Methods for Facilitating Microbial Growth on Pulp Mill Waste Streams and Characterization of the Biodegradation Potential of Cultured Microbes
Summary
Industrial wastes can be collected and modified to analyze microbial growth. Lignocellulose extraction techniques provide components to analyze specific biodegradation ability. Gas chromatography-mass spectrometry identifies fermentation products of microorganisms grown on pulping waste. These methods determine the metabolic capacity of microorganisms to degrade pulping waste.
Abstract
The kraft process is applied to wood chips for separation of lignin from the polysaccharides within lignocellulose for pulp that will produce a high quality paper. Black liquor is a pulping waste generated by the kraft process that has potential for downstream bioconversion. However, the recalcitrant nature of the lignocellulose resources, its chemical derivatives that constitute the majority of available organic carbon within black liquor, and its basic pH present challenges to microbial biodegradation of this waste material. Methods for the collection and modification of black liquor for microbial growth are aimed at utilization of this pulp waste to convert the lignin, organic acids, and polysaccharide degradation byproducts into valuable chemicals. The lignocellulose extraction techniques presented provide a reproducible method for preparation of lignocellulose growth substrates for understanding metabolic capacities of cultured microorganisms. Use of gas chromatography-mass spectrometry enables the identification and quantification of the fermentation products resulting from the growth of microorganisms on pulping waste. These methods when used together can facilitate the determination of the metabolic activity of microorganisms with potential to produce fermentation products that would provide greater value to the pulping system and reduce effluent waste, thereby increasing potential paper milling profits and offering additional uses for black liquor.
Introduction
The pulping of wood is a chemically intensive process that has been optimized over many years to create a system with minimal waste. However, some outputs of this process could be used to produce higher value product(s). Black liquor is one such example. It is generated from the kraft process, which is the dominant chemical pulping method, representing 85% of world lignin production1. The kraft process (Figure 1) uses temperature (160-200 °C), pressure (120 psig), and the chemicals contained in white liquor (sodium hydroxide and sodium sulfide) to dissolve the lignin from the wood fibers2,3. Black liquor contains lignin, organic acids, and polysaccharide degradation byproducts4. It is incinerated to produce steam and recover chemicals in the recovery boiler that provides thermal energy for downstream paper making and pulping processes. The volume of black liquor generated by pulping can exceed the amount that the recovery boiler can effectively process. Disposing of the black liquor as effluent negatively affects aquatic flora and fauna and thus is not an option. Application of microbial organisms that could use black liquor for growth would be beneficial in terms of increasing chemical recovery and generation of value-added product(s) that would improve the overall life cycle analysis of the pulping system. Chemical and biological conversion of lignin derived monomers has successfully produced vanillin and cinnamic acid for use as food sweeteners and fragrance additives, phenol used for plastic and resins, and cyclohexane, which could be used for fuel5.
Previous work on biodegradation of this pulp waste has been focused on lignin depolymerization. The International Lignin Institute (ILI) reports that between 40-50 million tons of lignin is produced each year (http://www.ili-lignin.com/aboutlignin.php). Only 1.5% of that lignin is used for commercial industrial processes6. Lignin depolymerization by laccase and peroxidase enzymes produced by white rot fungi of the Phanerochaete and Trametes genera has been studied at length7. Soil bacteria known to degrade aromatic compounds such as Nocardia and Rhodococcus8, Pseudomonas putida mt-29, and Streptomyces viridosporus T7A10 have also been shown to be capable of lignin degradation. Bacterial degradation of pulping waste is promising because some bacteria can thrive in the saline and alkaline (pH 10-14) conditions that characterize the pulping waste effluents11. While lignin is the main component of black liquor, microorganisms may also degrade the other components that make up black liquor. These techniques do not exclusively identify lignin degrading microorganisms, but serve to identify microorganisms that can be applied directly to the pulping waste black liquor instead of its further processed constituents.
Black liquor was collected and modified for microbial growth through neutralization and filter-sterilization. Microbial growth requirements were identified for an environmental microbial isolate by minimal media growth experiments on lignocellulosic components produced by a novel lignocellulose extraction protocol. Growth media were analyzed by gas chromatography-mass spectrometry (GC-MS) to determine the metabolic products of the environmental microbial isolate when grown on black liquor as the sole carbon source. The combination of these techniques provides an assessment tool to determine the metabolic capacity of a microorganism when grown on pulp mill wastes such as black liquor. Use of such techniques also offers insight into the value of application of specific microorganisms to pulp mill waste for the generation of byproducts.
Protocol
1. Collection of Black Liquor and Preparation of Black Liquor for Growth Cultures
- Collect a black liquor sample from the outlet valve attached to the kraft digester in a sterile glass bottle and allow it to cool to room temperature before proceeding with the following steps.
Neutralization - Add 100 ml of black liquor sample to a 500 ml beaker equipped with a stir bar.
- Place the beaker on a stir plate and adjust the speed to medium.
- Slowly add drops of phosphoric acid until the black liquor becomes viscous (viscosity similar to glycerol).
- Aliquot all of the black liquor solution by slowly pouring into 50 ml conical tubes and centrifuge at 9,300 x g for 30 min.
- Place the supernatant into the beaker and measure the pH using pH test ribbons. The pellet of precipitated lignin can be discarded and treated as nonhazardous solid waste.
- Continue to add phosphoric acid and centrifuge the solution until the pH strips indicate a neutral pH of the supernatant.
Sterilization - Pass the solution through a 0.22 µm filter to remove any contaminating bacteria and place the filtered solution into a sterile container for storage. Store at room temperature.
2. Lignocellulose Extraction
Carbohydrate Separation
- Place 5 g of switchgrass, pine, or Bermuda grass (milled to 1 mm or smaller particle size) into a 500 ml Erlenmeyer flask. Then add 200 ml of distilled or deionized water, 7.5 g of NaClO2, 2.5 ml of glacial acetic acid, and a stir bar to the flask.
- Place the flask in an oil bath placed on top of a stir plate at 80 °C for 1 hr. Adjust the speed of the stir plate to medium.
- Add an additional 7.5 g of NaClO2 and 2.5 ml of glacial acetic acid to the flask. Continue stirring at 80 °C in the oil bath for 30 min.
- Repeat the previous step 2x (Note: a total of 30 g of NaClO2 and 10 ml of glacial acetic acid is added to the flask containing the lignocellulosic biomass). Cool the solution to room temperature.
- Place a sheet of filter paper into a Büchner funnel. Place the funnel on top of a side arm flask and attach it to a vacuum.
- Slowly pour the solution into the funnel.
- Wash the solid material 3x with 100 ml of distilled or deionized water. Discard the filtrate after washing the solid material.
- Reserve 1 g of the solid material and place it onto a clean sheet of filter paper.
- Dry this solid material overnight in a 50 °C oven. After removing the sample from the oven, place into a storage vial labeled ‘holocellulose’.
- Add the remaining solid material to 250 ml of 10% NaOH (v/v) in a 500 ml Erlenmeyer flask. Cap the flask with a stopper.
- Place the solution in an incubator at 70 °C for 1 hr shaking at 250 rpm.
- Cool the solution for 30 min at room temperature.
- Remove the solid material using filter paper and Büchner funnel attached to a side arm flask.
- Transfer the solid material on a clean sheet of filter paper to a 50 °C oven and allow it to dry overnight.
- After the residue has dried, place it in a storage vial and label it ‘cellulose’.
- Transfer the filtrate from step 2.13 to a 500 ml Erlenmeyer flask and add 30 ml of acetic acid and 250 ml of isopropyl alcohol. Cap the flask and place the solution on the bench top for at least 8 hr at room temperature.
- Transfer all of the solution to 50 ml conical tubes and centrifuge the solution at 9,300 x g for 30 min. Carefully pipette the solution out of the conical tube and discard.
- Resuspend the pellet in 30 ml of distilled or deionized water by vortexing.
- Repeat steps 2.17-2.18 15x to thoroughly wash the pellet.
- Remove the supernatant and save the pellet for freeze drying. Once freeze-dried, collect the sample and place it in a vial labeled ‘hemicellulose’.
Lignin separation
Lignin separation protocol based on Karaaslan et al.12 - Add 5 g of switchgrass, pine, or Bermuda grass (milled to 1 mm or smaller particle size) to 500 ml of a solution of 0.25 M NaOH and 30% ethanol. Place the slurry in an incubator at 75 °C with shaking at 250 rpm for 2 hr.
- Cool the solution to room temperature.
- Place a sheet of filter paper in a Büchner funnel on top of a side arm flask. Attach to a vacuum and slowly pour the solution into the funnel.
- Discard the solid material.
- Add a stir bar to the flask containing the filtrate and place the flask on top of a stir plate adjusted to medium speed. Add concentrated hydrochloric acid dropwise to the solution until a pH of 2.0 is obtained (approximately 20 ml).
- Allow the acidified solution to sit overnight at room temperature.
- Centrifuge the solution at 9,300 x g for 30 min in conical tubes. Carefully pipette the solution out of the conical tube and discard.
- Resuspend the pellet in 30 ml of distilled or deionized water by vortexing.
- Repeat steps 2.27-2.28 15x to thoroughly wash the pellet.
- After centrifugation, remove the supernatant and save the pellet for freeze drying. Once freeze-dried, place the sample in a vial labeled ‘lignin’.
3. Preparation of Microbial Growth Cultures
Agar Plates
- Add 0.2% (w/v) sterile lignocellulose extraction products to M9 minimal media13 agar before pouring plates.
- Streak solidified plates with approximately 200 ul of a bacterial culture grown in M9 minimal media with an O.D. of approximately 2.0.
- Incubate plates at 37 °C and monitor growth.
Liquid Cultures - Add 50 ml of sterile Luria-Bertani (LB) media13 or M9 minimal media13 to a sterile 125 ml flask. Add black liquor at 10% (v/v) or 0.2% (w/v) of lignocellulose extraction products.
- Inoculate the media with 0.1% (v/v) of a bacterial culture at an O.D. of approximately 2.0.
- Incubate the cultures at 37 °C with shaking at 200 rpm.
- Assess growth every 6 hr during the culturing period by measuring optical density (λ = 600 nm). Place a 1 ml aliquot of the culture into a cuvette. Determine optical density of culture blanked against uninoculated media.
- Transfer bacteria to an anaerobic serum bottle at mid log phase.
- To prepare the anaerobic serum bottle: seal the serum bottle with a butyl rubber stopper and an aluminum crimp top under aerobic conditions and autoclave. Do not modify the headspace within the serum bottle after sterilization.
- Transfer the entire 50 ml culture from the 125 ml flask into the sealed sterile serum bottle using a syringe.
- Incubate at 37 °C with shaking at 200 rpm.
4. Sample Preparation for GC-MS
Prepare samples according to Raj, Reddy, and Chandra14. Negative control used for comparison consists of M9 minimal media with 10% black liquor and is also referred to as the uninoculated sample.
- Centrifuge uninoculated and inoculated bacterial cultures (50 ml) at 9,300 x g for 30 min after 450 hr of incubation.
- Transfer 10 ml of the supernatant into a glass test tube. (Note: Plastic is incompatible with the chemicals in the following steps.)
- Acidify the supernatant with concentrated HCl to pH 1-2.
- Add 3 volumes of ethyl acetate (30 ml), cap the tube and mix by inverting 4-6x.
- Place a small amount of anhydrous Na2SO4 (1-2 g) in a clean glass test tube.
- Carefully collect the organic layer (top) from the ethyl acetate solution by pipetting with a disposable, glass Pasteur pipette.
- Place the organic layer in the test tube with anhydrous Na2SO4.
- Dewater the organic layer over anhydrous Na2SO4 by gently tapping the test tube to mix. Gradually add small amounts of anhydrous Na2SO4and mix until there are no large clumps.
- Place a sheet of filter paper into a Büchner funnel on top of a side arm flask attached to a vacuum.
- Pour the solution into the funnel. Discard the solid material.
- Transfer the solution to an evaporating flask and evaporate the filtrate using a rotary evaporator.
- Place 3 mg of ethyl acetate extraction residue into a 2 ml amber chromatography vial.
- Dissolve the residue with the addition of 100 µl of 1,4-dioxane and 10 µl pyridine.
- Add 50 µl of N,O-Bis(trimethylsilyl) trifluoroacetamide with trimethylcholorsilane 99:1% (BSTFA). Then place in an incubator at 60 °C with shaking for 15 min.
- Store samples at 4 °C until use.
5. GC-MS
Equip the gas chromatograph (GC) interfaced with a mass spectrometer (MS) with a nonpolar capillary column and helium as the carrier gas with a flow rate of 1 ml/min.
- After the oven temperature is stabilized at 50 °C, inject 1 µl of the sample at a 1/50 split ratio.
- Hold the column at 50 °C for 5 min and increase to 280 °C at 5 °C/min. Maintain the final temperature of 280 °C for 20 min.
- Maintain the transfer line between the gas chromatograph and mass spectrometer at 300 °C.
- Select a solvent delay of 15 min.
- Record electron ionization mass spectra between the range of 10-500 (m/z) at electron energy of 70 eV.
- Derivatize and chromatograph standard as above. Identify compounds by comparing retention times of purchased standards or data in the National Institute of Standards and Technology (NIST) mass spectral database.
Representative Results
The collection and modification of black liquor generated in the kraft pulping process (Figure 1) will allow one to use this pulping waste to determine the biodegradation capacity of a single bacterial isolate or mixed culture. Figure 2 shows aerobic growth measured by optical density of the microbial environmental isolate cultured in LB media alone and LB media supplemented with 10% neutralized black liquor. These results indicate that this bacterium grows in the presence of neutralized black liquor. The growth of the environmental isolate without black liquor initially grows more quickly (with a generation time of 20 hr during exponential phase) but growth is not sustained beyond 62 hr. Growth of the microbial environmental isolate in the presence of black liquor is slow (with a generation time of 35 hr during exponential phase); however, the culture continues to increase in optical density until after 230 hr of growth. The growth curve of the microbial environmental isolate grown in the presence of black liquor also depicts biphasic growth, which suggests that the black liquor contains more than one carbon source that can be used by the microbial environmental isolate and that there is a preference of nutrient utilization exhibited by the bacterium. Further experiments are necessary to determine its ability to utilize black liquor for growth requirements. One may also determine growth of a bacterium on nonneutralized black liquor or determine the effect of increased concentrations of black liquor on growth.
After demonstrating growth in the presence of black liquor, one may determine carbon source utilization by minimal media growth experiments. Commercial substrates that make up the components of lignocellulose can be used as carbon sources in the minimal media. The lignocellulose extraction protocol presented here provides a model for determining the growth requirements of the bacterium. Figure 3 shows growth of the environmental microbial isolate on M9 minimal media agar supplemented with each component of the lignocellulose extraction. The presence of colonies on the minimal media agar plates indicates the ability of the environmental isolate to degrade holocellulose, cellulose, hemicellulose, and lignin. Colonies were present on M9 agar (without any carbon source added) and this may suggest the ability of the environmental isolate to degrade agar. Instead of plates, minimal media growth curves for liquid cultures could also be used to determine the specific rate of growth on each lignocellulose fraction so that agar, which could be a potential carbon source, would not have to be added.
The analysis of extracted and derivatized bacterial cultures by GC-MS reveals that metabolic products are produced and black liquor components are degraded. Figure 4A shows the spectra of TMS derivatives present in the uninoculated sample. Figure 4B shows the spectra of TMS derivatives produced by the environmental microbial isolate when grown anaerobically in M9 minimal media with 10% nonneutralized black liquor. Three replicates of each type of sample were run and produced similar results; the spectra shown in Figure 4 represent one of the replicates. Comparison of the two spectra reveals differences in the components present in the media, suggesting the presence of fermentation products and degradation of black liquor components. Figure 4 shows that compounds are produced as a result of microbial growth. The peak with a retention time of 39.34 min appears only in the inoculated sample. The peak with a retention time of 21.84 min increased in the inoculated sample compared to the uninoculated sample. The peak with a retention time of 25.79 min decreased in the inoculated sample. These results can then be compared with mass spectrometry libraries or standard compounds prepared the same way to determine the identity of the compounds produced by microbial fermentation. Figure 4C shows the spectra of a guaiacol standard, which was used to identify the peak with a retention time of 25.79 min.
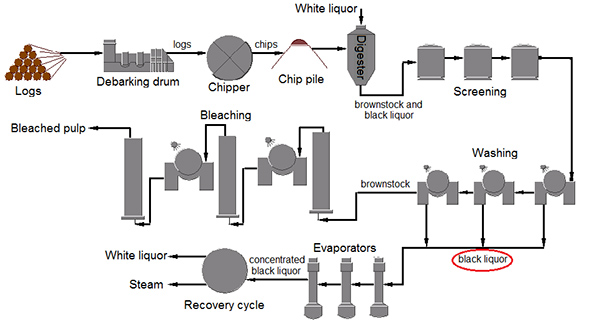
Figure 1. Flow diagram for a kraft pulp mill20. The image depicts the processing of wood into pulp. The kraft process aims to separate lignin from its polysaccharide constituents resulting in the production of bleached wood pulp and black liquor (circled in red). Click here to view larger image.
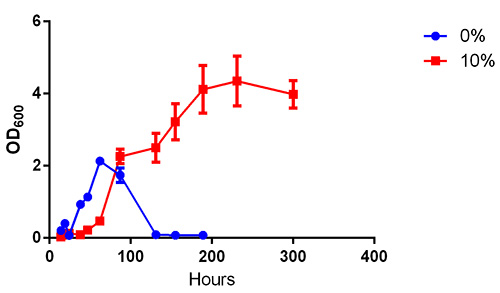
Figure 2. Growth of the environmental microbial isolate. Growth studies conducted aerobically in LB media (blue circles) or in LB media supplemented with 10% neutralized black liquor (red squares) at 37 °C with shaking at 200 rpm. n = 3.
Click here to view larger image.
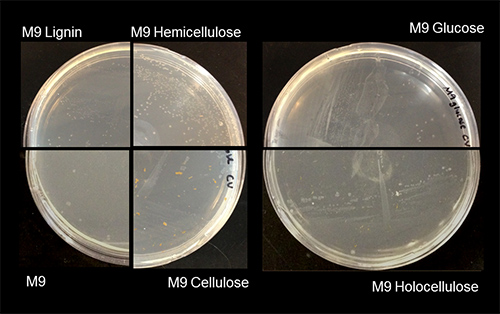
Figure 3. Determination of the ability of an environmental microbial isolate to use specific wood-derived carbon sources for growth. An overnight culture of an environmental microbial isolate was plated on M9 minimal media agar supplemented with lignocellulose extraction products. M9 minimal media supplemented with glucose served as a positive control while M9 minimal media without a carbon source served as a negative control. The environmental microbial isolate, producing white semi-translucent colonies, can use holocellulose, cellulose, hemicellulose, and lignin as the sole carbon source for growth. M9 holocellulose and cellulose plates exhibit opaque yellow-brown particles, which are the insoluble fraction of the holocellulose and cellulose added to the media.
Click here to view larger image.
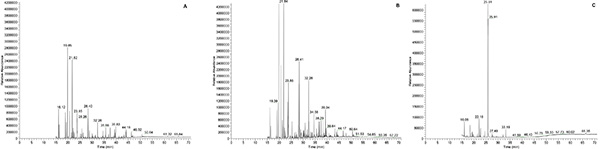
Figure 4. Spectra of TMS derivatives. A) uninoculated M9 minimal media 10% black liquor B) environmental microbial isolate grown for 450 hr in M9 minimal media supplemented with 10% black liquor under anaerobic conditions at 37 °C with shaking at 200 rpm. C) Trimethylsilyl derived guaiacol. Click here to view larger image.

Figure 5. Comparison of bleaching methods. A) Dried hollocellulose using NaClO2 bleaching. B) dried hollocellulose using NaOH bleaching. Comparison of color indicates increased delignification when NaClO2 is used as a bleaching agent.
Click here to view larger image.
Discussion
This protocol describes a combination of techniques that aims to identify microorganisms that can degrade pulping waste, the carbon sources utilized during growth on pulping waste, and the microbial metabolic products produced when grown on pulping waste. We have shown the success of this protocol with the microbial environmental isolate: a facultative anaerobe that can grow on 10% black liquor and use the lignocellulose extraction components as sole carbon sources for growth. This protocol could be used to determine the potential to degrade pulping waste by other microorganisms or microbial consortiums that have lignocellulolytic potential.
Approximately 1 L of black liquor was collected at a time; however, the amount collected should be determined by the needs of the experiments and the access one has to collect black liquor. While collecting black liquor, be sure to wear safety glasses and wear heat-resistant gloves to hold the collection bottle. In this protocol, the black liquor was neutralized to facilitate growth of the microbial environmental isolate used in this experiment; however, it may be that a different pH would enable optimum growth of other microorganisms. It might also be that other microorganisms are able to grow in a higher percentage of pulping waste. The dark color of this pulping waste interferes with optical density readings at high concentrations, and therefore, different methods should be used to determine cell growth (such as viable cell count or microscopy techniques).
The extraction process outlined here can be applied to other forms of biomass with variable particle sizes, but these changes may affect the yield. Minimal media agar studies were used here to provide qualitative information about the ability of this microbial environmental isolate to degrade the lignocellulose extraction components. This method was chosen for its simplicity. The minimal media agar studies could be supplemented with liquid minimal media growth studies to determine the specific growth rate on the lignocellulose extraction components. A comparison of the generation time would allow one to quantify carbon source preference. The results presented show that the microbial environmental isolate can degrade agar, and minimal media growth curves can be used to distinguish the ability of the microbe to degrade agar from the ability to degrade the lignocellulose extract. Anaerobic growth conditions were used to analyze metabolic products because it was the view of the authors that fermentation might produce higher value byproducts. Microbial growth cultures were transferred to a serum bottle during midlog phase. The serum bottle was sealed under aerobic conditions, which allowed the cells to continue growing aerobically until all the oxygen was depleted in the serum bottle. Since the microbial environmental isolate is a facultative anaerobe, we used this approach because the oxygen present in the serum bottle will be quickly used by the microorganism. However, if a different microorganism is being used that is strictly aerobic or anaerobic, the growth conditions should be modified appropriately. This approach was chosen in an attempt to ensure that a greater amount of fermentation products were produced because of the high cell density characterized by mid log phase. The preparation of the spent culture media for GC analysis uses silylation to ensure stability of the metabolic products on the GC column used. Pyridine is essential for the silylation reaction. Incomplete silylation will result in instability of the compounds on the column. Incomplete silylation results in one major peak on the GC spectra that matches with trimethylsilyl derivatizing agent. If this occurs, add an additional 10 µl of pyridine and place the sample in an incubator at 60 °C with shaking for 15 min.
The experiments described are dependent upon access to black liquor. Black liquor samples used in this work were obtained from the Forest Biomaterials Department at North Carolina State University. The percentage of solids (see materials table) was determined by weight after drying in a 90 °C oven for 18 hr. The pH of black liquor ranges from 10-14; neutralization was used to facilitate growth of microorganisms. Neutralization of black liquor results in the precipitation of lignin. Bacterial conversion of black liquor into value-added products would be the ideal application and thus requires that the bacteria used to degrade this pulping waste product could utilize black liquor without extensive modifications. Fermentation products such as ethanol, butanol, and acetate are only some examples of compounds that could be produced from microbial degradation of black liquor, and there could be methods other than GC-MS to determine the products produced such as LC-MS or HPLC.
Unlike previous experiments analyzing the ability of microorganisms to grow on kraft lignin15,16,17,18, the primary interest of this protocol is to determine if microorganisms can utilize pulping waste. The extraction technique presented is advantageous as it offers a way to extract each of the constituent components from a lignocellulose source. This novel method uses NaClO2 bleaching, which offers increased purity over conventional extraction techniques. Lignin remaining bound to the cellulose and hemicellulose fibers gives a brownish color19. Therefore, the increased whiteness of the NaClO2 extraction method suggests increased purity (Figure 5). The centrifugation steps (2.19 and 2.29) can be completed in multiple days. To do so, remove the supernatant and store the pellet at room temperature.
Environmental and industrial wastes offer a ready supply of resources that could be converted into valuable products. Black liquor is an example of waste from the pulping process. Black liquor is currently used to create steam and energy in the recovery boiler; however, the complex structure of lignocellulose provides sufficient chemical diversity to support conversion of its carbons into a variety of value-added products. The combination of techniques described is aimed at identifying and characterizing the microbial degradation of black liquor with the goal of identifying a microorganism that has the ability to produce valuable byproducts from pulping waste.
Declarações
The authors have nothing to disclose.
Acknowledgements
We would like to thank Jim McMurray for providing the black liquor and Dr. David Tilotta and August Meng for their help with GC-MS. Support for Stephanie L. Mathews was provided by a USDA National Needs Fellowship (award number 2010-38420-20399).
Materials
| 1,4-Dioxane (Certified ACS) | Fisher | D111 | Flammable, preoxidizable chemical |
| Black Liquor | Department of Forest Biomaterials at North Carolina State University | N/A | pH 12.72, 13.67% solids |
| Ethanol (microbiology grade) | Fisher | BP2818 | |
| Ethyl Acetate (ACS reagent grade) | EMD | EX0240-9 | Flammable |
| Glacial acetic acid (Certified ACS) | EMD | AX0073 | Corrosive |
| Guaiacol | Sigma Aldrich | PHR1136 | Harmful by ingestion, corrosive |
| HP-5 capillary column | Agilent | 19091J-577 | 60 m x 0.18 mm internal diameter, 0.18 μm thickness |
| Hydrochloric acid (certified ACS) | EMD | HX0603P | |
| N,O-Bis(trimethylsilyl)trifluoroacetamide with trimethylcholorsilane 99:1% (BSTFA) | Fluka | 15238 | Flammable, causes skin burns and eye damage with contact |
| Na2SO4 (FCC grade) | VWR | BDH8026 | |
| NaClO2 (80%) | Sigma Aldrich | 244155 | Flammable, toxic |
| NaOH (certified ACS) | EMD | 1.06498.1000 | Corrosive |
| Alkacid pH test ribbons | Fisher | A979 | |
| Phosphoric Acid (certified ACS) | Fisher | A242 | |
| Polaris Q mass spectrometer | Thermo Electron Corporation | ||
| Pyridine (99%) | Alfa Aesar | A12005 | Flammable, toxic in contact with skin |
| Switchgrass | Cherry Research Farm Goldsboro, NC | N/A | Harvested August 2011 |
| Thermo Finnigan trace gas chromatograph | Thermo Electron Corporation | ||
| Whatman no. 1 filter paper | Whatman | 1001-150 |
Referências
- Tejado, A., Pena, C., Labidi, J., Echeverria, J. M., Mondragon, I. Physico-chemical characterization of lignin from different sources for use in phenol-formaldehyde resin synthesis. Bioresource Technol. 98, 1655-1663 (2007).
- Brannvall, E., Elk, M., Gellerstedt, G., Henriksson, G. Overview of pulp and paper processes. Pulp and paper chemistry and technology. 2, 1-12 (2009).
- Biermann, C. J. Pulping fundamentals. Handbook of pulping and papermaking. , 55-100 (1996).
- Sjöström, E. . Wood chemistry: fundamentals and applications. , (1924).
- Philbrook, A., Alissandratos, A., Easton, C. J. Biochemical processes for generating fuels and commodity chemicals from lignocellulosic biomass. Environ. Biotechnol. , 39-63 (2013).
- Sanchez, R., Ferrer, A., Serrano, L., Toledano, A., Labidi, J., Rodriguez, A. Hesperaloafunifera as a raw material for integral utilization of its components. BioResources. 6 (1), 3-21 (2011).
- Font, X., Caminal, G., Gabarrel, X., Romero, S., Vicent, M. T. Black liquor detoxification by laccase of Trametesversicolorpellets. J. Chemical Tech. Biotech. 78, 548-554 (2003).
- Zimmerman, W. Degradation of lignin by bacteria. J. Biotechnol. 13, 119-130 (1990).
- Ahamad, M., Taylor, C. R., Pink, D., Burton, K., Eastwood, D., Bending, G. R., Bugg, T. D. H. Development of novel assay for lignin degradation: comparative analysis of bacterial and fungal lignin degraders. Mol. Biosystems. 6, 815-821 (2010).
- Ramchandra, M., Crawford, D. L., Hertel, G. Characterization of an extracellular lignin peroxidase of the lignocellulolyticactinomycete Streptomyces viridosporus. Appl. Environ. Micro. 54, 3057-3063 (1988).
- Mishra, M., Thakur, I. S., Satyanarayana, T., Johri, B. N., Prakash, A. . Microorganisms in environmental management: microbes and the environment. , (2012).
- Karaaslan, A. M., Tshabalala, M. A., Wood Bushcle-Diller, G. hemicellulose chitosan-based semi-interpenetrating network hydrogels: mechanical, swelling and controlled drug release properties. BioResources. 5 (2), 1036-1054 (2010).
- Lech, K., Brent, R., Ausubel, F. M., Brent, R., Kingston, R. E., Moore, D. D., Seidman, J. G., Smith, J. A., Kevin, S. . Short protocols in molecular biology. , (1992).
- Raj, A., Krishna Reddy, M. M., Chandra, R. Identification of low molecular weight aromatic compounds by gas chromatography-mass spectrometry (GC-MS) from kraft lignin degradation by three Bacillus sp. Int. Biodeterior. Biodegradation. 59, 292-296 (2007).
- Chandra, R., Raj, A., Purohit, H. J., Kapley, A. Biodegradation of kraft lignin by three pure and mixed aerobic bacterial cultures isolated from pulp and paper sludge. , (2005).
- Chen, Y., Chai, L., Tang, C., Yang, Z., Zheng, Y., Shi, Y., Zhang, H. Kraft lignin biodegradation by Novosphingobium sp. B-7 and analysis of the degradation process. Bioresource Technol. 123, 682-685 (2012).
- Bandounas, L., Wierckx, N. J., de Winde, J. H., Ruijssenaars, H. J. Isolation and characterization of novel bacterial strains exhibiting ligninolytic potential. BMC Biotechnol. 11 (94), (2011).
- Chandra, R., Abhishek, A., Sankhwar, M. Bacterial decolorization and detoxification of black liquor from rayon grade pulp manufacturing industry and detection of their metabolic products. Bioresource Technol. 102, 6429-6436 (2011).
- Gellerdtedt, G., Ek, M., Gellerstedt, G., Henriksson, G. Chemistry of Bleaching of Chemical Pulp. Pulp and Paper Chemistry and Technology. 2, 201-237 (2009).
- Kringstad, K. P., Lindstrom, K. Spent liquors from pulp bleaching. Environ. Sci. Tech. 18, 236-247 (1984).

