Cell Population Analyses During Skin Carcinogenesis
Summary
Carcinogenesis is a process involving interactions between cancer cells and the cancer microenvironment. To dissect the molecular events, one needs to isolate different cell populations at different stages during cancer development. Using a mouse model for basal cell carcinoma, we describe a protocol for cell analyses during carcinogenesis.
Abstract
Cancer development is a multiple-step process involving many cell types including cancer precursor cells, immune cells, fibroblasts and endothelial cells. Each type of cells undergoes signaling and functional changes during carcinogenesis. The current challenge for many cancer researchers is to dissect these changes in each cell type during the multiple-step process in vivo. In the last few years, the authors have developed a set of procedures to isolate different cell populations during skin cancer development using K14creER/R26-SmoM2YFP mice. The procedure is divided into 6 parts: 1) generating appropriate mice for the study (K14creER+ and R26-SmoM2YFP+ mice in this protocol); 2) inducing SmoM2YFP expression in mouse skin; 3) preparing mouse skin biopsies; 4) isolating epidermis from skin; 5) preparing single cells from epidermis; 6) labeling single cell populations for flow cytometry analysis. Generation of sufficient number of mice with the right genotype is the limiting step in this protocol, which may take up to two months. The rest of steps take a few hours to a few days. Within this protocol, we also include a section for troubleshooting. Although we focus on skin cancer, this protocol may be modified to apply for other animal models of human diseases.
Introduction
As the most common type of human cancer, basal cell carcinoma (BCC) affects about 2 million Americans per year 1. Accumulating evidence indicate that abnormal activation of hedgehog (Hh) signaling is the driving force underlying BCC development (Reviewed in 2,3). Alterations of Hh signaling in BCCs include inactivating mutations of PTCH1 4-6, gain-of-function mutations of SMO 7-9, and aberrant expression of Hh pathway transcription factors GLI1 10 and GLI2 11 or rare inactivated mutations of negative regulator Su(Fu)12. Through all these and other studies, Federal Drug Administration (FDA) has recently approved the use of Hh signaling inhibitor Vismodegib to treat metastatic and locally advanced BCCs13-15.
Despite all these achievements 16, we still do not understand the molecular and cellular mechanisms by which Hh signaling drives carcinogenesis. Establishing animal models using tissue-specific activation of Hh signaling is still important for these studies and for our understanding of drug resistance. In mice, wild-type mice do not develop BCCs, even under heavy doses of carcinogens, UV or ionizing radiation. In contrast, Ptch1+/- mice are susceptible to BCC development 17,18. The penetrance of BCC development in Ptch1+/- mice is over 50% 18,19 although Ptch1+/- mice rarely develop full-grown BCCs if kept under normal conditions. Due to the embryonic lethality of Ptch1-/-, tissue-specific knockout of PTCH1 is generally used for the study 20. In addition, conditional skin-specific expression of oncogenic SmoM2YFP (Krt14-creER:R26-SmoM2YFP or Krt14-cre:R26-SmoM2YFP) leads to formation of multiple microscopic BCCs at a very early age, providing an easy genetic assay for Hh signaling downstream of SMO 21.
There are three major issues in our knowledge of BCC biology. First, the cellular origin of BCCs is not entirely clear. While some studies support the budge of hair follicle as the stem cell site 22-24, Youssef et al. 25 localized the murine cell of origin of cutaneous Hh-driven tumors to be in the inter-follicular epidermis region, not in the hair follicle, by using cell-specific Cre to activate expression of a ROSA26-driven transgenic mutant SMO. Second, cellular interactions during BCC development are not well understood. It is known that keratinocytes with activated Hh signaling can lead to formation of BCCs, but other cellular changes are not well-understood. Furthermore, treatment of Smo antagonists in mice can lead to drug resistance 26-28. Thus novel targets for BCC are greatly needed. Understanding these three issues requires analyses of cellular changes in mice during carcinogenesis. While several methods have been published for keratinocyte culture, flow cytometry and skin stem cell isolation 29-31, there are currently no comprehensive procedures for cell analyses in mouse BCCs. By combining methods for mouse model of BCCs, separation of epidermis, generation of single cells from tissue and cell analyses, we will provide readers with a set of procedures to study cell signaling, cellular and molecular interactions during BCC development. We believe that this protocol will allow readers to see how each procedure is accomplished. In addition, we provided some data to illustrate what the results should look like, and troubleshooting to help readers to overcome difficulties during performing these procedures.
Protocol
This study has been approved by the IACUC Committee at Indiana University School of Medicine.
1. Generate Mice with Correct Genotypes
- Establish a mouse model of BCCs. Mate K14-CreER mice (Jackson laboratory stock number 005107) with R26-SmoM2YFP mice (Jackson laboratory stock number 005130) (18) to generate K14-creER+ and R26-SmoM2YFP+ mice.
- Perform genotyping. Immerse tail specimens (0.5 cm long) in directPCR tail lysis solution with 1 mg/ml proteinase K at 55 °C overnight with shaking. The next day, remove tissue debris by centrifugation at top speed in a micro-centrifuge (to keep the supernatant for genotyping). Use 1 μl of the supernatant for each PCR reaction with the primers and conditions recommended by the vendor. Select the mice with the right genotypes (at age of 3-6 weeks, buprenorphine at 0.05-0.1 mg/kg will be applied via SQ every 8-12 hr for 2-3 days after tail snip) for step 2.
2. Induce Expression of SmoM2 in Skin Keratinocytes
Induce SmoM2 expression in keratinocytes by oral administration of tamoxifen (5 μg/kg body weight in 50 ml of vegetable oil in each feeding) for 5 consecutive days via oral gavage with a feeding needle (curved 20 gauge).
3. Preparing Skin Specimens
In general, phenotypes are more severe on the ear and the tail in K14creER/R26SmoM2GFP mice. To prepare for cell analyses, we first harvest the mouse skin after animal sacrifice with an institutional approved procedure (CO2 plus cervical dislocation).
4. Isolating Epidermis
- After sacrifice mice with an approved procedure (see step 3), remove fur using a trimmer. Immerse skin specimens in dispase solution (5 mg/ml in DMEM with 10% FBS) at 37 °C for 1-2 hr (1 hr for mice less than 8 weeks old and 2 hr for mice old than 8 weeks).
- Following dispase treatment, separate epidermis from dermis by forceps.
5. Generate Single Cells
- To obtain single cell population, mince epidermis by scissors and then immerse in collagenase IV solution (1 mg/ml in DMEM with 10% FBS) for 1-2 hr at 37 °C in a 50 ml tube. Try to gently swirl the tubes every 20 min to help cell disassociation.
- Inspect cell disassociation under microscope. When single cells can be detected in the collagenase medium, incubate the specimen for an additional 30 min to increase the cell yield. In general, the epidermis from one mouse can yield up to 107 cells.
- Filter the tissue mixture through cell strainer (pore size 70 μm), spin cells down at 1,500 rpm via bench top centrifugation, and wash once time with PBS.
6. Label Cells and Perform Flow Cytometry Analyses
- Resuspend the cells in 10% FBS in PBS at 2.5 x 106 cells/ml to block non-specific binding (on ice for 30 min)
- Take an aliquot of cells to each of 1.5 ml microtubes for specific antibody labeling. Add different antibodies to each tube at a final concentration of 2 μg/ml. Mix antibodies with cells via a gentle vertex for several seconds and leave them on ice for 30 min, then wash with 1 ml PBS. For BCC specimens, we used the antibodies for following biomarkers: CD11b-PE plus Gr1-APC (myeloid cells or myeloid-derived suppressor cells); vimentin-FITC (fibroblast) (see reagents for details).
- For intracellular staining, we use a kit and follow the vendor’s manual (see Reagents for details). We use fresh cells to analyze cell surface markers. After surface marker staining, wash cells, and resuspend them in PBS. Add DAPI to the cell mixture at a final concentration of 1 μg/ml. DAPI will stain dead cells that will be gated out from further analyses.
- Analyze the data with specific software. We first select single cells based on the FSC vs. SSC plot, and use DAPI negative population (live cells) for further analysis.
Representative Results
It is known that mice do not develop basal cell carcinomas except with Hh signaling activation. Figure 1 shows the skin phenotype following induced expression of oncogenic smoothened, SmoM2YFP. Figure 2 shows analysis of myeloid cells in normal and tumor-bearing mice. CD11b+ Gr1+ cells are derived from myeloid cells, and some of them are myeloid derived suppressor cells. In normal situation, the CD11b+Gr1+ cell population is hardly detectable, but will increase in response to inflammation or tumor development. We showed that induced expression of SmoM2YFP in skin results in an increase in CD11b+Gr1+ cells. Currently, the molecular mechanisms underlying this change in BCCs are not clear.
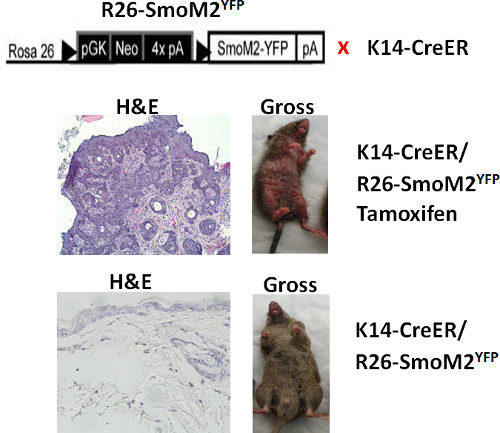
Figure 1. Morphological changes after SmoM2YFP induction in mouse skin. The top shows a diagram of mouse genotypes. The middle panel: the K14creER+ and R26-SmoM2YFP+ mouse treated with tamoxifen showed grossly abnormality in the skin (right) as well as skin tumors in the H&E section (left). The bottom panel: the mouse without SmoM2YFP induction showed normal looking skin (right) and no abnormal morphology in the H&E section (left).
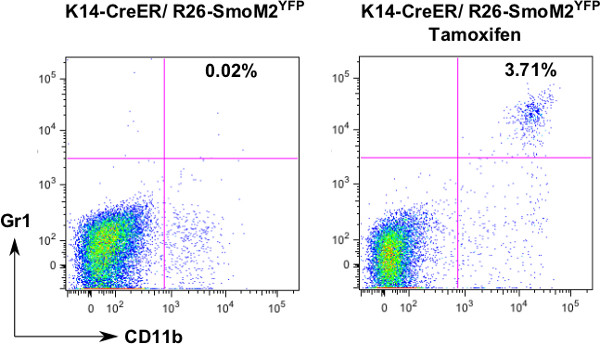
Figure 2. Flow analysis of CD11b+Gr1+ cells. To examine the change of CD11b+Gr1+ cells during skin cancer development, we isolated single cells and stained with fluorescence-labeled antibodies to CD11b and Gr1. After analysis with FlowJo, we noticed an increase of this cell population in skin tissues from tamoxifen-treated mice.
Discussion
We have described a method for cell population analysis in BCCs. Tumor tissues are consistent of many cell types, and each cell type behaves differently at a given time of carcinogenesis, which is very hard to recapture even under the best in vitro conditions. Using this protocol, we showed that development of basal cell carcinoma is accompanied by expansion of epidermal stem cells as well as recruitment of myeloid cells. In comparison with immunohistochemistry or immunofluorescent analyses, cell population analyses give a more quantitative assessment of cell population changes since specific antibodies to a variety of cell types are now available.
For this protocol, 10 weeks are needed to complete the study because phenotypic change from gene induction requires time. It is thus important to plan the experiment ahead of time. For cell isolation and analyses, two whole days may be needed. Because live cells are used for analyses, it is important to reserve a FACSCalibur ahead of time. We have also listed common problems when working with this protocol (see below).
In our experience, below are common problems we encountered:
- Poor separation of epidermis: This may be caused by incomplete dispase treatment (Please increase the time of diapase treatment) or insufficient dispase solution (The skin needs to be totally immersed in dispase solution). We use at least 5 ml of dispase solution for one mouse. The volume may vary based on the size of skin.
- Low yield of cell number: This may due to incomplete digestion with collagenase IV. Please first check collagenase concentration or expiration date. This can also be caused by insufficient amount of epidermis used. Furthermore, enzyme digestion should be performed at to 37 °C with shaking (please adjust temperature before perform digestion).
- Cell clumps: This is very common for skin cells (pipette cells a few times after going through strainer (before centrifugation), or existence of Mg2+ and Ca2+ in the solution (please use Mg2+ and Ca2+-free PBS for cell wash).
- Too many dead cells: This may be a result of degraded tissues (please use fresh tissues), or over-digestion (avoid extended incubation with dispase and collagenase IV).
This protocol can be combined with bone marrow transplantation or tissue transplantation to examine the cell origin. For example, transplantation of GFP-expressing bone marrow cells into lethally irradiated mice will allow us to examine the contribution of bone marrow cells to tumor-associated fibroblasts and myeloid cells. Similarly, skin tumors can be transplanted into a new mouse host to examine tumor-host interactions.
In addition to examine cell population changes during carcinogenesis, this protocol will also allow researchers to examine effects of drug treatments to the cells in the tumor environment. Although this protocol is designed to use for cell population analyses during skin carcinogenesis, slight modifications can be made for other cancer types.
In summary, cell population analyses in mouse models of skin cancer can give us the dynamic cellular changes during carcinogenesis, leading to a better understanding of cell biology in neoplastic transformation and different cellular events in the transformation process.
Declarações
The authors have nothing to disclose.
Acknowledgements
This study was supported by NCI (R01-94160 and R01-155086), IU Simon Cancer Center and Wells Center for Pediatric Research.
Materials
| Reagents | Company | Cat# | Comments |
| directPCR lysis reagent (tail) | Viagen Biotech | 102-T | store @ 4 °C |
| proteinase K | Sigma | P2308 | store @ -20 °C |
| ApliTag 360 taq polymerase | Applied biosystems | N8080155 | store @ -20 °C |
| Tamoxifen | Sigma | T5648 | store @ 4 °C |
| Feeding needle (20 gauge) | Fisher scientific | 01-290-9B | |
| Dispase | Roche Diagnostic | 04942078001 | store @ 4 °C |
| Collagenase IV | Worthington | LS004189 | store @ -20 °C |
| Cell strainer | Fisher scientific | 08-771-2 | |
| Anti-CD11b-PE | eBioscience | 12-0112-81 | store @ 4 °C |
| Anti-Gr1-APC | eBioscience | 17-5931-81 | store @ 4 °C |
| Anti-vimentin-FITC | eBioscience | 11-9897-80 | store @ 4 °C |
Referências
- Rogers, H. W., et al. Incidence estimate of nonmelanoma skin cancer in the United States. Arch. Dermatol. 146, 283-287 (2006).
- Epstein, E. H. Basal cell carcinomas: attack of the hedgehog. Nat. Rev. Cancer. 8, 743-754 (2008).
- Yang, L., Xie, G., Fan, Q., Xie, J. Activation of the hedgehog-signaling pathway in human cancer and the clinical implications. Oncogene. 29, 469-481 (2010).
- Johnson, R. L., et al. Human homolog of patched, a candidate gene for the basal cell nevus syndrome. Science. 272, 1668-1671 (1996).
- Hahn, H., et al. A mammalian patched homolog is expressed in target tissues of sonic hedgehog and maps to a region associated with developmental abnormalities. J. Biol. Chem. 271, 12125-12128 (1996).
- Hahn, H., et al. Mutations of the human homolog of Drosophila patched in the nevoid basal cell carcinoma syndrome. Cell. 85, 841-851 (1996).
- Xie, J., et al. Activating Smoothened mutations in sporadic basal-cell carcinoma. Nature. 391, 90-92 (1998).
- Reifenberger, J., et al. Missense mutations in SMOH in sporadic basal cell carcinomas of the skin and primitive neuroectodermal tumors of the central nervous system. Cancer Res. 58, 1798-1803 (1998).
- Lam, C. W., et al. A frequent activated smoothened mutation in sporadic basal cell carcinomas. Oncogene. 18, 833-836 (1999).
- Nilsson, M., et al. Induction of basal cell carcinomas and trichoepitheliomas in mice overexpressing GLI-1. Proc. Natl. Acad. Sci. U.S.A. 97, 3438-3443 (2000).
- Sheng, H., et al. Dissecting the oncogenic potential of Gli2: deletion of an NH(2)-terminal fragment alters skin tumor phenotype. Cancer Res. 62 (2), 5308-5316 (2002).
- Reifenberger, J., et al. Somatic mutations in the PTCH, SMOH, SUFUH and TP53 genes in sporadic basal cell carcinomas. Br. J. Dermatol. 152, 43-51 (2005).
- Guha, M. Hedgehog inhibitor gets landmark skin cancer approval, but questions remain for wider potential. Nat. Rev. Drug Discov. 11, 257-258 (2012).
- Sekulic, A., et al. Efficacy and safety of vismodegib in advanced basal-cell carcinoma. N. Engl. J. Med. 366, 2171-2179 (2012).
- Tang, J. Y., et al. Inhibiting the hedgehog pathway in patients with the basal-cell nevus syndrome. N. Engl. J. Med. 366, 2180-2188 (2012).
- Aszterbaum, M., et al. Ultraviolet and ionizing radiation enhance the growth of BCCs and trichoblastomas in patched heterozygous knockout mice. Nat. Med. 5, 1285-1291 (1999).
- Pazzaglia, S., et al. Modulation of patched-associated susceptibility to radiation induced tumorigenesis by genetic background. Cancer Res. 64, 3798-3806 (2004).
- Athar, M., et al. Inhibition of smoothened signaling prevents ultraviolet B-induced basal cell carcinomas through regulation of Fas expression and apoptosis. Cancer Res. 64, 7545-7552 (2004).
- Mancuso, M., et al. Basal cell carcinoma and its development: insights from radiation-induced tumors in Ptch1-deficient mice. Cancer Res. 64, 934-941 (2004).
- Ellis, T., et al. Patched 1 conditional null allele in mice. Genesis. 36, 158-161 (2003).
- Mao, J., et al. A novel somatic mouse model to survey tumorigenic potential applied to the Hedgehog pathway. Cancer Res. 66, 10171-10178 (2006).
- Wang, G. Y., Wang, J., Mancianti, M. L., Epstein, E. H. Basal cell carcinomas arise from hair follicle stem cells in Ptch1(+/-) mice. Cancer Cell. 19, 114-124 (2011).
- Grachtchouk, M., et al. Basal cell carcinomas in mice arise from hair follicle stem cells and multiple epithelial progenitor populations. J. Clin. Invest. 121, 1768-1781 (2011).
- Kasper, M., et al. Wounding enhances epidermal tumorigenesis by recruiting hair follicle keratinocytes. Proc. Natl. Acad. Sci. U.S.A. 108, 4099-4104 (2011).
- Youssef, K. K., et al. Identification of the cell lineage at the origin of basal cell carcinoma. Nat Cell Biol. 12, 299-305 (2010).
- Yauch, R. L., et al. Smoothened mutation confers resistance to a Hedgehog pathway inhibitor in medulloblastoma. Science. 326, 572-574 (2009).
- Buonamici, S., et al. Interfering with resistance to smoothened antagonists by inhibition of the PI3K pathway in medulloblastoma. Sci. Transl. Med. 2, 51ra70 (2010).
- Dijkgraaf, G. J., et al. Small molecule inhibition of GDC-0449 refractory smoothened mutants and downstream mechanisms of drug resistance. Cancer Res. 71, 435-444 (2011).
- Guo, Z., Draheim, K., Lyle, S. Isolation and culture of adult epithelial stem cells from human skin. J. Vis. Exp. (49), e2561 (2011).
- Li, L., Fukunaga-Kalabis, M., Herlyn, M. The three-dimensional human skin reconstruct model: a tool to study normal skin and melanoma progression. J. Vis. Exp. (54), e2937 (2011).
- Anacker, D., Moody, C. Generation of organotypic raft cultures from primary human keratinocytes. J. Vis. Exp. (60), e3668 (2012).

