Automated Separation of C. elegans Variably Colonized by a Bacterial Pathogen
Summary
The wormsorter facilitates genetic screens in Caenorhabditis elegans by sorting worms according to expression of fluorescent reporters. Here, we describe a new usage: sorting according to colonization by a GFP-expressing pathogen, and we employ it to examine the poorly understood role of pathogen recognition in initiating immune responses.
Abstract
The wormsorter is an instrument analogous to a FACS machine that is used in studies of Caenorhabditis elegans, typically to sort worms based on expression of a fluorescent reporter. Here, we highlight an alternative usage of this instrument, for sorting worms according to their degree of colonization by a GFP-expressing pathogen. This new usage allowed us to address the relationship between colonization of the worm intestine and induction of immune responses. While C. elegans immune responses to different pathogens have been documented, it is still unknown what initiates them. The two main possibilities (which are not mutually exclusive) are recognition of pathogen-associated molecular patterns, and detection of damage caused by infection. To differentiate between the two possibilities, exposure to the pathogen must be dissociated from the damage it causes. The wormsorter enabled separation of worms that were extensively-colonized by the Gram-negative pathogen Pseudomonas aeruginosa, with the damage likely caused by pathogen load, from worms that were similarly exposed, but not, or marginally, colonized. These distinct populations were used to assess the relationship between pathogen load and the induction of transcriptional immune responses. The results suggest that the two are dissociated, supporting the possibility of pathogen recognition.
Introduction
Automatic worm sorting is much like FACS, operating by measuring a fluorescent signal in a worm (typically provided by transgenic expression of reporter proteins) as it passes straightened in a tube, allowing redirection either to a collection tube/well or to a waste container according to gating parameters set by the researcher1. The wormsorter can facilitate research in many ways; an example of employing it as an analytical tool is a study that followed spatiotemporal patterns of promoter activity for almost 1,000 genes2.
However, the major use of the wormsorter is in genetic screens, following target gene expression levels or localization of fluorescent protein along the axis of the worm3-5.
Here, we describe a new application for the wormsorter, in following colonization of the worm by a fluorescently tagged pathogen. With this as a tool we focused on the relationship between pathogen colonization/load and the immune response, to gain new insights into the mechanisms responsible for initiation of immune responses in the worm.
In virtually all organisms studied to date, initiation of innate immune responses to microbial pathogens depends on recognition of pathogen-associated molecular patterns (PAMPs), and/or danger/damage-associated molecular patterns (DAMPs)6,7. The first are conserved microbial structures that include components of the microbial cell wall, its flagellum, or its lipid bilayer6; the second, include both released molecules (e.g. ATP8), altered proteins or other markers of altered cellular processes9,10. Both types of signals are recognized by proteins designated as pattern recognition receptors (PRRs), which upon specific binding of a pattern molecule activate a chain of events leading to a protective response. C. elegans has been extremely useful as a tractable model to dissect various aspects of host-pathogen interactions, but one thing that is not well understood is how immune responses are initiated in the worm. None of the putative receptors that are orthologous to pattern recognition receptors (PRRs) in other organisms have been shown to bind PAMPs, and many of the orthologs of PRRs that are pivotal for immune responses in other organisms show a surprisingly limited contribution to worm pathogen responses and resistance. For example, the Drosophila Toll receptor, which is essential for resisting Gram positive pathogens, is represented in C. elegans by a sole homolog, tol-1, which contributes to protection from the Gram negative pathogen Salmonella Typhimurium11, but not from other tested Gram-negative, or -positive pathogens11,12 . These observations, combined with data indicating that immune responses could be induced by disrupting cellular protein translation has led some to suggest that C. elegans primarily detects DAMPs9,13,14. Nevertheless, reports describing ability of dead pathogens to induce immune responses suggest that PAMP binding may be have an important role in pathogen recognition in C. elegans15,16. Previous work focusing on immune responses in age-synchronized genetically identical C. elegans populations, demonstrated large individual variability in intestinal colonization by the bacterial Gram-negative pathogen Pseudomonas aeruginosa.
However, transcriptional profiling studies treated these variably-colonized populations as one entity17,18. Taking advantage of this variability, we developed a protocol focusing an automated wormsorter to separate differentially colonized populations of Caenorhabditis elegans exposed to GFP-expressing P. aeruginosa. Examining gene expression in differently-colonized populations facilitated assessment of the relationship between pathogen load (and the associated damage) and immune responses and provided new insights about pathogen recognition in C. elegans19. Below we describe the protocol, which could be applied to sort worms infected with any fluorescently labeled pathogen.
To potential users it should be noted that the number of worms required to be sorted out depends on the nature of the subsequent analyses and protocols in use. For example, in the case of microarray gene expression analysis, >1,000 worms will be required to obtain enough RNA, if standard protocols are used, but ~100 worms would suffice if amplification is employed, allowing fast collection of material and thus minimizing stress to the worms.
Protocol
1. Obtaining a Synchronized Culture of Young Adult Animals
- Grow worms on several NGM plates seeded with OP50-1 E. coli bacteria (10x concentrated from a saturated culture) until many worms have reached the gravid stage.
- Treat gravid animals with egg-prep solution to obtain a synchronized culture (eggs).
- Plate eggs on several 60-mm NGM plates seeded with 10x concentrated OP50-1 at a density of roughly 150-200 eggs/plate.
- Incubate plates at 25 °C for 2 days (until worms have reached the L4 – Young Adult stage).
2. Preparing Pseudomonas aeruginosa Plates and Infecting Worms
- On the day of the egg-prep (step 1.1) inoculate a PA14::GFP culture into 2 ml of King’s B media containing rifampicin at a final concentration of 100 μg/ml. Incubate the culture at 37 °C with agitation O/N.
- Plate the PA14::GFP culture onto as many Slow Killing Plates (SKP) as needed for the assay. On each 150 mm SKP Petri dish pipette 75-100 μl of saturated culture and spread evenly using a sterile glass spreader. Incubate the plates at 37 °C for 20-24 hr.
- Remove plates from incubator and allow them to cool to RT (~20 min). Using M9 buffer, wash worms from step 1.4 onto PA14::GFP plates in a minimal volume of liquid. When using 150 mm plates, several hundred worms can be placed on a single plate.
- Incubate plates at 25 °C for 18-21 hr. For P. aeruginosa-exposed young adult worms, this is the time window displaying the optimal distribution of colonized vs noncolonized animals.
3. Setting up the Wormsorter
Before performing the sample sort, ensure that the machine is functioning properly. Details can be obtained at http://www.unionbio.com/support/documents.aspx?id=43, and the essential steps are outlined below.
- Set the sheath valve pressure to approximately 5 psi (4.5-5.5 range).
- To verify that sheath fluid flow rate is appropriate, place a 15 ml conical tube beneath the dispenser, turn on the sheath valve, and turn off the sorter valve. Hold the tube beneath the dispenser for 60 sec. The volume in the tube should be 9-10 ml. If it is outside this range, adjust the sheath valve pressure accordingly.
- To ensure that sheath fluid flow rate (set as in step 3.2) will allow accurate sorting, test the sort rate using 40 μm control particles:
- Turn off the sheath valve. Add about 25 ml of control particle solution to the reservoir.
- Turn on the sheath valve, and the sample valve.
- On the computer software, navigate to the "control particle mode" and click acquire.
- Make sure the particle flow rate is ~5-8 particles/sec, with a TOF (Time Of Flight) approximating the width of the particle, 40 μm. Such a TOF indicates that only a single 40 μm object is passing through at a time. If values are outside of this range, adjust the settings (following directions above) accordingly.
4. Sorting Colonized vs. Noncolonized Worms
- Using M9, wash worms into 15-ml conical tubes. Allow adult worms to sink to the bottom of the tube by gravity and remove the supernatant. Fill the tube with fresh M9. Repeat this process 3-4x. This removes a large proportion of larvae as well as excess bacteria and takes about 10 min.
- Add worms to the wormsorter reservoir with a gently-mixing small stir bar.
- Adjust initial signal gain by setting the Green PMT to 600 and the Red and Yellow PMT to 200.
- Begin acquiring data to adjust settings.
- Aim for a sort rate of 25-30 events (worms) per sec. If the number rate is too high, dilute the worms in the reservoir with M9 accordingly. If the rate is too low, add more worms in a small volume of M9.
- If the experiment requires a very stringent separation of colonized vs noncolonized worms, set the "coincidence check" to "pure". This ensures that in the event two objects occur too close to each other or in the same drop, the machine rejects the drop rather than collecting it.
- To sort the population of interest, draw gates around the axes indicating size (to obtain adult worms) and fluorescence intensity (i.e. high for colonized, low for noncolonized). The values for fluorescence gating must be determined empirically.
- Place a multi-well plate or a Petri dish underneath the dispenser to collect worms then open the sort valve to begin sorting worms.
- Collect a small population of animals to verify under the microscope that the sorted population is the population of interest. If not, adjust the gating parameters accordingly and continue with sample collection.
Representative Results
When age-matched, genetically identical C. elegans are exposed to P. aeruginosa, a wide distribution is observed in levels of colonization (Figure 1A). With the help of the protocol described here efficient separation of noncolonized from colonized worms can be achieved (Figure 1B). Unlike noncolonized worms, colonized worms show signs of damage, such as sluggishness and reduced defecation19. The latter may be a reason why worms colonized by P. aeruginosa have difficulty clearing infection. This has direct implications for the described protocol suggesting that worms sorted as noncolonized were not previously colonized. To directly test whether this assertion is correct, hand-picked colonized worms were subjected to similar washing steps as those in the sorting experiment and subsequently examined under a fluorescent microscope; only 4/56 cleared the pathogen. Therefore, it is likely that the proportion of previously-colonized worms among those sorted as noncolonized is negligible. However, it is advisable to monitor changes in colonization during washing steps to prevent effects of mistaken sorting on analysis, which may be more of an issue for other pathogens than P. aeruginosa.
Previous work employing the protocol described here used sorted worms for a microarray gene expression analysis and observed essentially identical responses to P. aeruginosa in colonized and noncolonized worm populations that had been exposed to the pathogen for the same amount of time19. Figure 2 demonstrates the same trend using quantitative (q)RT-PCR to measure expression of four genes, known to be part of the C. elegans immune response: lys-2 encodes a lysozyme, F08G5.6 and F55G11.2 are two uncharacterized genes, that respond specifically to infection, of which the first also contributes to infection resistance, and pgp-5, which responds to infection as well as to heavy metal stress. All four genes were induced similarly by P. aeruginosa exposure, regardless of the colonization status of the worms. This corroborates previously published results indicating that colonization and its associated damage are not required for an immune response, instead pointing at pathogen-associated molecular patterns as the signal. That pgp-5 is induced in noncolonized animals, which are not expected to be experiencing extensive damage, suggests an overlap in the regulatory programs controlling immune responses and general stress responses in C. elegans.
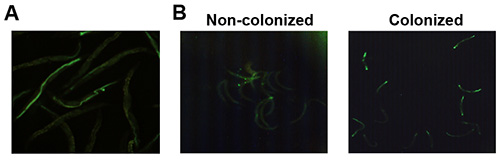
Figure 1. Colonized and noncolonized C. elegans separated using a wormsorter. (A) Representative image of wild-type adult C. elegans exposed to GFP-expressing P. aeruginosa for 18 hr. (B) Representative images of a noncolonized and colonized worms separated using the wormsorter. Please click here to view a larger version of this figure.
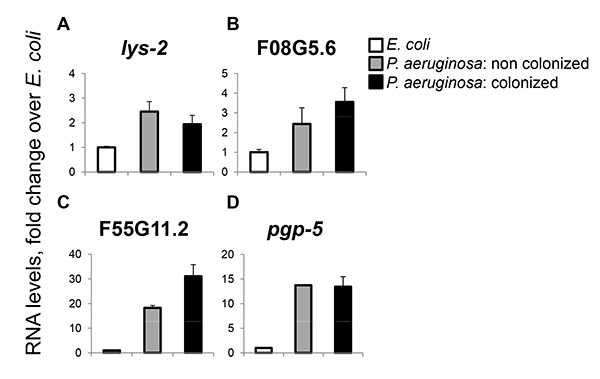
Figure 2. Colonization is not required for the transcriptional response to P. aeruginosa. RNA levels of lys-2 (A), F08G5.6 (B), F55G11.2 (C), and pgp-5 (D) measured by qRT-PCR in wild-type animals exposed to E. coli or P. aeruginosa for ~18 hr. Shown are means and SD of duplicate measurements from one experiment, which is a representative of three. Please click here to view a larger version of this figure.

Table 1. Materials and reagents needed for a wormsorter experiment.
Discussion
The method we describe takes advantage of fluorescent labeling of entities outside of the worm, to follow interactions between the worm and its environment. In the case we present, separation was based on labeling of a pathogen and was employed to separate worms with heavy pathogen load from those with no (or light) load. Subsequent gene expression analysis found no difference in immune responses between the two groups suggesting that they were independent of pathogen load. The signal that initiates the response was shown elsewhere to be physically associated with the bacteria, rather than being secreted, suggesting that this signal may be a PAMP. Together, these results supported the hypothesis that PAMP recognition plays a role in initiating immune response in C. elegans19.
One advantage of the sorting protocol described above is that both colonized and noncolonized worms are isolated from a single population. This depends on having a population with an optimal distribution of animals that are colonized vs. noncolonized. We have found that when infecting young-adult, wild type worms with P. aeruginosa, this corresponded to an exposure time of roughly 18 hr. This exposure-time produced a population where the majority of animals were colonized to an "intermediate" degree, but several hundred worms showed full colonization, and a similar number were noncolonized. While this represents optimal distribution for our purposes, others may define their own optimal distribution.
During sorting, it is advisable to have "quality controls" built into each experiment. We did that in two ways. One, for gene expression studies, we hand-picked a small number of (colonized and noncolonized) worms under a fluorescent dissecting microscope, which can be used for qRT-PCR on a limited set of genes. These serve as the "gold standard" for what gene expression in a truly pure population would look like. Secondly, it is good practice to sort 20 or more worms to a plate for each automatically sorted population to verify under a fluorescent microscope that the instrument is performing as expected. Depending on the downstream application, different degrees of "fidelity" of the sorting protocol may be required. When sorted worms do not conform to the desired degree of colonization, gating parameters should be adjusted (step 4.7). It should be noted that the more stringent the gates, the higher the initial number of worms needed, and experiments should be scaled up accordingly.
The experiment described here could be carried out with any pathogen of interest (as long as it is fluorescently tagged). Following sorting, subsequent analyses do not need to adhere to gene expression analysis, and could focus on survival or even behavioral analysis. Furthermore, similar protocols could be used for analytical purposes, to follow metabolite absorption, or to quantify pathogen load, the latter providing a simpler alternative to CFU counts, which are highly variable20,21. The increase in numbers and in speed could facilitate quantifying pathogen load under different conditions, including genetic disruptions in the host or the pathogen, and different environmental conditions. Moreover, since the wormsorter can be equipped to detect multiple fluorescent molecules, tracking colonization need not be limited to a single type of bacteria. This capability would be useful for ecological studies, allowing different microbes to compete for colonization. In laboratory studies of pathogenesis, C. elegans is typically grown on a bacterial monoculture. However, the study of mechanisms of innate immunity in the wild, where pathogens exist amongst hundreds of other microorganisms that may impact immunity is beginning to garner attention22,23.
In summary, using the wormsorter for a wide range of applications allows experiments to be performed in a short time, and/or on large scale. Expanding the repertoire of such applications could advance our understanding of C. elegans physiology. In particular, in the field of C. elegans innate immunity, survival is an often-used metric for testing the contributions of various genes. However, colonization is more proximal to the events that initiate immunity while still providing a functional output. Using protocols as described here to isolate and/or analyze populations of interest is a valuable tool to study various aspects of the early events of immune responses in the worm.
Declarações
The authors have nothing to disclose.
Acknowledgements
The authors thank the Ellison Medical Foundation for their support. We wish also to thank members of the Abby Dernburg laboratory for assistance with using the wormsorter.
Materials
| M9 Buffer | Prepared in house | Recipe at wormbook.org |
| Rifampicin | Sigma | R3501 |
| Egg prep solution | Prepared in house | 50ml water ; 40ml bleach ; 10ml of 10N Sodium Hydroxide |
| NGM plates | Prepared in house | Recipe at wormbook.org |
| SKP plates | Prepared in house | Recipe same as NGM only 0.35% peptone instead of 0.25% |
| Control test particles | Union Biometrica | 310-5071-001 |
Referências
- Pulak, R. Techniques for analysis, sorting, and dispensing of C. elegans on the COPAS flow-sorting system. Methods Mol. Biol. , 275-286 (2006).
- Dupuy, D., et al. Genome-scale analysis of in vivo spatiotemporal promoteractivity in Caenorhabditis elegans. Nat. Biotechnol. 25, 663-668 (2007).
- Squiban, B., Belougne, J., Ewbank, J., Zugasti, O. Quantitative and Automated High-throughput Genome-wide RNAi Screens in elegans. J. Vis. Exp. (60), (2012).
- Doitsidou, M., Flames, N., Lee, A. C., Boyanov, A., Hobert, O. Automated screening for mutants affecting dopaminergic-neuron specification in C. elegans. Nat. Methods. 5, 869-872 (2008).
- Pujol, N., et al. Distinct innate immune responses to infection and wounding in the C. elegans epidermis. Curr. Biol. 18, 481-489 (2008).
- Medzhitov, R., Janeway, C. Innate immune recognition: mechanisms and pathways. Immunol. Rev. 173, 89-97 (2000).
- Matzinger, P. Tolerance, danger, and the extended family. Annu. Rev. Immunol. 12, 991-1045 (1994).
- Mariathasan, S., et al. Cryopyrin activates the inflammasome in response to toxins and ATP. Nature. 440, 228-232 (2006).
- Melo, J. A., Ruvkun, G. Inactivation of conserved C. elegans genes engages pathogen- and xenobiotic-associated defenses. Cell. 149, 452-466 (2012).
- Chen, G. Y., Nunez, G. Sterile inflammation: sensing and reacting to damage. Nat. Rev. Immunol. 10, 826-837 (2010).
- Tenor, J. L., Aballay, A. A conserved Toll-like receptor is required for Caenorhabditis elegans innate immunity. EMBO Rep. 9, 103-109 (2008).
- Pujol, N., et al. A reverse genetic analysis of components of the Toll signaling pathway in Caenorhabditis elegans. Curr. Biol. 11, 809-821 (2001).
- McEwan, D. L., Kirienko, N. V., Ausubel, F. M. Host translational inhibition by Pseudomonas aeruginosa Exotoxin A Triggers an immune response in Caenorhabditis elegans. Cell Host Microbe. 11, 364-374 (2012).
- Dunbar, T. L., Yan, Z., Balla, K. M., Smelkinson, M. G., Troemel, E. R. C. C. elegans detects pathogen-induced translational inhibition to activate immune signaling. Cell Host Microbe. 11, 375-386 (2012).
- Irazoqui, J. E., et al. Distinct pathogenesis and host responses during infection of C. elegans by P. aeruginosa and S. aureus. PLoS Pathog. 6, (2010).
- Pukkila-Worley, R., Ausubel, F. M., Mylonakis, E. Candida albicans infection of Caenorhabditis elegans induces antifungal immune defenses. PLoS Pathog. , (2011).
- Shapira, M., et al. A conserved role for a GATA transcription factor in regulating epithelial innate immune responses. Proc. Natl. Acad. Sci. U.S.A. 103, 14086-14091 (2006).
- Troemel, E. R., et al. p38 MAPK regulates expression of immune response genes and contributes to longevity in C. elegans. PLoS Genet. , 183 (2006).
- Twumasi-Boateng, K., Shapira, M. Dissociation of immune responses from pathogen colonization supports pattern recognition in C. elegans. PLoS One. , 7 (2012).
- Jakobsen, H., et al. The alkaloid compound harmane increases the lifespan of Caenorhabditis elegans during bacterial infection, by modulating the nematode’s innate immune response. PLoS One. 8, (2013).
- Portal-Celhay, C., Bradley, E. R., Blaser, M. J. Control of intestinal bacterial proliferation in regulation of lifespan in Caenorhabditis elegans. BMC Microbiol. 12, 49 (2012).
- Troemel, E. R., Felix, M. A., Whiteman, N. K., Barriere, A., Ausubel, F. M. Microsporidia are natural intracellular parasites of the nematode Caenorhabditis elegans. PLoS Biol. 6, 2736-2752 (2008).
- Montalvo-Katz, S., Huang, H., Appel, M. D., Berg, M., Shapira, M. Association with soil bacteria enhances p38-dependent infection resistance in Caenorhabditis elegans. Infect. Immun. 18, 514-520 (2013).

