Slice It Hot: Acute Adult Brain Slicing in Physiological Temperature
Summary
In this paper we show a method for preparing acute brain slices in physiological temperature, using a conventional physiological solution without special modifications for the cutting (such as adding sucrose) and without intracardial perfusion of the animal before slice preparation.
Abstract
Here we present a protocol for preparation of acute brain slices. This procedure is a critical element for electrophysiological patch-clamp experiments that largely determines the quality of results. It has been shown that omitting the cooling step during cutting procedure is beneficial in obtaining healthy slices and cells, especially when dealing with highly myelinated brain structures from mature animals. Even though the precise mechanism whereby elevated temperature supports neural health can only be speculated upon, it stands to reason that, whenever possible, the temperature in which the slicing is performed should be close to physiological conditions to prevent temperature related artifacts. Another important advantage of this method is the simplicity of the procedure and therefore the short preparation time. In the demonstrated method adult mice are used but the same procedure can be applied with younger mice as well as rats. Also, the following patch clamp experiment is performed on horizontal cerebellar slices, but the same procedure can also be used in other planes as well as other posterior areas of the brain.
Introduction
The aim of the presented method is to get high-quality acute brain slices for in vitro electrophysiological experiments, especially when using adult or even old animals.
The acute brain slicing method, as described by Skrede and Westgaard1 in two elegant sentences, has become one of the foundations of modern neuroscience research and is employed in innumerable variations worldwide. The quality of the slices is reflected in number of living neurons per slice, the period of time during which the cells keep their electrophysiological and morphological properties as well as in the integrity of the tissue. Moreover, the maximal duration for stable recordings depends on the quality of the slices. Thus, along the decades, the original slicing method has been further developed by individual research groups to enhance slice recovery after cutting2-10, often by complex modifications of the composition of cutting or recovery solutions (such as adding ascorbate, thiourea or even H2O2) as well as intra-cardiac pre-perfusion of the animal with cooled physiological solutions.
As has been recently shown11, physiological temperature during slicing seems to be more beneficial than cooling to neuronal health; the improvement is most striking when working with adult (2-8 month) rodents. Avoiding dramatic temperature changes prevents artifacts due to temperature-dependent processes in the cells, such as plasticity13 and ion-channels kinetics13,14. Such changes could influence membrane voltage and intracellular calcium signaling, spike threshold, and spike shape.
The “hot” acute slice preparation method presented here is a general procedure for obtaining high-quality acute brain slices from any brain region, including the cerebellum, the cortex and hippocampus, brainstem nuclei16 as well as the olfactory bulb, both in rats and mice.
Notably, the physiological temperature slicing procedure requires that the cutting blade vibrates nearly perfectly horizontally and is without any structural defects. Such precision might not be attainable with older slicer models; in such cases, we recommend performing the slice preparation in freezing-cold conditions as the low temperature seems to make the tissue more resistant to mechanical damage, even if at the cost of metabolic aberrations.
Protocol
All experimental procedures described in this protocol were approved by the Hebrew University's Animal Care and Use Committee.
1. Preparing the Solutions and Tools for Slicing
- Prepare 1 L of standard physiological solution (SPS) containing the ions described in Table 1.
- Prepare a stock solution containing the salts at 10 times the final concentration in advance in a storage glass bottle filled with 1,000 ml of deionized water (conductance of 0.055 µS/cm). Add salts (NaCl, KCl, KH2PO4, and MgSO4), and stir until completely dissolved. Store the stock solution in 4 °C.
- Make the final SPS solution on the day of the experiment. Add 100 ml of the stock solution to 700 ml of purified water, dissolve the glucose (3.6 g) and NaHCO3 (2.18 g) using magnetic stir bar and then add purified water up to 1 L.
- Equilibrate the solution by gassing it with 95% O2 / 5% CO2 for ~20 min. After this, add 2 ml of 1 M CaCl2 solution.
- Assemble the following tools (Figure 1).
- Prepare big scissors or other tool for decapitation, small surgical scissors for opening the skull, fine tip forceps for lifting the skull to expose the brain, scalpel with blade for dissecting the desired part of the brain to be sliced and a small spatula for manipulating the dissected brain parts.
- In addition, prepare small pieces of filter paper for removing excess liquid from the brain, two small Petri dishes for the brain to be dissected in two glass 500 ml beakers for containing warmed purified water and SPS, and cyanoacrylate glue for gluing the brain to the slicing stage.
- Prepare a small brush for handling the slices during the slicing and a wide-mouthed glass Pasteur pipette for moving the slices from the slicing bath to the recovery chamber. Disinfect the tools to prevent bacteria growth in the warm slice baths. Furthermore, use clean laboratory gloves.
- Prepare a submerged slice recovery chamber such as described by Gibb and Edwards15. Continuously gas the SPS in it with 95% O2 / 5% CO2 and keep at 36 °C. Make sure that the gassing does not result in small bubbles trapped in the bath that could damage slices.
- Warm ~300 ml of the SPS to 36 °C on a heater plate with magnetic stirrer or in a water bath. If a heater plate is used, take care to prevent the solution from overheating, which may result in ion precipitation. In case it happens, warm fresh SPS instead of cooling the old one.
- Bring ~500 ml of purified water to boil using an electric kettle and, in a beaker, combine 300 ml of it with cool water to obtain slightly warmer water than the slicing temperature. Use this warmed purified water during the decapitation and use the remaining of the boiled water later for maintaining the physiological temperature of the slicing bath.
- Anaesthetize the mouse by injecting 0.1 ml of pentobarbital (60 mg/ml) intraperitoneally. After a few minutes check that the animal does not respond to strong toe or tail pinches to ascertain the lack of sensation.
2. Dissecting the Brain
- Decapitate the mouse quickly by cutting the neck with the big scissors near the back of the skull, and let the head fall into the beaker with warmed purified water to rinse off excess blood.
- Expose the foramen magnum in the skull under the neck muscles and skin, possibly removing more of the neck with the small scissors.
- Pull the skin on the top of the skull to be able to clearly see the skull sutures in order to guide opening of the skull.
- Cut the skull open by inserting the lower tip of the small scissors through the foramen magnum and immediately turning them towards the lateral side. Gently cut along the lateral edge of the parietal bone up to a location behind the eyes and the frontoparietal suture; then, turn towards the center of the skull and cut across the midline to the other side of the skull (see green dashed line in Figure 2A).
- Using fine tip forceps, lift the skull from the frontoparietal corner and pull it diagonally up and sidewards to expose the brain (see yellow dashed arrow in Figure 2A). Make sure that the skull is not attached to any bone or skin of the head, so that the brain will stay in place when the skull is lifted. In case that the brain is moving with the skull, use the small scissors to remove any connective tissue between the skull and the brain.
- When the brain is exposed, do not allow the brain to be dry. Thus, perform the next steps while keeping the skull and the brain in a Petri dish filled with warmed, gassed SPS.
- Detach the part of the brain used for experiment from the rest of the brain using a scalpel and a spatula. In case of using the cerebellum, detach from the forebrain by a single cut through the midbrain and pons (along red lines in Figures 2B-D) and move it to a small Petri dish that contains fresh, gassed SPS.
- Remove the brainstem to form a straight and wide base for gluing the brain to cutting stage when slicing the cerebellum in horizontal plane. Briefly place the cerebellum on a wet piece of filter paper so that the side that was cut from the forebrain is facing down.
- Cut the brainstem with a scalpel to form the base (as indicated with the blue line in Figure 2B), and return the cerebellum to the SPS. When other planes of cutting are needed, trim the cerebellum block accordingly (blue lines in Figures 2C & D).
3. Slicing the Brain
- Prepare the cutting stage by ascertaining that it is dry, before applying a small drop of superglue in the middle of the stage.
- Lift the brain block from the SPS using a spatula with correct side up, and remove any excess liquid from the brain using small pieces of filter paper. Then, with a single movement, slide the brain from the spatula onto the drop of glue on the stage.
- To prevent the brain from drying and the glue from running up on the sides of the tissue, apply a few drops of SPS with a Pasteur pipette and place the cutting stage in the slicing chamber. Align the brain block so that the regions of interest (e.g., cerebellar cortex) are facing the blade and thus will be subjected to least amount of mechanical pressure before being sliced.
- Cut each slice according to the manufacturer’s instructions while constantly gassing the SPS in the cutting chamber as well as maintaining the temperature at 34-37 °C. Keep the temperature of the cutting chamber at this range by filling the external chamber of the slicer with warmed purified water and replacing the water whenever the temperature falls too low.
NOTE: The slicing parameters must be experimentally found for each type of tissue and neurons that are being examined. In case of the Campden SMZ 700 slicer with ceramic blades and cerebellar Golgi cells, use 0.75 mm vibration amplitude at 65 Hz and advancing speed of 0.05 mm/sec; for cerebellar nuclear neurons higher amplitude and frequency (1 mm and 90 Hz, respectively) and slower advance speed (0.01-0.02 mm/sec) are recommended. For both Golgi and cerebellar nuclear neurons, slice thickness should be 300 µm. - During the slicing, do not allow the slices to fold on themselves. Prevent this by gently supporting them with a soft brush, preferably touching the slice only in regions that are not of interest for the experiment. Take care to prevent the brush from touching the vibratome blade; especially in case of the ceramic blades, even a light contact can damage the blade.
- Move each slice from the slicing chamber to a recovery/holding chamber using a wide-mouthed, fire-polished glass Pasteur pipette.
- Let the slices rest in the chamber for at least 1 hr before starting the experiment. This allows for degradation of damaged or dying tissue and cells and thus results in a cleaner slice surface. During the first hour, keep the temperature of the SPS in the chamber in the range of 34-36 °C; ensure that the slices are not touched by air bubbles that can damage the slices or make them float.
4. Experiment
- After 1 hr, let the recovery chamber temperature cool down to RT (17-25 °C).
NOTE: This might prolong the time period during which the slices can be used by slowing down the growth of bacteria as well as cellular metabolism. This period depends on the brain region and cell type. For example, certain types of Golgi cells can be found to be healthy in slices 8 hr after cutting whereas other types last only 6 hr. - After 1 hr has passed from the slicing, use the slices for various electrophysiological experiments.
Representative Results
Slices prepared in the described manner can be used for various electrophysiological and optogenetic experiments. In Figure 3A and 3C, we show a representative example of a horizontal cerebellar slice and a coronal cerebral cortical slice, respectively, viewed under differential interference (DIC) optics. In the cerebellar slice, several types of cerebellar neurons can be easily recognized by their location and cell body shape, allowing targeted electrophysiological recordings. In Figure 3B and 3D, example traces from whole-cell patch-clamp recordings in current clamp mode from a spontaneously active cerebellar Golgi cell and cortical pyramidal cell are shown. Further examples of the quality of electrophysiological and optogenetic recordings obtained from hot-sliced brains can be found in Huang and Uusisaari11 and Lefler et al.16.
| concentration [mM] | weight (g/L) | |
| NaCl | 124 | 72.5 |
| KCl | 3 | 2.23 |
| KH2PO4 | 1.2 | 1.63 |
| MgSO4*6H2O | 1.9 | 4.3 |
| Glucose | 20 | 3.6 |
| NaHCO3 | 26 | 2.18 |
| CaCl2 | 2 | 1.109 |
Table 1. Composition of the standard physiological solution.
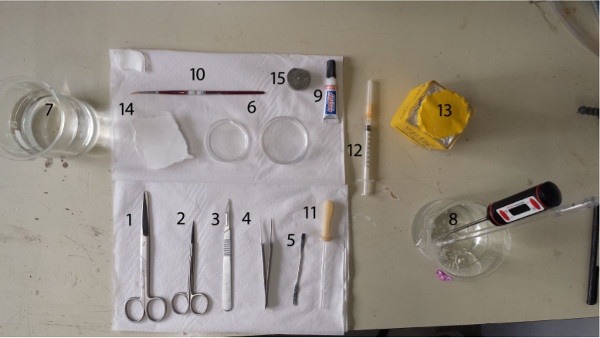
Figure 1. Arrangement of the tools. 1. Big scissors, 2. Small surgical scissors, 3. Scalpel with blade #11, 4. Fine tip forceps, 5. Small spatula, 6. Small Petri dishes, 7. Glass beaker for deionized water, 8. Glass beaker for warmed gassed physiological solution, 9. Super glue, 10. Thin brush, 11. Pasteur pipette, 12. Small syringe, 13. Pentobarbital, 14. Filter papers, 15. Cutting stage.
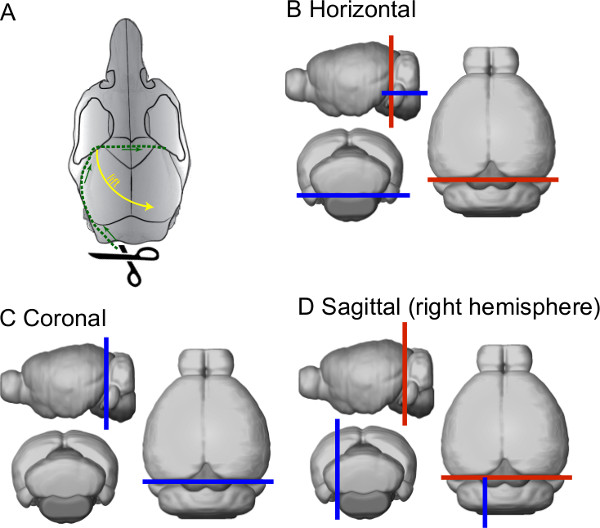
Figure 2. Schematic representation of the skull and brain dissection. (A) Drawing depicting the opening of the skull to expose the brain. (B–D) Schematic drawings describing the planes of cutting the brain for the three major slicing planes. The blue line represents the plane onto which the brain will be glued on.
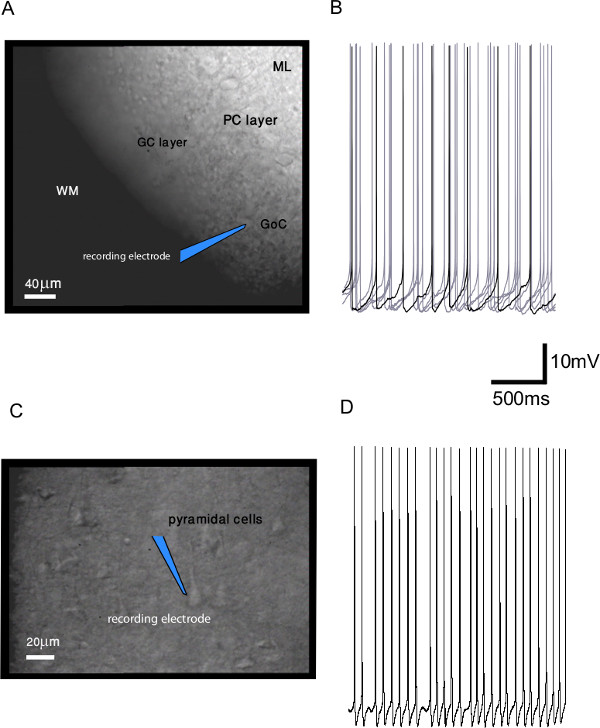
Figure 3. Representative results of the method. (A) a horizontal cerebellar slice obtained with the demonstrated method. The three major layers of the cerebellar cortex (granule cell layer, GC layer; Purkinje neuron layer, PN layer; molecular layer, ML) as well as white matter (WM) are indicated. A patch-clamp electrode is schematically drawn onto a Golgi cell (GoC) (scale bar: 40 μm). (B) example recording from the Golgi cell shown in A. The recording is obtained with a patch-clamp electrode in current-clamp mode. (In black: representative trace out of six grey traces). (C) a coronal cerebral cortical slice obtained with the demonstrated method. Cortical pyramidal cells are shown together with a schematic drawing of a patch-clamp electrode. (D) example patch clamp recording, in current clamp mode, from the pyramidal cell shown in (C). Please click here to view a larger version of this figure.
Discussion
We demonstrate a method for preparing acute brain slices from mice in physiological instead of ice-cold temperature.
It has been shown11 that the quality of slices obtained in warm conditions is superior when compared with those prepared with cold conditions, provided that the slicer blade has minimal vertical vibration. Slicing in physiological temperature may prevent physiological artifacts caused by the low temperature, such as those related to changes in metabolic processes17-19 that may manifest in aberrations of single-cell as well as network behavior. Furthermore, even though the slicing procedure should obviously be completed without unnecessary delays, there is no need to hurry with the slicing (as it is when slicing in ice-cold conditions in order to finish cutting before solutions become too warm, and to prevent the damage from long-lasting freezing of the tissue). Thus, slower cutting speeds can be used. This can be beneficial in case of slicing very delicate structures. Last, omitting the cooling step of both the solutions and of the brain significantly reduces the amount of time required for the procedure as well as its complexity. The cooling of the solution to ice-cold temperature requires roughly 40 min more than the warming of the solution to physiological temperature, leaving more time for the experiment itself.
It has been shown20 that the intensity of fluorescence in cortical neurons in GAD67-GFP mice is stronger when examining slices cut in 20 °C compared with those cut in 0 °C. This further supports the notion that slicing in ice-cold temperature exerts a harmful influence on the viability of neurons. On the other hand, in the same paper it was shown that the density of fluorescent neurons was reduced in sliced obtained at 37 °C compared to those cut at 20 °C. The controversy with the results might arise from the fact that the z-deflection was not taken under considerations. As mentioned above, the z-deflection has a strong influence on the tissue quality when slicing in physiological temperature, in addition to the quality of the blade and the speed and other settings of the slicer.
One of the crucial parts of the procedure involves fine-tuning of the slicer blade before slicing. Unlike when using ice-cold solutions where the slicing likely involves both horizontal cutting as well as vertical “cracking” of the rigid lipid membranes, under physiological conditions any movement of the slicer blade in the z-direction will cause damage in the tissue. To minimize this, the blade should be as hard and straight as possible; in our experience, conventional razor blades have too many imperfections to be usable. High-quality stainless steel blades specifically designed for vibratome usage are better, but for best results we suggest single-beveled ceramic blades. Furthermore, we strongly recommend spending significant time for perfect horizontal alignment of the blades as this directly influences slice quality. We use the Campden 700SMZ slicer that allows tuning of the blade alignment so that the vibration in the z-plane is less than 0.5 μm. Indeed, the most crucial limitation of the hot slicing method is its dependence on slicer blade quality and stability. If these requirements cannot be met, it is advisable to use the traditional ice-cold cutting method instead, despite the disadvantages in slicing in ice-cold solution such as varying osmolarity from being partly frozen.
The method demonstrated is used to obtain horizontal slices of the cerebellum; with simple modifications the same method can be used to get slices in either the coronal or the sagittal plane, from many other regions of the fore – or midbrain as well as the brainstem. For slices of the most anterior parts of the forebrain and olfactory system, the skull should be cut more anterior to prevent damage to these parts. We purposefully keep the method as simple and short as possible and do not employ pre-perfusion of the animal, nor do we substitute any components in the physiological solution for cutting. This allows the method to be modified according to the specific neuronal subtype of interest. It is likely that modifications of the cutting solutions (as described in the references in the Introduction) can further improve the viability of slices and robustness of results. In general, the method should be compatible with most other common physiological solutions commonly used for electrophysiological recording.
In the method shown here, slices are cut 300 μm thick. The optimal slice thickness depends on the sliced brain region and the cells of interest. For network integrity considerations, the slices should not be thinner than 250 μm, and because of diffusion limitations the upper limit of the slice thickness is ~400-450 μm.
A critical step in this method regards gluing the brain to the cutting stage, as even small instability in the tissue can result in uneven or unusable slices. Thus, care should be taken to make sure that the brain surface has no excess SPS when it is lowered onto the glue drop; also, too much glue will result in the brain lying unevenly on the stage and possibly detaching from the stage during slicing. Also, excess glue might creep up on the tissue sides and, in addition to introducing inhomogeneity into the slices, might damage the blade.
There are a few difficulties that might rise when changing the slicing protocol from the ice-cold method to the demonstrated hot method. One of the difficulties is the appearance of bacteria in the slices. For this reason it is important to disinfect the tools, the chamber, and the slicing bath with ethanol before the procedure. Also, turning off the heating system in the recovery bath after 1 hr slows the bacteria growth.
Finally, it should be emphasized that the improved health of the neurons in slices cut in physiological temperature might also result in changes in their intrinsic properties. Therefore, it is advisable to perform an initial comparison of the results obtained from slices with both cold and warm methods, especially if the experiments are part of a longer project with earlier works done using the cold method.
Declarações
The authors have nothing to disclose.
Acknowledgements
We would like to acknowledge the significant contribution Dr. Shiwei Huang (Australian National University) in validating the method. Furthermore, we would like to thank Ms. Kasia Pietrajtis for helpful comments regarding Golgi cells and Mr. Vitaly Lerner for the cortex experimental data. This work was supported by PITN-GA-2009-238686 (CEREBNET), FP7-ICT (REALNET), ELSC and ISF.
Materials
| Name | Company | Catalog # | Comments |
| Pentobarbital | CTS | 170066 | Concentration: 60 mg / ml in physiological saline. |
| Big scissors | FST | 14001-16 | Any large scissors or a guillotine with sufficiently sharp edges can be used for decapitation |
| Iris scissors | Prestige medical | 48,148 | Any fine tip scissors can be used, provided the scissor blades are not longer than 1.5 – 2 cm |
| Fine tip forceps | FST | 11254-20 | |
| Scalpel | FST | 91003-12 | |
| Scalpel blade #11 | FST | 10011-00 | |
| Small spatula | Fisher | 2350 | |
| Filter paper | Any laboratory brand can be used. | ||
| Petri dishes | Duroplan | Z231509-1 | |
| Glass beakers | SCHOT | 10022846 | |
| Pasteur pipette | Maple Leaf Brand | 14672-029 | |
| Super glue | LOCTITE | 4091361/1 | |
| Slicer | Campden | 7000-smz | |
| Ceramic slicing blade | Campden | 7550-1-C | |
| Magnetic heater/stirrer | For heating up the SPS for the procedure | ||
| Electric kettle | For heating up water for temperature control | ||
| Slice recovery chamber + heating unit | Warner instruments | BSC-HT + BSC-BUW | Home-built models may also be used. |
| Thermometer | For monitoring SPS temperature during dissection and slicing |
Referências
- Skrede, K., Westgaard, R. The transverse hippocampal slice: a well-defined cortical structure maintained in vitro. Brain Research. 35, 589-593 (1971).
- Aghajanian, G., Rasmussen, K. Intracellular studies in the facial nucleus illustrating a simple new method for obtaining viable motoneurons in adult rat brain slices. Synapse. 3, 331-338 (1989).
- Gueritaud, J. Electrical activity of rat ocular motoneurons recorded in vitro. Neurociência. 24, 837-852 (1988).
- Lipton, P., et al. Making the best of brain slices: comparing preparative methods. Journal of Neuroscience Methods. 59, 151-156 (1995).
- Richerson, G., Messer, C. Effect of composition of experimental solutions on neuronal survival during rat brain slicing. Experimental Neurology. 131, 133-143 (1995).
- Brahma, B., Forman, R., Stewart, E., Nicholson, C., Rice, M. Ascorbate inhibits edema in brain slices. Journal of Neurochemistry. 74, 1263-1270 (2000).
- Moyer, J. R., Brown, T. H. Patch-clamp techniques applied to brain slices. In: Patch-clamp analysis: advanced techniques. Springer. , 135-193 (2002).
- Ye, J. H., Zhang, J., Xiao, C., Kong, J. Q. Patch-clamp studies in the CNS illustrate a simple new method for obtaining viable neurons in rat brain slices: glycerol replacement of NaCl protects CNS neurons. J. Neuroscience Methods. 156, 251-259 (2006).
- Bischofberger, J., Engel, D., Li, L., Geiger, J., Jonas, P. Patch-clamp recording from mossy fiber terminals in hippocampal slices. Nature Protocols. 1, 2075-2081 (2006).
- Zhao, S., et al. Cell type–specific channelrhodopsin-2 transgenic mice for optogenetic dissection of neural circuitry function. Nature Methods. 8, 745-752 (2011).
- Huang, S., Uusisaari, M. Y. Physiological temperature during brain slicing enhances the quality of acute slice preparations. Front. Cell. Neurosci. 7, (2013).
- Ohe, G. C., Darian-Smith, C., Garner, C. C., Heller, H. C. . The Journal of Neuroscience. 26 (41), 10590-10598 (2006).
- Voets, T., et al. The principle of temperature-dependent gating in cold- and heat-sensitive TRP channels. Nature. 430, 748-754 (2004).
- Coulter, D. A., Huguenard, J. R., Prince, D. A. Calcium currents in rat thalamocortical relay neurones: kinetic properties of the transient, low-threshold current. The Journal of Physiology. 414, 587-604 (1998).
- Gibb, A. J., Edward, F. A. Patch clamp recording from cells in slice tissues. Microelectrode Techniques: the Plymouth workshop handbook. , (1994).
- Lefler, Y., Yarom, Y., Uusisaari, M. Y. Cerebellar Inhibitory Input to the Inferior Olive Decreases Electrical Coupling and Blocks Subthreshold Oscillations. Neuron. 81 (6), 1389-1400 (2014).
- Bourne, J. N., Kirov, S. A., Sorra, K. E., Harris, K. M. Warmer preparation of hippocampal slices prevents synapse proliferation that might obscure LTP-related structural plasticity. Neuropharmacology. 52, 55-59 (2007).
- Kirov, S. A., Sorra, K. E., Harris, K. M. Slices have more synapses than perfusion-fixed hippocampus from both young and mature rats. J. Neurosci. 19, 2876-2886 (1999).
- Kirov, S. A., Petrak, L. J., Fiala, J. C., Harris, K. M. Dendritic spines disappear with chilling but proliferate excessively upon rewarming of mature hippocampus. Neurociência. 127, 69-80 (2004).
- Tanaka, Y., Tanaka, Y., Furuta, T., Yanagawa, Y., Kaneko, T. The effects of cutting solutions on the viability of GABAergic interneurons in cerebral cortical slices of adult mice. J. Neurosci. Methods. 171, 181-125 (2008).

