The Use of Gas Chromatography to Analyze Compositional Changes of Fatty Acids in Rat Liver Tissue during Pregnancy
Summary
Pregnancy leads to significant changes to the fatty acid composition of maternal tissues. Lipid profiles can be obtained via gas chromatography to allow identification and quantification of fatty acids in individual lipid classes among rats fed various high and low fat diets during pregnancy.
Abstract
Gas chromatography (GC) is a highly sensitive method used to identify and quantify the fatty acid content of lipids from tissues, cells, and plasma/serum, yielding results with high accuracy and high reproducibility. In metabolic and nutrition studies GC allows assessment of changes in fatty acid concentrations following interventions or during changes in physiological state such as pregnancy. Solid phase extraction (SPE) using aminopropyl silica cartridges allows separation of the major lipid classes including triacylglycerols, different phospholipids, and cholesteryl esters (CE). GC combined with SPE was used to analyze the changes in fatty acid composition of the CE fraction in the livers of virgin and pregnant rats that had been fed various high and low fat diets. There are significant diet/pregnancy interaction effects upon the omega-3 and omega-6 fatty acid content of liver CE, indicating that pregnant females have a different response to dietary manipulation than is seen among virgin females.
Introduction
Gas chromatography (GC) is a well-established technique used to identify and quantify the incorporation of fatty acids into lipid pools and cell membranes1,2 during supplementation or physiological conditions such as obesity (and related diseases such as diabetes) or pregnancy3-5. It is also suitable for analyzing the types and quantities of fats in foods. This is useful when characterizing experimental diets, as well as ensuring that the food industry complies with regulations. For example, GC can be used to confirm the identity and quantity of fatty acids within a product such as a dietary supplement to ensure that labeling is correct and regulations are adhered to6,7. Analysis of fatty acids can provide valuable insights into lipid metabolism in health and disease, the impact of dietary change, and the effect of changes in physiological state8. Use of GC to study samples during pregnancy has provided important information on changes in fatty acid and complex lipid homeostasis3.
In advance of the chromatographic separation, lipids are typically extracted from the sample using the solubility of lipids in solvent mixtures of chloroform and methanol. Sodium chloride is added to facilitate the separation of the mixture into aqueous and organic lipid containing phases9,10. Complex lipid classes of interest can be separated from the total lipid extract by solid phase extraction (SPE). This separation technique elutes lipid classes based upon their polarity or binding affinity. Triacyglycerols (TAG) and cholesteryl esters (CE) are eluted first as a combined fraction, further classes, phosphatidylcholine (PC), Phosphatidylethanolamine (PE), and non-esterified fatty acids (NEFA) are eluted by increasing the polarity of the eluting solvent. The separation of TAG from CE exploits the binding of TAG only to a fresh SPE cartridge, allowing CE to be eluted. TAG can then be eluted by increasing the polarity of the eluting solvent9,10. This method allows multiple samples to be separated simultaneously with a higher yield than is achieved with thin layer chromatography, which means that relatively small sized samples (e.g. <100 µl plasma or serum, <100 mg tissue) can be analyzed11,12.
GC is a well-established technique first described in the 1950s; it was suggested that the mobile phase in the then liquid-liquid systems could be replaced with vapor. It was initially used for petroleum analysis but rapidly expanded into other areas such as amino acid analysis and lipid biochemistry, which is still of major interest. Advances in GC equipment and technology such as the development of capillary columns from the previously used packed columns has led to our current techniques in which fatty acids are able to be separated more efficiently at lower temperatures resulting in GC being routinely used to identify and quantify fatty acids in a wide range of investigations13.
GC requires fatty acids to be derivatized in order that they may become sufficiently volatile to be eluted at reasonable temperatures without thermal decomposition. This usually involves the substitution of a functional group containing hydrogen to form esters, thioesters or amides for analysis. Methyl esters are commonly studied derivatives, which are produced by methylation. In this method the ester bonds in complex lipids are hydrolyzed to release free fatty acids, which are transmethylated to form fatty acid methyl esters (FAME). The resulting profile of FAME, determined by GC, is referred to as the fatty acid composition and may be easily compared between different experimental groups9,10. The technique allows both the proportions of individual fatty acids and their concentrations to be measured.
In addition to the use of GC for analyzing fatty acids in nutrition studies and within the food industry, the technique can be used across a wide range of analytical fields. For example, environmental analyses using GC include measuring water contamination by insecticides and soil analyses measuring chlorobenzene content. In toxicology, GC has also been used to identify illegal substances in urine and blood samples of individuals; such a sports performance enhancers12 and the ability to separate complex mixtures of hydrocarbons makes this technique popular in the petroleum industry for petrochemical analysis12.
Pregnancy is associated with significant changes to the fatty acid composition of maternal tissues, specifically in the content of omega-3 (n-3) and omega-6 (n-6) polyunsaturated fatty acids (PUFA)3. In the current study, we exemplify the use of GC in the measurement of fatty acids by describing its use in the analysis of the fatty acid composition of liver tissue taken from virgin and pregnant rats fed low and high fat diets with different oil sources. The experimental diets provided here were a low fat soybean oil based diet, a high-fat soybean oil-based diet (130.9 g total fat/kg total fat) or a high-fat linseed oil-based diet (130.9 g total fat/kg diet), provided for 20 days. The full nutrient and fatty acid composition of these diets have been described previously14. The soybean oil diets are rich in linoleic acid (18:2n-6) and contain some α-linolenic acid (18:3n-3) while the linseed oil diet is rich in α-linolenic acid. These high-fat diets represent different rations of linoleic to α-linolenic acids (rations of 8:1 and 1:1, respectively). The method for isolation of individual lipid classes and analysis by GC is well established and validated, and has been published previously10 but without the detailed technical description found herein.
Protocol
1. Animal Procedures
- All animal work should be carried out in accordance with the Home Office Animals (Scientific Procedures) Act (1986).
- Mate Wistar rats aged 10 weeks old by monogamous breeding, and confirm pregnancy by the appearance of a vaginal plug. Record this as day 1 of gestation, and commence experimental diet. For virgin females, house each rat individually, and commence experimental diet.
- After feeding the experimental diets for 20 days euthanize rats by CO2 asphyxiation followed by cervical dislocation.
- Use dissection forceps and scissors to expose the abdominal cavity and excise the liver by cutting the ligaments, which connect the liver to the diaphragm, anterior wall of the abdomen, stomach and duodenum. Wash the liver in PBS and freeze in liquid nitrogen before storing at -80 °C.
2. Preparation of a Total Lipid Extract9
- Add molecular sieves (to fill 1/10 of solvent container) to all solvents to create ‘dry’ solvents. Carry out all solvent work within a fume hood.
- Cut approximately 100 mg frozen liver and weigh. Place the tissue into a tube in an ice bucket and add 0.8 ml ice cold 0.9% NaCl. Homogenize the tissue.
- Add internal standards dissolved in 1 ml/mg of dry chloroform: methanol (2:1, v/v) containing butylated hydroxytoluene (BHT; 50 mg/l) as anti-oxidant. For 100 mg of rat liver add 100 μg of CE standard (Cholesteryl heptadecanoate 17:0). Caution: Chloroform and BHT are hazardous.
- Add 5.0 ml dry chloroform: methanol (2:1, v/v) containing BHT (50 mg/L).
- Add 1.0 ml 1 M NaCl, mix thoroughly by vortexing until mixture looks uniform. Samples can be capped and stored at -20 °C at this stage for up to a week.
- Centrifuge at 1,000 x g for 10 min, low brake at room temperature.
- Collect lower phase using glass Pasteur pipette, transfer to new screw cap glass tube and dry under nitrogen at 40 °C. Samples can be capped and stored at -20 °C at this stage for up to a week.
3. Separation of Lipid Classes by Solid Phase Extraction (SPE)10
- Connect the SPE tank to a vacuum pump and place aminopropyl silica SPE cartridge on the tank.
- Place new screw-cap glass tube labeled TAG and CE in the tank rack under the column to collect first fraction.
- Dissolve the total lipid extract in 1.0 ml dry chloroform and vortex.
- Apply sample to the column using a glass Pasteur pipette and allow to drip through into the screw-cap tube under gravity. When no further drips fall, remove the remaining liquid by vacuum.
- Elute the TAG and CE fraction under vacuum, wash the column with 2 x 1.0 ml washes of dry chloroform.
- When all liquid is removed, dry the TAG and CE fraction under nitrogen at 40 °C. Samples can be capped and stored at -20 °C at this stage for up to a week.
- Place new screw-cap glass tube labeled PC into the tank tray under the column.
- Elute the PC fraction under vacuum with the addition of 2 x 1.0 ml dry chloroform: methanol (60:40, v/v) until all liquid is removed from the column.
- Remove and dry PC fraction under nitrogen at 40 °C. Samples can be capped and stored at -20 °C at this stage for up to a week.
- Place a new screw-cap glass tube labeled PE into the tank tray and elute PE fraction with the addition of 1.0 ml dry methanol under vacuum.
- Remove and dry PE fraction under nitrogen at 40 °C. Samples can be capped and stored at -20 °C at this stage for up to a week.
- Place new screw-cap glass tube labeled NEFA into the tank tray and elute NEFA fraction under vacuum by the addition of 2 x 1.0 ml washes of dry chloroform: methanol: glacial acetic acid (100:2:2, v/v/v). Caution: Glacial acetic acid is hazardous.
- Remove collected NEFA fraction and dry under nitrogen at 40 °C. Samples can be capped and stored at -20 °C at this stage for up to a week.
- Place a new aminopropyl silica SPE cartridge on the SPE tank and place a screw-cap glass tube in the tank tray under the cartridge to collect waste.
- Wash the column with 3 washes of dry hexane under vacuum and then a final 1.0ml wash under gravity. Do not allow the cartridge to become dry (turn the cartridge column channels to a closed position when hexane level is close to the cartridge matrix). Caution: Hexane is hazardous.
- Replace waste tube with new screw-cap glass tube labeled CE.
- Dissolve the dried TAG and CE fraction (prepared in step 3.6) in 1.0 ml of dry hexane and vortex. Apply this to the column using a glass Pasteur pipette and allow to drip through under gravity.
- When no further drips fall, remove the remaining liquid under vacuum.
- Under vacuum, wash the column with 2 x 1.0 ml washes of dry hexane to elute CE and dry collected fraction under nitrogen at 40 °C. Samples can be capped and stored at -20 °C at this stage for up to a week.
- Place new screw-cap glass tube labeled TAG in the tank tray and elute TAG with the addition of 2 x 1.0 ml washes of dry hexane: methanol: ethyl acetate (100:5:5) under vacuum.
- Dry collected fraction under nitrogen at 40 °C. Samples can be capped and stored at -20 °C at this stage for up to a week. Caution: Ethyl acetate is hazardous.
4. Preparation of FAME from CE10
- Add 0.5 ml of dry toluene to the separated CE fraction (collected in step 3.19) and vortex. Caution: Toluene is hazardous.
- Prepare methylation reagent (dry methanol with 2% (v/v) H2SO4), of which 1.0 ml is required per sample. Dispense volume of dry methanol into glass or suitable plastic container with lid and add the required amount of H2SO4 dropwise then mix by inversion. Caution: Sulfuric acid is hazardous.
- Add 1.0 ml of the methylating reagent to the samples dissolved in dry toluene, cap the tubes securely, and mix gently.
- Heat the samples for 2 hr at 50 °C.
- After 2 hr remove tubes from heat. Once cool add 1.0 ml neutralizing solution (0.25 M KHCO3 0.5M K2CO3). Caution: Potassium bicarbonate and potassium carbonate are hazardous.
- Add 1.0 ml dry hexane and vortex.
- Centrifuge at 250 x g for 2 min, low brake at room temperature.
- Collect upper phase, which contains the FAME, and transfer into a new non screw-cap disposable glass tube.
- Dry the collected FAME under nitrogen at 40 °C. Samples can be capped and stored at -20 °C at this stage for up to a week.
5. Removal of Free Cholesterol Contamination from CE FAME14
(Free Cholesterol can contaminate the sample; see Figure 1 for example chromatograph traces with and without free cholesterol removal).
- Place a waste tube into SPE tank and place a silica gel SPE cartridge onto the tank.
- Wash column with 3 x 1 ml washes of dry hexane under vacuum and 1 x 1 ml under gravity.
- Remove waste washes and add new waste tube into tank.
- Dissolve CE FAME in 1 ml dry hexane, vortex.
- Apply to the column using a glass Pasteur pipette and allow to drip through under gravity.
- Wash column with 3 x 1 ml washes of hexane under vacuum.
- Remove waste washes and place new non screw cap tube into tank labeled CE FAME.
- Elute the CE FAME with 2 x 1 ml dry hexane: diethyl ether (95:5 v/v) washes.
- Dry under nitrogen at 40 °C. Samples can be capped and stored at -20 °C at this stage for up to a week. Caution: Diethyl ether is hazardous.
6. Transfer of FAME into GC Auto Sampler Vial
- Add 75 μl dry hexane to sample, vortex, and transfer to a GC auto sample vial.
- Add a further 75 μl dry hexane to sample, vortex and transfer to the same GC auto sample vial. Samples can be capped and stored at -20 °C at this stage for up to a month.
7. Analysis Using the Gas Chromatograph14
- Analyze FAME on a gas chromatograph. Example set up: 30 m x 0.25 μm x 0.25 mm BPX-70 fused silica capillary column with temperature protocol:
Initial temperature 115 °C, hold 2 min, ramp 10 °C/min to 200 °C, hold 18.5 min, ramp 60 °C/min to 245 °C, hold 4 min.
Column: Helium gas, flow rate 1.0, pressure 14.6 and velocity 29.
Injector: Temperature = 300 °C.
Detector: Hydrogen flow 40.0, air flow 184.0, make up gas Helium, flow 45.0, temperature = 300 °C. - Set split ratio as appropriate (e.g. 25:1 for CE FAME analysis).
- Determine the area under each peak using appropriate software and identify FAME by comparison with standards. See Figure 2 for example chromatograms.
- Use the area under the peak data to calculate the contribution of individual fatty acids as a percentage of total fatty acids.
- Calculate absolute concentrations of fatty acids by dividing the area of internal standard by the amount added. Divide the area of each fatty acid by this result to obtain absolute concentrations of each fatty acid within the amount of tissue used.
Representative Results
The success of this method is dependent on following the protocol precisely and on using clean solvents and reagents in order to reduce ‘noise’ and contamination that can appear on a chromatogram. Contaminated samples are more challenging to analyze, lowering the accuracy of the area under the curve calculations. If the protocol is followed successfully a chromatogram with clear symmetrical, well defined peaks and with minimal background noise should be obtained as illustrated in Figure 3. If contamination has occurred the chromatogram will show additional peaks and exhibit non-symmetrical (skewed) peaks as illustrated in Figure 4. Contamination from free cholesterol will occur when running FAME derived from CE (see Figure 1, unless the cholesterol is removed (as described in Protocol 5).
The use of a prepared calibration mix allows for the identification of the FAME in the sample. The calibration mix is run using the same instrument settings as the samples so that the chromatogram can be compared to the sample and peaks correctly identified based upon their retention times as illustrated in Figure 2.
Peak area is used to calculate the percentage of specific fatty acids within the total. Once data have been collected, it is useful to inspect them for outliers as shown in Figure 5. Outlier samples can then be further investigated and the extraction and/or analysis repeated if necessary.
Adding an internal standard to samples (as described in protocol step 2.3) allows the quantification of fatty acids within the sample by calculations using the area of known quantity of the internal standard peak relative to the area of the peak of interest, and adjusted for the original sample volume or weight. In Table 1, FAME within rat liver CE are described as a percent of total fatty acids (g/100 g total fatty acid) within liver CE. These data describe the CE fatty acids in the liver of virgin or pregnant rats fed for 20 days on one of three different diets. Statistical analysis using two-factor ANOVA (factors: diet, pregnant vs virgin) reveals significant effects of diet and pregnancy upon the proportion of several fatty acids, as well as significant diet x pregnancy interactions (Table 1). As an illustration, Figure 6 shows that the arachidonic acid (AA; 20:4n-6) content of liver CE in pregnant rats is more influenced by diet than that of virgin females.

Figure 1. Comparative gas chromatograms of identical samples illustrating the importance of free cholesterol removal in rat liver tissue. The untreated sample shows evidence of free cholesterol contamination by the addition undesirable peaks as circled. These can occur anywhere throughout the sample potentially disrupting peaks of interest and affecting the accuracy of area under the curve calculations and the resulting quantification of those fatty acids. The chromatogram produced from the sample where free cholesterol was removed shows well defined, distinguishable peaks with no background interference, which would produce results with high accuracy. Click here to view larger image.

Figure 2. Example of the method used to identify sample CE FAME from rat liver tissue using a prepared calibration mix. A prepared calibration mix is run using the same instrument settings as the samples so that the chromatograms can be compared to identify fatty acids within the sample. The accompanying chromatogram for the calibration mix allows the FAME in the mix to be labeled. This labeled trace can then be compared to the chromatogram from the sample where easily identifiable large peaks can be compared by their retention times to those on the calibration mix then labeled appropriately. For example highlighted on the trace. is 16:0 to illustrate the comparison of retention times from the calibration mix above to the fatty acids in the sample trace below allowing correct labeling of fatty acids. Click here to view larger image.

Figure 3. Good example of a gas chromatogram depicting cholesteryl ester fatty acids (as methyl esters) from rat liver tissue. Peaks of interest are easily distinguished as they are symmetrical, well defined and there is very little background noise. All peaks are un-interrupted and there are no sloping peaks allowing easy integration and accurate area under the curve calculation. Click here to view larger image.

Figure 4. Example of a contaminated NEFA gas chromatogram obtained from human plasma. There are many unexpected peaks, as circled at the beginning of the sample, which can affect the integration of genuine peaks. The peaks are interrupted and undefined as seen towards the end of the sample and have become sloped as circled. This will affect the accuracy of the area under the curve calculations and the quantification of these fatty acids. Click here to view larger image.

Figure 5. Identification of outlier data among fatty acid composition of liver CE in virgin rats fed a high-fat soybean oil diet (n=6). This illustrates how percentage data of analyzed fatty acids can be used to determine ambiguous results. These data indicate that sample 125 may be an outlier, and requires further investigation. The five major fatty acids within CE (16:0, 18:0, 18:1n-9, 18:2n-6, 20:4n-6) are not shown. Click here to view larger image.

Figure 6. Arachidonic acid content of liver CE among virgin and pregnant rats fed experimental diets. Values are means ± SD, n=6. Means without a common letter differ, P<0.05. * different from virgin females within matched dietary group, P<0.05. This is a visual representation of the results from Table 1 showing the differences in liver CE content of virgin rats fed various diets compared to pregnant rats fed those same diets. Differences can be seen in each dietary group between the virgin and pregnant rats. Click here to view larger image.
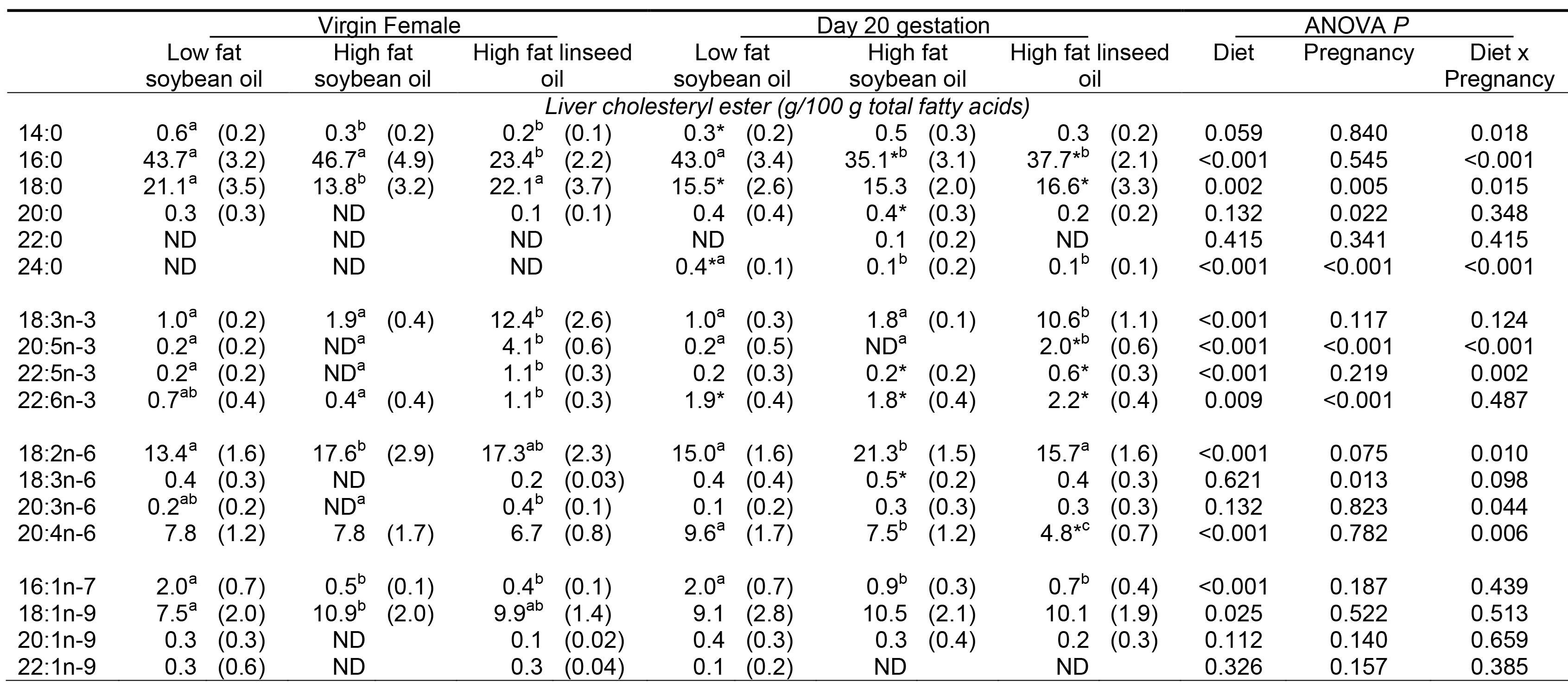
Table 1. Fatty acid composition of rat liver cholesteryl esters of virgin and pregnant rats fed experimental diets. Values are means (SD), n=6. ND indicates not detected (mean < 0.1%). Means without a common letter within virgin or pregnant females differ, P<0.05. * different from virgin females within matched dietary group, P<0.05. This table shows the mean values of n-3 and n-6 fatty acids present in the liver tissue of virgin and pregnant rats when fed low and varying high fat diets. The results were statistically analyzed using analysis of variance (ANOVA) with a significance level of P<0.05 for differences between virgin and pregnant rats, the type of diet and diet x pregnancy interaction. ANOVA results suggest there are significant differences in the levels of arachidonic acid (20:4n-6) between virgin and pregnant rats within matched dietary groups.
Discussion
Gas chromatography is an accurate technique to use for fatty acid analysis, and its high reproducibility deems this technique suitable for clinical analyses. Appropriate GC columns must be used to enable identification of fatty acids of interest, with available columns having variations in the polarity of the stationary phase, column length and internal diameter. The use of a fused silica capillary column in this method of analysis provides good thermal stability and high reproducibility of retention times due to its high surface inertness and good resolution8.
Critical steps within this protocol include lower and upper phase collection steps (protocol steps 2.7 and 4.8). It is important that as much of the correct phase is collected as possible without contamination with any of the unwanted phase. The presence of contaminants in a sample will result in an undesirable chromatographic output, as illustrated in Figure 2. When collecting CE during SPE it is imperative that the cartridge columns are kept saturated with solvent (step 3.15) following the washes with hexane to ensure the sample penetrates the column to allow successful separation of CE from TAG. The further separation of CE to remove free cholesterol is important to avoid contamination appearing on chromatograph traces as illustrated in Figure 1. It is also important to ensure steps requiring removal of liquid under vacuum are followed precisely so all liquid is completely removed from the column to ensure a good yield.
The main limitation of GC is that complex lipids such as phospholipids and triacyglycerols need to be saponified prior to derivitization to form FAME prior to analysis, so information on the specific structures of these lipids and typical combinations of fatty acids is lost10. All steps in this protocol must be carried out in a fume hood due to the use of solvents, which limits the suitability of surroundings this method can be performed in. This method can also be time consuming for analyzing a small number of samples, typically taking two working days to get from sample of interest to data output, unless fully automated procedures are used. However, when processing larger numbers of samples, each stage can be done in batches to maximize time effectiveness of this technique and availability of equipment to other users. Certain techniques within the protocol require practice and manual dexterity such as upper and lower phase extractions (steps 2.7 and 4.8), which could be a problem for people with problematic joints or who are prone to negative effects of repetitive movements.
Steps within this method can be easily added or removed to facilitate the collection of different fractions for a wide range of samples; for example, PE collection may not be required when analyzing plasma, but is of interest in cell and tissue samples10. This method can also be modified for the analysis of cells of total lipid extracts, where the SPE steps can be omitted if desired. One such variation is that described for red blood cells, which omits the total lipid extraction step as well as SPE steps15. In contrast to the current protocol, the method used in this study uses 250 µl of a methylating reagent (14% boron trifluoride), which is added directly to red cells along with 250 µl of hexane and heated for 10 minutes at 100 ˚C. The neutralization step is omitted and instead water and hexane are added; the sample is then centrifuged and the hexane upper phase collected and transferred directly into a GC auto sampler vial without drying under nitrogen and re-dissolving in hexane.
GC is a versatile method and provides reliable results with a range of available modifications15 and can be used to analyze a wide variety of samples. It has advantages over mass spectrometry (MS) when analyzing n-6 and n-3 fatty acid metabolism as it is able to distinguish between structurally similar fatty acids as it uses retention time for labeling as opposed to atomic mass. MS is able to identify fatty acids within a sample but unable to distinguish double bond positions in stereoisomers and therefore unable to tell certain fatty acids apart. If required, both methods can be used in tandem by GC-MS16. This technique is employed during investigation into lipid metabolism and function in the field of lipidomics. The use of GC in tandem with MS has led to great advances as it allows manipulation of fatty acid identification by use of different stationary phases in GC to discriminate between fatty acids that MS alone cannot. GC can also be used in combination with electron impact mass spectrometry (EI-MS), which allows for the identification of fatty acids when they are combined with alternative chemical derivatives such as picolinyl esters. This widens the use of the technique and continues to improve lipid profiling as a research method in a range of areas such as physiology, clinical biomarker detection, and pathology, as well as lipid biochemistry17.
Declarações
The authors have nothing to disclose.
Acknowledgements
The authors would like to acknowledge the contribution of Meritxell Romeu-Nadal to the rat study.
Materials
| Methanol: | Fisher Scientific | M/4056/17 | 'CAUTION' Fumes – HPLC Grade |
| Chloroform: | Fisher Scientific | C/4966/17 | 'CAUTION' Fumes – HPLC Grade |
| BHT: | Sigma- Aldrich | W218405 | 'CAUTION' Dust fumes – Anhydrous |
| NaCl | Sigma- Aldrich | S9888 | Anhydrous |
| Hexane | Fisher Scientific | H/0406/17 | 'CAUTION' Fumes – HPLC Grade |
| Glacial acetic acid | Sigma- Aldrich | 695084 | 'CAUTION' Burns – 99.85% |
| Sulfuric acid | Sigma- Aldrich | 339741 | 'CAUTION' Burns – 99.999% |
| Potassium carbonate | Sigma- Aldrich | 209619 | 99% ACS Reagent grade |
| Potassium bicarbonate | Sigma- Aldrich | 237205 | 99.7% ACS Reasgent grade |
| Ethyl acetate | Fisher Scientific | 10204340 | 'CAUTION' Fumes – 99+% GLC SpeciFied |
| Toluene | Fisher Scientific | T/2300/15 | 'CAUTION' Fumes |
| Diethyl ether | Sigma- Aldrich | 309966 | 'CAUTION' Fumes |
| Nitrogen (oxygen free) cylinder | BOC | 44-w | 'CAUTION' Compressed gas – explosion risk |
| Aminopropyl silica SPE cartridges | Agilent | 12102014 | Cartridge – Bead mass 100mg |
| Silica gel SPE cartidges | Agilent | 14102010 | Cartridge – Bead mass 100mg |
| Molecular seives | Sigma- Aldrich | 334324 | Pellets, AW-300, 1.6mm |
| Glass pasteur pipettes | Fisher Scientific | FB50251 | |
| Borosilicate glass test tube | Fisher Scientific | FB59527 | Non-screw cap |
| Screw thread glass test tubes | Fisher Scientific | FB59555 | 13mm |
| Caps for screw thread test tubes | Fisher Scientific | FB51353 | To fit 13mm tube |
| Solida phase extraction (SPE) tank | Agilent | VacElut 20 Manifold | |
| Luer stopcocks for SPE tank | Agilent | 12131005 | |
| Vacuum pump | Sigma- Aldrich | 2656-194GB-1EA | |
| GC vials | Kinesis | STV 12-03TS | Short thread 9mm, TPX 0.2ml fused glass insert |
| GC vial lids | Kinesis | SCC09-0.2B | Short thread 9mm blue |
| GC inlet liners | SGE Analytical science | 092002 | Split/splitless Focus liner ID 4mm, OD 6.3mm length 78.5mm |
| GC septa | SGE Analytical science | 041856 | 11mm, MN material |
| GC column | SGE Analytical science | 054612 | Length 30m, ID 0.22mm, Film thickness 0.25µm |
| Gas chromatograph | HP 6890 series |
Referências
- Browning, L. M., et al. Incorporation of eicosapentaenoic and docosahexaenoic acids into lipid pools when given as supplements providing doses equivalent to typical intakes of oily fish. Am. J. Clin. Nutr. 96 (4), 748-758 (2012).
- Cao, J., Schwichtenberg, K. A., Hanson, N. Q., Tsai, M. Y. Incorporation and clearance of omega-3 fattyacids in erythrocyte membranes and plasma phospholipids. Clin. Chem. 52 (12), 2265-2272 (2006).
- Lauritzen, L., Carlson, S. E. Maternal fatty acid status during pregnancy andlactation and relation to newborn and infant status. Matern. Child Health. 7 (2), 41-58 (2011).
- Kelsall, C. J., et al. Vascular dysfunction induced in offspring by maternal dietary fat involves altered arterial polyunsaturated fatty acid biosynthesis. PLoS One. 7 (4), (2012).
- Karpe, F., Dickmann, J. R., Frayn, K. N. Fatty acids, obesity, and insulin resistance: time for a re-evaluation. Diabetes. 60 (10), 2441-2449 (2011).
- Mossoba, M. M., Moss, J., Kramer, J. K. Trans fat labelling and levels in U.S. foods: assessment of gas chromatographic and infrared spectroscopic techniques for regulatory compliance. J. AOAC Int. 92 (5), 1284-1300 (2009).
- Chee, K. M., et al. Fatty acid content of marine oil capsules. Lipids. 25 (9), 523-528 (1990).
- Folch, J., Lees, M., Sloane-Stanley, G. H. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 226 (1), 497-509 (1957).
- Burdge, G. C., Wright, P., Jones, E. A., Wootton, S. A. A method for separation of phosphatidylcholine, triacylglycerol, non-esterified fatty acids and cholesterol esters from plasma by solid-phase extraction. Br. J. Nutr. 84 (5), 781-787 (2000).
- Seppänen-Laakso, T., Laakso, I., Hiltunen, R. Analysis of fatty acids by gas chromatography, and its relevance to research on health and nutrition. Anal. Chim. Acta. 465 (1), 39-62 (2002).
- Beesley, T. E., Buglio, B., Scott, R. P. W. . Quantitative chromatographic analysis. , (2000).
- Bartle, K. D., Myers, P. History of gas chromatography. Trends Anal. Chem. 21 (9), 9-10 (2002).
- Childs, C. E. . The effect of gender, pregnancy and diet upon rat tissue fatty acid composition and immune function. , 378 (2008).
- Harris, S. W., Pottala, J. V., Ramachandran, S. V., Larson, M. G., Robins, S. J. Changes in erythrocyte membrane Trans and marine fatty acids between 1999 and 2006 in older Americans. J. Nutr. 142 (7), 1297-1303 (2012).
- Roberts, L. D., McCombie, G., Titman, C. M., Griffin, J. L. A matter of fat: An introduction to lipidomic profiling method. J. Chromatogr. B. 871 (2), 174-181 (2008).

