Retropinacol/Cross-pinacol Coupling Reactions – A Catalytic Access to 1,2-Unsymmetrical Diols
Summary
A novel account for the synthesis of unsymmetrical 1,2-diols based on a retropinacol/cross-pinacol coupling mechanism is described. Due to the catalytic execution of this reaction a considerable improvement compared to conventional cross-pinacol couplings is achieved.
Abstract
Unsymmetrical 1,2-diols are hardly accessible by reductive pinacol coupling processes. A successful execution of such a transformation is bound to a clear recognition and strict differentiation of two similar carbonyl compounds (aldehydes → secondary 1,2-diols or ketones → tertiary 1,2-diols). This fine-tuning is still a challenge and an unsolved problem for an organic chemist. There exist several reports on successful execution of this transformation but they cannot be generalized. Herein we describe a catalytic direct pinacol coupling process which proceeds via a retropinacol/cross-pinacol coupling sequence. Thus, unsymmetrical substituted 1,2-diols can be accessed with almost quantitative yields by means of an operationally simple performance under very mild conditions. Artificial techniques, such as syringe-pump techniques or delayed additions of reactants are not necessary. The procedure we describe provides a very rapid access to cross-pinacol products (1,2-diols, vicinal diols). A further extension of this new process, e.g. an enantioselective performance could provide a very useful tool for the synthesis of unsymmetrical chiral 1,2-diols.
Introduction
The pinacol coupling reaction is a general and commonly used method for the preparation of symmetrically vicinal diols (1,2-diols, pinacols). For comprehensive reviews in this field see references Hirao1, Chatterjee and Joshi2, Ladipo3, and Gansäuer and Bluhm4. In contrast to that, only a few reports were published to refer an efficient realization of cross-pinacol coupling reactions to yield the corresponding unsymmetrical 1,2-diols (titanium(IV) chloride/manganese5, samarium(II) iodide6, magnesium/trimethylchlorosilane7, vanadium(II)8, zirconium/tin9, and ytterbium10). Thus, the intermolecular cross-pinacol coupling reaction still remains a big challenge in organic chemistry, especially the catalytic execution of this transformation.
The formation of cross coupling products is kinetically disfavored under conditions of a classical pinacol coupling. To obtain sufficient amounts of the unsymmetrical product delayed addition of one carbonyl compound is possible. There exist a few examples which are developing this concept, but they are based on several specific experimental manipulations and hence cannot be generalized. In addition, the required excess of one carbonyl compound in these transformations resulted in a laborious separation of a complex product mixture11. An alternative for this purpose is represented by the precomplexation of one reactant rendering equimolar amounts of an additional reagent necessary.
Various examples of a reversible pinacol reaction have been described12. These lead to the consideration that such conditions might be an optimal starting point for the selective synthesis of cross coupling products. Since a low-valent metal as well as a reactive radical species is formed simultaneously in situ, unsymmetrical diols could be formed exclusively in the presence of a suitable carbonyl reactant. To the best of our knowledge such a method has not been reported before (Porta et al. described a comparable pinacol cleavage and subsequent coupling by the additional deployment of stoichiometric amounts of AIBN (2,2′-azo-bis-isobutyronitrile) to generate the required radicals)13.
Herein a protocol is visualized which provides a rapid and operationally simple access to unsymmetrical 1,2-diols. The unsymmetrical pinacol products are mostly accessible in excellent yields (>95%). Undesired symmetrically pinacol products are not observed. This new cross-pinacol methodology is based upon a retropinacol/cross-pinacol coupling sequence. It will be demonstrated in the following by representative reactions of benzopinacole (1,1,2,2-tetraphenyl-1,2-ethanediol, 1) with 2-ethylbutyraldehyde (in the aldehyde series) and with diethylketone (in the ketone series).
Protocol
1. Preparation of Titanium(IV) tert-butoxide/Triethylchlorosilane Solution
- Dissolve 400 mg (400 μl) titanium(IV) tert-butoxide (1 mmol) in 10 ml of dry dichloromethane. Add 150 mg (170 μl) triethylchlorosilane (1 mmol) to this solution at RT. 1 ml of this dichloromethane-solution contains 0.1 mmol titanium(IV) tert-butoxide and 0.1 mmol triethylchlorosilane.
2. Pinacol-reaction of Tetraphenyl-1,2-ethanediol (1) with 2-Ethylbutyraldehyde
- Solve 366 mg of tetraphenyl-1,2-ethanediol (1, 1 mmol) and 300 mg (370 μl) of freshly distilled 2-ethylbutyraldehyde (3 mmol) in 3 ml dry dichloromethane.
- Add 0.5 ml of the separately prepared titanium(IV) tert-butoxide/triethylchlorosilane solution (0.05 mmol).
- Stir the resulting mixture at RT in a sealed reaction tube.
- Confirm the reaction is completed by thin layer chromatography (eluent: hexane/acetone – 9/1) on silica gel TLC plates (60 F254). The end of reaction is reached at the time when tetraphenyl-1,2-ethanediol 1 can no longer be detected (∼12 hr). The Rf-value of the product is 0.3 14.
- Dilute the resulting reaction mixture with 50 ml of dichloromethane.
- Wash the diluted reaction mixture successively by 20 ml saturated aqueous ammonium chloride and sodium hydrogen carbonate solution in a separatory funnel.
- Isolate the organic layer by a separatory funnel.
- Dry the organic layer by stirring over dry magnesium sulfate.
- Filtrate the suspension by a fluted paper filter and collect the filtrates.
- Remove dichloromethane from the filtrate in vacuo at 40 °C using a rotary evaporator (10-30 mmHg). Evaporation of solvents will require 20 min.
- Purify the remaining residue by flash column chromatography through a column of silica gel (0.035-0.070 mm, ACROS) with a gradient of hexane/acetone (starting from 19:1 and going down to 16:4) to acquire 280 mg of 1,2-diol 2f (0.99 mmol).
- Confirm the identity of the 1,2-diol 2f by 1H nuclear magnetic resonance spectroscopy (NMR) using CDCl3 as solvent. For a 300 MHz NMR spectrometer, the 1H NMR spectrum of the diol is as follows: δ =0.78 (t, 3H, J = 7.4 Hz), 0.87 (t, 3H, J = 7.3 Hz), 1.18-1.40 (m, 4H), 1.75-1.81 (m, 1H), 1.91 (s, 1H, OH), 3.12 (s, 1H, OH), 4.68 (d, 1H, J = 1.2 Hz), 7.19-7.37 (m, 6H), 7.44-7.46 (m, 2H), 7.61-7.63 (m, 2H).
3. Pinacol-reaction of Tetraphenyl-1,2-ethanediol (1) with Diethyl Ketone
- Solve 366 mg of tetraphenyl-1,2-ethanediol (1,1 mmol) and 345 mg (423 μl) of diethyl ketone (4 mmol) in 3 ml dry dichloromethane.
- Add 1 ml of the separately prepared titanium(IV) tert-butoxide/triethylchloro-silane solution (0.1 mmol).
- Stir the resulting mixture at RT in a sealed reaction tube.
- Confirm the reaction is complete by thin layer chromatography (eluent: hexane/acetone, 9:1) on silica gel TLC plates (60 F254). The end of reaction is reached at the time, when tetraphenyl-1,2-ethanediol 1 cannot be detected (∼12 hr). The Rf of the product is 0.3 14.
- Dilute the resulting reaction mixture with 50 ml of dichloromethane.
- Wash the diluted reaction mixture successively by 20 ml saturated aqueous ammonium chloride and sodium carbonate solution in a separatory funnel.
- Isolate the organic layer by a separatory funnel.
- Dry the organic layer by stirring over dry magnesium sulfate.
- Filtrate the suspension by a fluted paper filter and collect the filtrates.
- Remove dichloromethane in vacuo at 40 °C using a rotary evaporator (10-30 mmHg). Evaporation of volatile constituents will require 30 min.
- Purify the remaining residue by flash column chromatography through a column of silica gel (0.035-0.070 mm, ACROS) with a gradient of hexane/acetone (starting from 19:1 and going down to 16:4) to acquire 250 mg of 1,2-diol 4f (0.93 mmol).
- Confirm the identity of the product by 1H nuclear magnetic resonance spectroscopy (NMR) using CDCl3 as solvent. For a 300 MHz NMR spectrometer, the 1H NMR spectrum of the diol 4f is as follows: δ = 0.92 (t, 6H, J = 7.6 Hz), 1.78 (m, 4H), 2.03 (s, 1H, OH), 2.83 (s, 1H, OH), 7.26-7.35 (m, 6H), 7.69-7.71 (m, 4H).
Representative Results
In reactions of tetraphenyl-1,2-ethanediol 1 and acetone in the presence of catalytic amounts of titanium(IV)-alkoxides we observed the formation of 1,1-diphenyl-1,2-diol 4a and at the same time the formation of benzophenone 3 (Scheme 1). The corresponding symmetrical 1,2-diol formed by a competitive pinacol coupling of acetone was not detected. However, to obtain quantitative conversions extremely long and unacceptable reaction times were required under these conditions. A considerable increase in reaction rates was observed by the addition of trialkylchlorosilanes. Overall high yields within acceptable reaction times were noticed. Further on, a catalytic performance becomes possible that extremely simplifies the purification process of the products.
Best results were achieved by deploying 5-10 mol% triethylchlorosilane as well as titanium(IV) tert-butoxide. By means of this catalyst combination undesired competitive reactions were avoided (Meerwein-Ponndorf-Verley reactions, formation of silylethers or pinacol rearrangement). By deployment of bulky trialkylchlorosilanes longer reaction times were observed again.
Reactions were conducted in dichloromethane at RT. Other solvents like toluene or acetonitrile also proved to be applicable. Schlenk-conditions (inert conditions, argon atmosphere) were not required, but the reaction tube should be properly sealed. The catalytic species was inactivated by exposure of air. But it can easily be regenerated afterwards by flushing with a nitrogen or argon atmosphere. Also, the order of addition of reactants and reagents was unsubstantial. Deployment of α-unbranched aldehydes resulted in a partial formation of corresponding acetales (2a, 2b and 2p, Table 1). In most other cases the diols were isolated with excellent yields.
The deployment of ketones extended significantly the product scope of this method (Table 2). A minor increase of catalyst loading (10 mol%) was required to afford the corresponding 1,2-diols 4a–s in good to quantitative yields. Again, no symmetrical diols were formed under these reaction conditions.
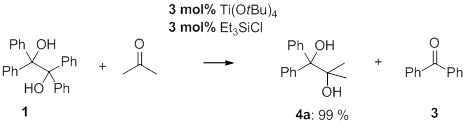
Scheme 1. Retropinacol/cross-pinacol reaction of tetraphenylethane-1,2-diol with acetone.
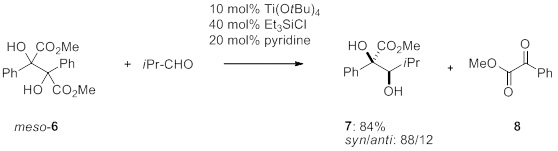
Scheme 2. Retropinacol/cross-pinacol reaction of 2.3-diphenyl-dimethyl-tartrate with isobutyraldehyde.
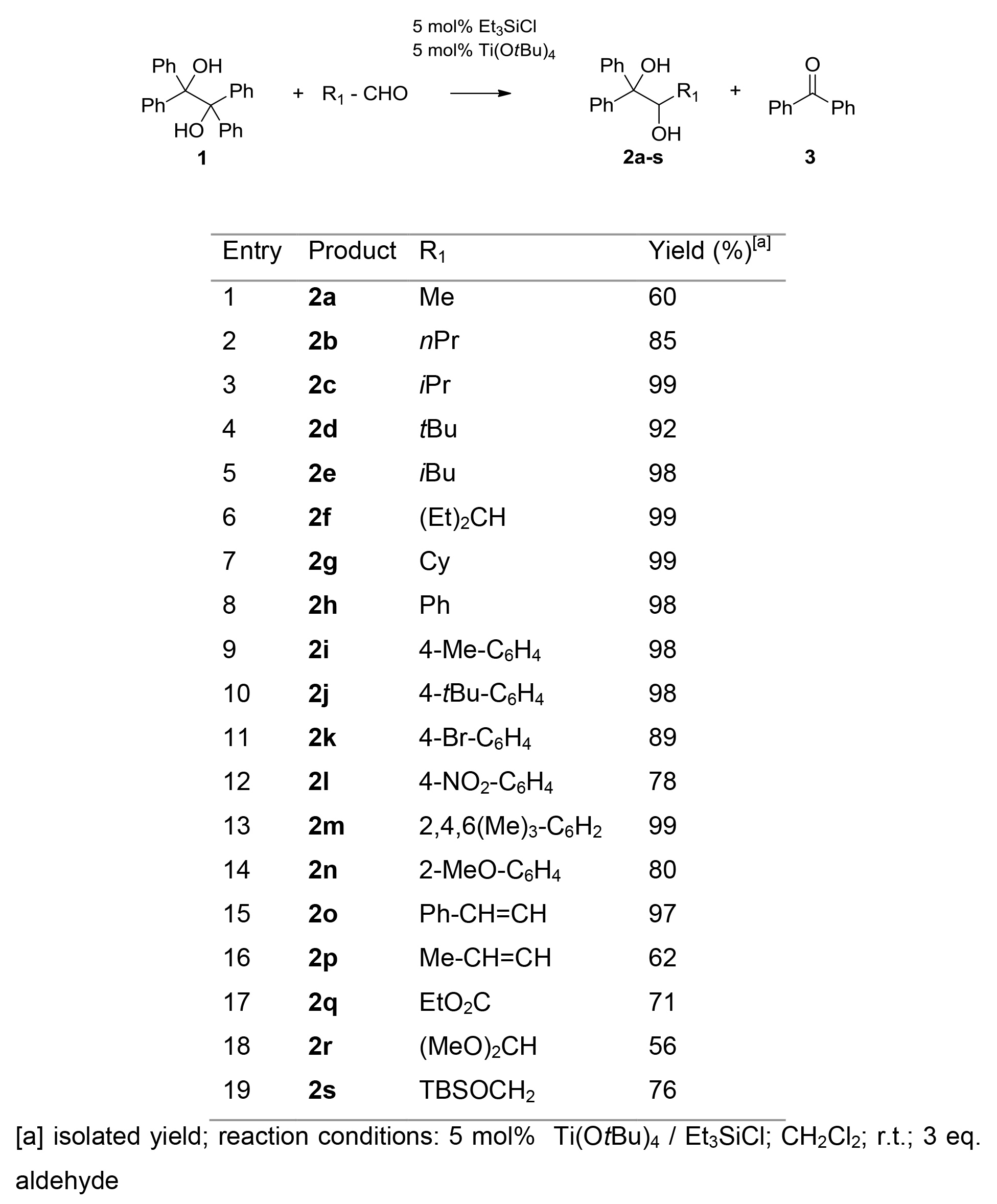
Table 1. Retropinacol/cross-pinacol coupling reactions with aldehydes.
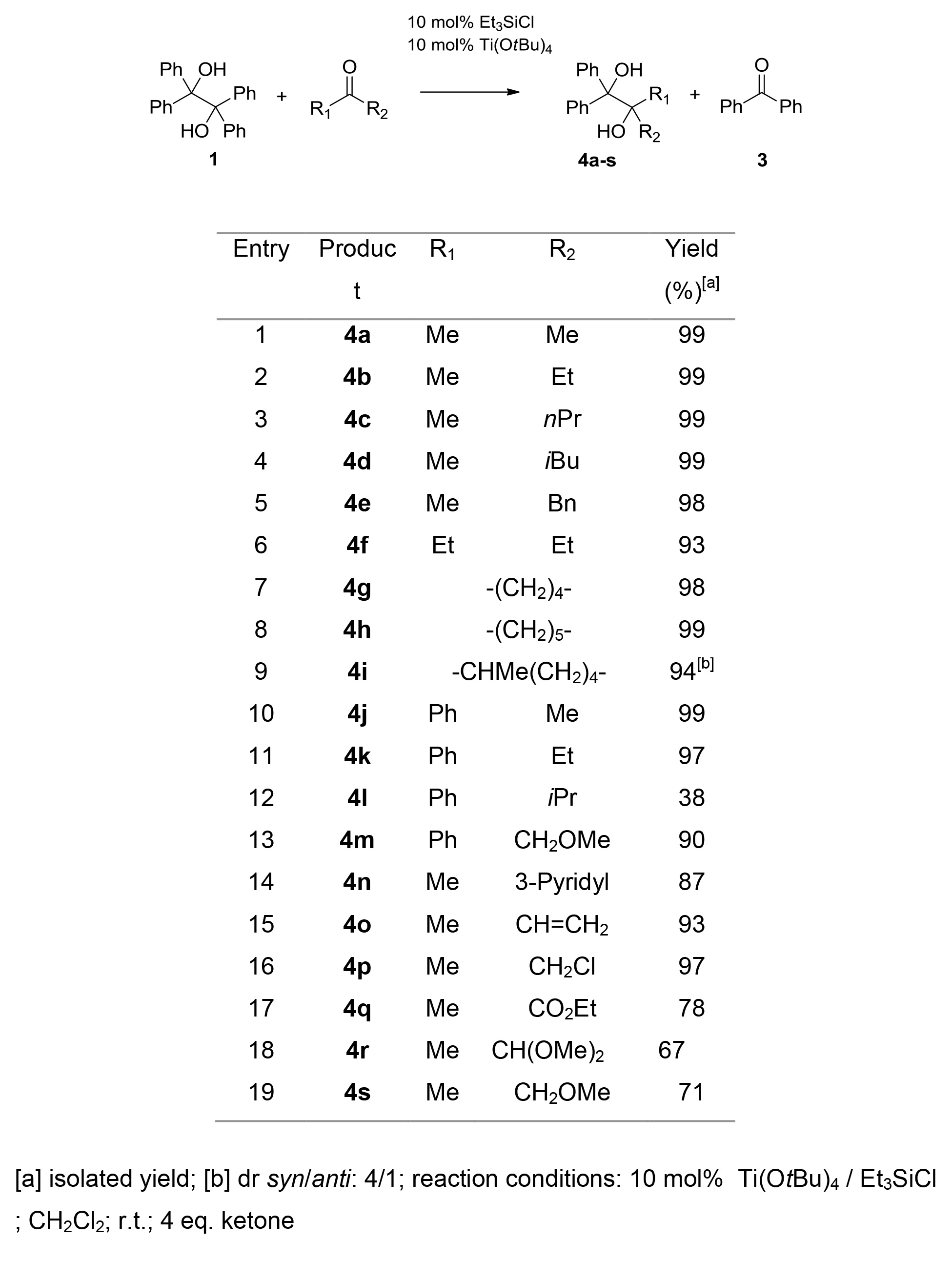
Table 2. Retropinacol/cross-pinacol coupling reactions with ketones.
Discussion
An overall decrease in reaction times and higher yields is observed by deployment of electron-rich carbonyl compounds (compare entry 3 with 17, Table 1 or entry 19 with 13, Table 2). In addition, in reactions of ketones with bulky substituents a decrease in yields is observed under comparable conditions (compare entry 12 with 11, Table 2).
Although a wide range of carbonyl compounds can be applied in this novel process, different starting geminal diols request an optimization of reaction conditions. This is true especially for functionalized 1,2-diols. To demonstrate this, we have tested 2,3-diphenyl-dimethyl-tartrate (6) as an alternative starting compound under similar reaction conditions. By increasing the amount of triethylchlorosilane a retropinacol/cross-pinacol coupling of dimethyl tartrate 6 could be achieved even with enolizable aldehydes (isobutyraldehyde) (Scheme 2).
Based on this straightforward extension of this novel methodology, it is assumed that the described retropinacol/cross-pinacol coupling concept can be generalized to the synthesis of further unsymmetrically vicinal 1,2-diols, e.g. in total synthesis of natural products.
Declarações
The authors have nothing to disclose.
Acknowledgements
The authors thank Deutsche Forschungsgemeinschaft, Bayer Pharma AG, Chemtura Organometallics GmbH Bergkamen, Bayer Services GmbH, BASF AG, and Sasol GmbH for financial support.
Materials
| 1.2-Dichloromethane | Sigma-Aldrich | 319929 | |
| Titanium(IV)tert-butoxide | VWR International | 200014-852 | |
| 2-Ethylbutyraldehyde | Sigma-Aldrich | 110094 | |
| Benzopinacol | Aldrich | B9807 | |
| Triethylchlorosilane | Aldrich | 235067 | |
| hexane, certified ACS | Fisher scientific | H29220 | |
| acetone, certified ACS | ACROS | 42324 | |
| Ammonium chloride | ACROS | 19997 | |
| Sodium hydrogen carbonate | ACROS | 12336 | |
| Magnesium sulphate | ACROS | 41348 | |
| silica gel 60 F254 TLC plates | VWR International | 1,057,140,001 | |
| silica gel, 0.035-0.070 for flash-chromatography | ACROS | 240360300 |
Referências
- Hirao, T. Catalytic reductive coupling of carbonyl compounds – The pinacol coupling reaction and. 279, 53-75 (2007).
- Chatterjee, A., Joshi, N. N. Evolution of the stereoselective pinacol coupling reaction. Tetrahedron. 62, 12137-12158 (2006).
- Ladipo, F. T. Low-valent titanium-mediated reductive coupling of carbonyl compounds. Curr. Org. Chem. 10, 965-980 (2006).
- Gansäuer, A., Bluhm, H. Reagent-controlled transition-metal-catalyzed radical reactions. Chem. Rev. 100, 2771-2788 (2000).
- Duan, X. -. F., Feng, J. X., Zi, G. -. F., Zhang, Z. -. B. A Convenient synthesis of unsymmetrical pinacols by coupling of structurally similar aromatic aldehydes mediated by low-valent titanium. Synthesis. , 277-282 (2009).
- Paquette, L. A., Lai, K. W. Pinacol macrocyclization-based route to the polyfused medium-sized CDE ring system of lancifodilactone. G. Org. Lett. 10, 3781-3784 (2008).
- Maekawa, H., Yamamoto, Y., Shimada, H., Yonemura, K., Nishiguchi, I. Mg- promoted mixed pinacol coupling. Tetrahedron Lett. 45, 3869-3872 (2004).
- Kang, M., Park, J., Pedersen, S. F. Pinacol cross coupling reactions of ethyl 2-alkyl-2-formylpropionates. stereoselective synthesis of 2,2,4- trialkyl-3-hydroxy-γ-butyrolactones. Syn. Lett. , 41-43 (1997).
- Askham, F. R., Carroll, K. M. Anionic zirconaoxiranes as nucleophilic aldehyde equivalents. application to intermolecular pinacol cross coupling. J. Org. Chem. 58, 7328-7329 (1993).
- Hou, Z., Takamine, K., Aoki, O., Shiraishi, H., Fujiwara, Y., Taniguchi, H. Nucleophilic Addition of lanthanoid metal umpoled diaryl ketones to electrophiles. J. Org. Chem. 53, 6077-6084 (1988).
- Groth, U., Jung, M., Vogel, T. Intramolecular chromium(II)-catalyzed pinacol cross coupling of 2-Mmethylene-α,ω-dicarbonyls. Syn. Lett. , 1054-1058 (2004).
- Appendino, G. Synthesis of Modified Ingenol Esters. Eur. J. Org. Chem. , 3413-3420 (1999).
- Spaccini, R., Pastori, N., Clerici, A., Punta, C., Porta, O. Key role of Ti(IV) in the selective radical-radical cross-coupling mediated by the Ingold-Fischer effect. J. Am. Chem. Soc. 130, 18018-18024 (2008).
- Leonard, J., Lyfo, B., Procter, G. . Advanced Practical Organic Chemistry. , (2013).
- Scheffler, U., Stoesser, R., Mahrwald, R. Retropinacol / cross-pinacol coupling reactions – a catalytic access to 1,2-unsymmetrical diols. Adv. Synth. Cat. 354, 2648-2652 (2012).

