the same number of cells, as estimated by the OD at 600 nm. Make any necessary adjustments by diluting more concentrated samples with additional 70% ASW. In preliminary experiments, spot cultures at various initial OD values to determine an optimal starting OD for a given set of strains or conditions. The best results are obtained for V. fischeri when spots are generated from cultures with an OD of approximately 0.2.
- Spot 10 μl of the washed cells onto LBS plates containing any necessary antibiotics. When spotting a culture onto a plate, make sure to steady the pipette (with your finger) just above the agar surface. Spot vertically, not at an angle, and eject the liquid slowly for uniform distribution of the spot. Typically each strain is spotted once per plate (up to 6 spots per plate), with multiple plates (2-3) per experiment.
- To ensure that the spotted culture remains evenly distributed, allow the spot to dry before moving the plate to the incubator. Invert the plates and incubate them at 28 °C.
- Monitor the morphology of the growing spot hourly beginning at 12-15 hours following inoculation. We use a dissecting scope (Zeiss stemi 2000-C) with a camera attachment (ProgRES C10PLUS) and the ProgRes CapturePro and ImageJ software programs to observe and document colony morphology and to assess the start and progression of wrinkled colony formation.
- For the specific setup listed in step 2.5, the spots are illuminated from underneath through a transparent glass stage with a CL 1500 EC cold light source, while the images are captured from above. This setup is optimal for imaging wrinkled colony development because V. fischeri colonies (spots) are translucent.
- In the absence of the specific equipment listed in step 2.5, monitor wrinkled colony morphology using any dissecting microscope that allows for full colony viewing and has an adjustable light source and, ideally, an attached camera. If necessary, a digital camera can also be utilized in the absence of an attached camera, but this is not optimal.
- To best visualize wrinkled colony development, it is necessary to adjust both the lighting intensity and angle of reflection underneath the bacterial colonies such that the three-dimensional morphology of the developing biofilms can be discerned. Determine the optimal lighting conditions that provide the strongest contrast between the spotted colony and the surrounding agar background, such that the architecture (wrinkles) of the colony is clearly distinguishable.
- In some cases, the color of the spotted colony must also be taken into consideration, as alterations in colony color can occur during biofilm formation. Adjust the intensity and angle of the lighting source to reveal these subtle changes in coloration. Once the appropriate settings are achieved, maintain them for the duration of the experiment.
- Note how much time elapses from the time of inoculation to the time at which wrinkled colony formation initiates for each strain or condition. Define the start of wrinkled colony formation as the time point at which the formation of patterning and 3D structures (i.e., striations forming at the outer edge or ‘ripples’ occurring in the center) is first apparent. Mutant cells may exhibit decreased or increased time to the start of wrinkled colony formation (e.g., Fig. 2).
- Document the start and development of wrinkled colony formation by capturing appropriate digital images. It is important to use the same magnification when collecting images throughout the experiment. Switch the view from the eyepiece of the microscope to the computer screen using the lever located on the back of the camera. When switching between eyepiece and computer screen viewing, adjust the view and focus accordingly.
- Before imaging spotted cultures, the lid of the Petri plate is typically removed to provide the clearest image. However, this is an optional step, as spotted cultures can be imaged through the lid using the setup outlined in steps 2.5 and 2.6.
- At each time point following the start of wrinkled colony formation, note the pattern of wrinkled colony development. The architecture may develop from the inside out, or the outside in. This assessment provides a mechanism to distinguish the biofilms formed by different strains or under different conditions.
- Measure the diameter of the developing colony at each time point. This can be done manually, or digitally using an associated software program. If done digitally, use the ProgRes CapturePro software program and first calibrate the scale bar to the specific magnification used in the experiment. Include the scale bar in each image captured.
- To calculate the colony diameter using the ImageJ software program, open each image file. Standardize the scale bar as follows: Select the “Straight line option” from the toolbar. Overlay the embedded scale bar with a straight line of the same length and width. Select “Set Scale” from the tab labeled “Analyze.” In the “known distance” box, insert the corresponding length (as determined from the original scale bar; i.e., 2 mm) and select “OK”.
- Use the “Straight line” option to insert a horizontal line across the spot. Under the Analyze tab, select “Measure”. In the new Results window, the calculated length will be provided. Obtain a second measurement by inserting a perpendicular line across the spot. Re-calculate the diameter. Record the average of the two measurements. Graph the average diameter for each spot at each time point using a software program such as Excel.
- The end of the experiment occurs either at a specified time point or when there is no further development of the biofilm. Once the end is reached, consolidate the images into a figure(s) to visualize the pattern development over time using a software program such as Powerpoint.
3. Representative Results
In these experiments we used V. fischeri as a model organism to study biofilm formation by evaluating the development of wrinkled colonies on a solid agar surface. Biofilm-producing strains of V. fischeri form colonies with extensive 3D architecture within 40 h (Fig. 1) 8,9,14. When examined over a time course, it becomes apparent that wrinkled colony formation by the control strain initiates as early as about 12 h post-inoculation (depending on the specific conditions) (Fig. 2) 9. In contrast, biofilm formation by a representative mutant is delayed by approximately 4 h, not initiating until approximately 16 h post-inoculation 9. Repeats of these experiments suggested that the timing was relatively consistent, making this assessment semi-quantitative 9. A second semi-quantitative measure of biofilm formation makes use of the change in diameter of the developing colony over time. As shown in Fig. 3A, a representative biofilm-competent strain forms colonies that increase in complexity and diameter relative to a representative strain that does not form biofilms. Careful measurements over time of the diameters of the colonies formed by these two strains revealed that the size of the biofilm-competent colonies increased at a greater rate than the biofilm-negative colonies, and at the end time point the two differed by almost 2-fold (Fig 3B). Thus, although images of a representative late time or “end-point” colony morphology frequently are shown in the literature, additional, semi-quantitative data can be collected that will permit a better understanding of the biofilm defect.
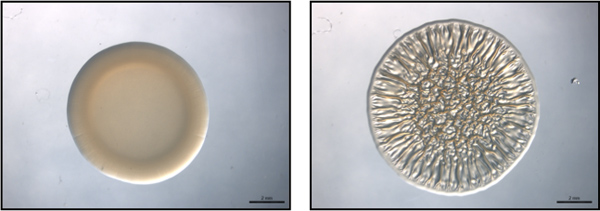
Figure 1. End-point assay. This figure is an example of an end-point assay using representative non-biofilm-forming (left) and biofilm-forming (right) strains of V. fischeri. These images were collected at 40 h post-spotting. These images were generated with wild-type V. fischeri containing a vector control (non-biofilm-forming) or an rscS overexpressing plasmid (biofilm-forming) and were part of the data set collected for Morris et al., 2011.
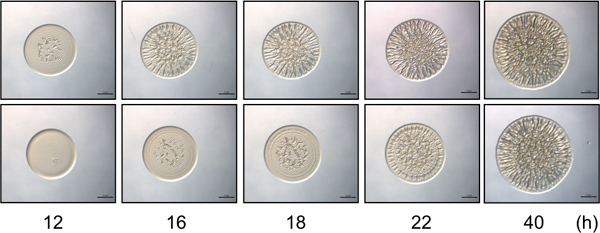
Figure 2. Time-course assay. The upper panel contains representative images of biofilm formation by a biofilm-competent control strain of V. fischeri over a selected time course. Initiation of biofilm formation is evident at 12 h. The lower panel contains representative images of a mutant strain of V. fischeri that exhibits a delay (4 h) in the start of wrinkled colony formation over time, with biofilm formation initiating at 16 h post-inoculation. Note that at 40 hours the strains look similar in the intensity and patterning of biofilm formation, while subtle differences between these strains are only observed at the earlier time points. These images were generated with rscS overexpressing wild-type and sypE mutant V. fischeri cells and were part of the data set collected for Morris et al., 2011.
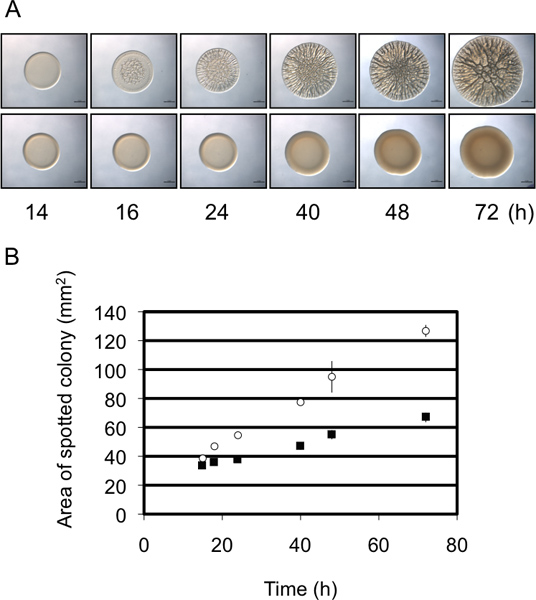
Figure 3. Colony diameter as a semi-quantitative analysis of biofilm formation. (A) Time course of biofilm formation by representative biofilm-forming (upper panel) and non-biofilm-forming (lower panel) strains of V. fischeri. Note that the biofilm-forming strain exhibits a greater increase in diameter over time relative to the non-biofilm forming strain. These images were generated with wild-type V. fischeri containing an rscS overexpressing plasmid (biofilm-forming) or a vector control (non-biofilm-forming) and were part of the data set collected for Morris et al., 2011. (B) A graphical representation of the increase in colony diameter over time by the two strains in panel A. These data were generated using the ImageJ and Excel software as outlined in the protocol.
Discussion
In this work, we describe a semi-quantitative method to assess biofilm formation using V. fischeri as a model organism. Specifically, we utilize a dissecting microscope with camera attachment to monitor biofilm formation and development as wrinkled colony formation over time on a solid agar surface. In this protocol, we outline two specific types of methods we commonly use to assess wrinkled colony formation. The first is the end-point assay, which allows us to observe the final, overall 3D architecture, patterning, and diameter of a spotted culture at a selected “final” time point. This approach is most useful for assessing mutant strains or conditions that lead to dramatic defects in biofilm formation. However, this approach does not distinguish between more subtle differences occurring at time points before the selected end-point. To more closely monitor wrinkled colony formation, we use a time course assay, which allows us to identify the start of wrinkled colony formation and watch its development over time. As a result of this approach, more subtle differences in timing of wrinkled colony formation, 3D architecture, and patterning can be identified. We used this time course assay to generate two semi-quantitative assays of biofilm formation. First, the time at which a strain begins to develop 3D architecture can be compared to that of control strains. We have found that the delay in biofilm formation of a particular mutant under the same conditions is fairly consistent 9. For example, in the data shown in Fig. 2, the mutant consistently exhibited about a 4 h delay in initiating biofilm formation. A second semi-quantitative measure of biofilm formation is the change in size of the diameter of the wrinkled colony (spot). We have found that the diameter of wrinkled colonies progressively differs from that of non-biofilm colonies, reaching about a 2-fold difference at the end time point (Fig. 3) (Morris and Visick, unpublished data). To date, we have not observed one phenotype without the other (i.e., biofilm formation without an increase in colony diameter) (unpublished data), although it remains possible some mutants will behave differently. Indeed, it has been reported for V. cholerae that some wrinkled colonies result in a substantial increase in colony diameter while others do not 15. Still, assessing the change in diameter over time could aid in the characterization of potential biofilm mutants and/or provide an additional quantitative measure of mutants with delays in development. Of the two measures of biofilm formation (time and diameter), determining the start of wrinkled colony formation is more sensitive, but also more subjective, than determining the diameter of the spot. Even so, both measures provide a semi-quantitative assessment of a phenotype that is extremely useful to biofilm researchers but not readily amenable to quantification.
When performing spotted cultured assays, it is important to consider the environmental conditions in which the spotted strains are cultured. Wrinkled colony formation is often influenced by various environmental conditions, including nutrient availability, temperature, and humidity. To reduce variability between experiments, it is helpful to standardize these conditions as much possible (i.e. standardizing the agar plates to a set volume and culturing the spotted strains at a controlled temperature). To further control for variability between spotting experiments, is important to include the appropriate control strains within each set of experiments. Finally, when interpreting the data from these assays, it is necessary to perform any one experiment multiple times (3+), especially when assessing subtle differences in wrinkled colony formation (e.g., a delay in biofilm formation or patterning differences). Some limitations of this protocol are: 1) determining whether cells have a defect in growth may be difficult: growth of cells in liquid culture may not accurately reflect growth rates on solid media, and an accurate determination of growth of biofilm-forming cells, which may stick together, may not be possible; 2) strains with growth defects will be problematic to analyze; 3) it may not be possible to distinguish diameter differences between strains with subtle biofilm phenotypes; 4) for strains that do not grow in a concentric ring it may not be possible to accurately measure changes in diameter; 5) while patterning can be observed during biofilm formation, there is no way to quantify the patterning of the resulting biofilm; and 6) there is no way to measure the Z-dimension of the biofilm with this experimental set-up. Despite these limitations, this protocol nevertheless provides a means to obtain numerical data to aid in assessing wrinkled colony formation.
In this protocol, we utilize a specific imaging system (i.e., a Zeiss dissecting microscope and ProgRes CapturePro imaging software) to observe and evaluate wrinkled colony formation. The imaging system described here is powerful: the ability to detect the start of wrinkled colony formation, and thus assess development with a time course approach, is greatly enhanced through the use of a dissecting microscope. However, if this technology is unavailable, the protocol can be adapted for use with other equipment, including a simple digital camera with a zoom focus. While this protocol focuses on assessment of wrinkled colony development, it could also be modified to evaluate pellicle formation, a form of biofilm that develops when cells are growing statically in liquid culture. This protocol may also prove useful in assessing other indicators of biofilm formation, including the incorporation of specific dyes into the spotted colonies during biofilm development. These include, but are not limited to, dyes such as Congo Red and calcofluor which can bind to cellulose, a common component of bacterial biofilms 16. Additionally, this protocol, although developed for use with V. fischeri, is not limited to this organism but can be generalized to studying biofilm formation in numerous different organisms, such as Bacillus subtilis 4, Vibrio cholerae 5 , Vibrio parahaemolyticus 6, and Pseudomonas aeruginosa 7, which all exhibit wrinkled colony formation. Finally, it also can be adapted to study other colony morphologies that have a developmental pattern, such as, potentially, the patterning that occurs during growth of Myxococcus xanthus 17 and aerial structure development in Pseudomonas aeruginosa 18. This protocol is thus of general use to microbiology and biofilm researchers.
Declarações
The authors have nothing to disclose.
Acknowledgements
This work was supported by NHI R01 grant GM59690 awarded to KLV.
Materials
| Name of Equipment | Company | Catalogue Number | Comments |
| Zeiss stemi 2000-C Dissecting Microscope | Zeiss | 45505300000000000 | (obtained through Lukas Microscope) |
| ProgRes C10PLUS | JENOPTIK Optical Systems GmbH | 017953-602-26 | Camera attachment (obtained through Lukas Microscope) |
| CL 1500 EC Cold Light Source | Zeiss | 4355400000000000 | (obtained through Lukas Microscope) |
| ProgRes CapturePro | JENOPTIK Optical Systems GmbH | Free software with registered camera | Computer program for image capture |
| Image J | NIH | Free software from: http://rsb.info.nih.gov/ij/download.html | Computer program for diameter measurements |
Referências
- Donlan, R. M., Costerton, J. W. Biofilms: survival mechanisms of clinically relevant microorganisms. Clin. Microbiol. Rev. 15, 167-167 (2002).
- Stoodley, P., Sauer, K., Davies, D. G., Costerton, J. W. Biofilms as complex differentiated communities. Annu. Rev. Microbiol. 56, 187 (2002).
- Branda, S. S., Vik, S., Friedman, L., Kolter, R. Biofilms: the matrix revisited. Trends Microbiol. 13, 20 (2005).
- Branda, S. S. Fruiting body formation by Bacillus subtilis. Proc. Natl. Acad. Sci. U.S.A. 98, 11621-11621 (2001).
- Beyhan, S. Regulation of rugosity and biofilm formation in Vibrio cholerae: comparison of VpsT and VpsR regulons and epistasis analysis of vpsT, vpsR, and hapR. J. Bacteriol. 189, 388 (2007).
- Enos-Berlage, J. L., Guvener, Z. T., Keenan, C. E., McCarter, L. L. Genetic determinants of biofilm development of opaque and translucent Vibrio parahaemolyticus. Mol. Microbiol. 55, 1160 (2005).
- Merritt, J. H., Brothers, K. M., Kuchma, S. L., O’Toole, G. A. SadC reciprocally influences biofilm formation and swarming motility via modulation of exopolysaccharide production and flagellar function. J. Bacteriol. 189, 8154-8154 (2007).
- Yip, E. S., Geszvain, K., DeLoney-Marino, C. R., Visick, K. L. The symbiosis regulator rscS controls the syp gene locus, biofilm formation and symbiotic aggregation by Vibrio fischeri. Mol. Microbiol. 62, 1586-1586 (2006).
- Morris, A. R., Darnell, C. L., Visick, K. L. Inactivation of a novel response regulator is necessary for biofilm formation and host colonization by Vibrio fischeri. Mol. Microbiol. 82, 114 (2011).
- Nyholm, S. V. Roles of Vibrio fischeri and nonsymbiotic bacteria in the dynamics of mucus secretion during symbiont colonization of the Euprymna scolopes light organ. Appl. Environ. Microbiol. 68, 5113 (2002).
- Yip, E. S., Grublesky, B. T., Hussa, E. A., Visick, K. L. A novel, conserved cluster of genes promotes symbiotic colonization and σ54-dependent biofilm formation by Vibrio fischeri. Mol. Microbiol. 57, 1485 (2005).
- Mandel, M. J. A single regulatory gene is sufficient to alter bacterial host range. Nature. 458, 215 (2009).
- Graf, J., Dunlap, P. V., Ruby, E. G. Effect of transposon-induced motility mutations on colonization of the host light organ by Vibrio fischeri. J. Bacteriol. 176, 6986 (1994).
- Geszvain, K., Visick, K. L. The hybrid sensor kinase RscS integrates positive and negative signals to modulate biofilm formation in Vibrio fischeri. J. Bacteriol. 190, 4437 (2008).
- Krasteva, P. V. Vibrio cholerae VpsT regulates matrix production and motility by directly sensing cyclic di-GMP. Science. 327, 866 (2010).
- Romling, U. Characterization of the rdar morphotype, a multicellular behaviour in Enterobacteriaceae. Cell Mol. Life. Sci. 62, 1234 (2005).
- Berleman, J. E., Chumley, T., Cheung, P., Kirby, J. R. Rippling is a predatory behavior in Myxococcus xanthus. J. Bacteriol. 188, 5888 (2006).
- Lee, K., Veeranagouda, Y. Ultramicrocells form by reductive division in macroscopic Pseudomonas aerial structures. Environ. Microbiol. 11, 1117 (2009).

