Sampling Blood from the Lateral Tail Vein of the Rat
Summary
Blood samples are useful for assessing biomarkers of physiological states or disease in vivo. Here we describe the methodology to sample blood from the lateral tail vein in the rat. This method provides rapid samples with minimal pain and invasiveness.
Abstract
Blood samples are commonly obtained in many experimental contexts to measure targets of interest, including hormones, immune factors, growth factors, proteins, and glucose, yet the composition of the blood is dynamically regulated and easily perturbed. One factor that can change the blood composition is the stress response triggered by the sampling procedure, which can contribute to variability in the measures of interest. Here we describe a procedure for blood sampling from the lateral tail vein in the rat. This procedure offers significant advantages over other more commonly used techniques. It permits rapid sampling with minimal pain or invasiveness, without anesthesia or analgesia. Additionally, it can be used to obtain large volume samples (upwards of 1 ml in some rats), and it may be used repeatedly across experimental days. By minimizing the stress response and pain resulting from blood sampling, measures can more accurately reflect the true basal state of the animal, with minimal influence from the sampling procedure itself.
Introduction
Biomarkers obtained from blood provide useful diagnostic, predictive, and stratifying measures in many clinical contexts, including cardiovascular disease1, cancer sciences2, and psychiatric disease3. They may also be used in basic science to assess the “state” of an organism, including the degree of hunger, inflammation, or stress present. Such measures can be influenced by variables that may or may not be critical to the question of interest, including the time of day that the sample is obtained and the gender of the subjects. It may also be influenced by the stress induced during the blood sampling procedures itself. Stress hormones and the perception of pain can rapidly alter the composition of the blood.
Rodents are the most commonly used laboratory animal, and multiple methods have been developed for blood collection. The ideal method of blood sampling should have minimal physiological impact on the animal, require no anesthesia, allow rapid and repeated sampling, and provide sufficient blood volume for numerous downstream applications. Popular techniques for collecting blood such as catheterization of the jugular vein or tail tip amputation do not meet these criteria.
The aim of this protocol is to describe a blood sampling technique for use in rats that is minimally stressful, does not require anesthesia, allows for multiple blood collections within a single subject, and provides a relatively large sample volume such that multiple assays may be performed on a single sample. The goal of this method is to obtain blood samples that are minimally influenced by the acute stress response.
Protocol
All experiments were done using adult male Long-Evans rats. All procedures were in accordance with the US National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee of the Massachusetts Institute of Technology and the Animal Care and Use Review Office of the USAMRMC.
1. Preparation
- Heparinise the catheter and syringe by placing the shielded needle in a 500 µl tube containing heparin (1,000 USP units/ml) and then aspirating and expelling heparin solution through the needle.
- Attach a butterfly catheter to the syringe. Keep the shield over the needle of the catheter to protect the sharp tip from damage.
- Withdraw a volume of heparin that is slightly greater than the volume of blood that will be collected. Detach the syringe and fill it with air.
- Re-attach the syringe to the catheter and use the air to expel excess heparin solution; ensure only trace amounts remain in the tubing, needle, and syringe.
- Place the sterile catheter, with the syringe still attached, on a sterile surface.
- Quickly secure the rat in a clean cloth ensuring that fore- and hindpaws are in a comfortable position and breathing is unrestricted.
- Secure the wrap with hook and loop tape; ensure that external genitalia are not constricted.
- Have an assistant gently and firmly restrain the rat (abdomen and base of tail) on a solid work surface with the tail hanging off the edge of the counter.
2. Blood Sampling
- Immerse the tail in 42 °C water for 40-50 sec to dilate blood vessels and dry the tail with a paper towel. Locate the tail vein to be bled (rotate the whole body with the tail to prevent twisting the tail).
Note: Sufficient warming of the tail is critical for the rapid collection of a blood sample. If the vasculature is constricted, proper placement of the catheter is difficult, and blood flow is vastly reduced. A heating pad may be used as an alternative to water immersion. - Identify the sampling point.
Note: The artery lies along the mid-dorsal aspect of the tail; do not use this for sampling.- Target either the left and right tail veins that lie lateral to the artery. Pigmentation of the tail, which varies by strain and increases with age, may obscure some of the vasculature. Target a portion of the vein in the lower portion of the tail.
- Wipe the target area with 2% chlorhexidine antiseptic solution.
- Create negative pressure in the syringe and catheter by withdrawing the plunger from zero to approximately 50 µl.
- Hold the tail gently and firmly near the tip to keep the tail straight throughout sample collection. Ensure that blood flow is not occluded by an overly tight grip.
- Slowly insert the catheter into the vein at a shallow angle approximately 5 cm from the tip of the tail. When the vein is penetrated, blood will flow into the catheter. Slowly withdraw the plunger of the syringe to collect the desired volume at a steady rate (~20 µl per sec).
- Consult the veterinary staff for information about the maximum blood volume that can be collected. The maximum amount of blood that should be collected depends on the weight and health status of the rat. Do not withdraw more than 15% of total blood volume in a 14 day period.
Note: Blood is much more difficult to collect from animals that were acutely stressed in the minutes prior to sample collection because stress hormones constrict the vasculature. For example, moving the rat's home cage to a novel room, taking several minutes to wrap the animal, or repeated insertion of the catheter into a vein are all likely to trigger an acute stress response. - Facilitate blood flow by ‘milking' the vein. Run a finger along the length of the vein, from the base towards the tip of the tail, but remain more than 2 cm from the tip of the inserted needle or the catheter may become dislodged from the vein.
- If blood cannot be successfully collected from the initial site of catheter penetration, re-insert the needle further up the vein. If blood was collected at the initial site, re-pressurize the needle by disconnecting and then reconnecting the catheter and syringe prior to re-insertion in the vein. In general, avoid additional penetrations.
- As multiple penetrations can cause tail vein collapse, in which the blood supply to the tail is cut off and the soft tail tissue becomes necrotized, euthanize the rat if there is tail vein collapse.
- Consult the veterinary staff for information about the maximum blood volume that can be collected. The maximum amount of blood that should be collected depends on the weight and health status of the rat. Do not withdraw more than 15% of total blood volume in a 14 day period.
- When adequate sample volume is collected, release pressure in the syringe by disconnecting and reconnecting the catheter. Aspirate slightly using the syringe plunger (~50 µl), and withdraw the needle from the vein.
Note: If the needle is withdrawn without first releasing the pressure in the syringe, blood will drip from the needle. - Briefly apply pressure to the insertion site to stop bleeding, and wipe the area with antiseptic solution. Return the rat to its home cage.
3. Processing the Blood Sample
- Aspirate air to ensure no blood remains inside the catheter needle, and use scissors to cut the catheter tubing just above the needle. Expel the blood into a sterile 1.5 ml microcentrifuge tube.
Note: If blood is pushed through the needle, the shearing force may cause red blood cells to rupture which can interfere with many downstream assays. Remove the needle to avoid hemolysis.- To collect blood plasma, use tubes that contain EDTA as an anticoagulant (here, use 10 µl of 0.1 M EDTA for 200-400 µl of blood; ensure the concentration of EDTA used does not interfere with the downstream assay) and place on ice.
- Spin whole blood samples at 2,100 x g in a refrigerated centrifuge (4 °C) for 10 min within 10 min of collection. Elute the plasma, avoiding disturbing the red and white blood cell layers.
- To collect blood serum, place samples (without anticoagulant) at room temperature for up to 30 min to enable clotting. Spin the collection tubes in a refrigerated centrifuge (4 °C) at 2,000 x g. The serum may then be eluted.
- To collect blood plasma, use tubes that contain EDTA as an anticoagulant (here, use 10 µl of 0.1 M EDTA for 200-400 µl of blood; ensure the concentration of EDTA used does not interfere with the downstream assay) and place on ice.
- Use samples immediately, or store at -80 °C for up to one year.
Representative Results
Blood plasma collected from the lateral tail vein as described in the protocol gives a plasma sample that was translucent and pale yellow in appearance. As shown in Figure 1, hemolysis in a sample imparts a red tint to the plasma. The acute stress response can rapidly alter the composition of blood. For example, circulating corticosterone concentration can markedly increase within 10 min of stressor exposure, as shown in Figure 2. The low basal levels of corticosterone obtained with this method prior to stressor exposure reveal that the sampling procedure itself is not a significant source of stress.
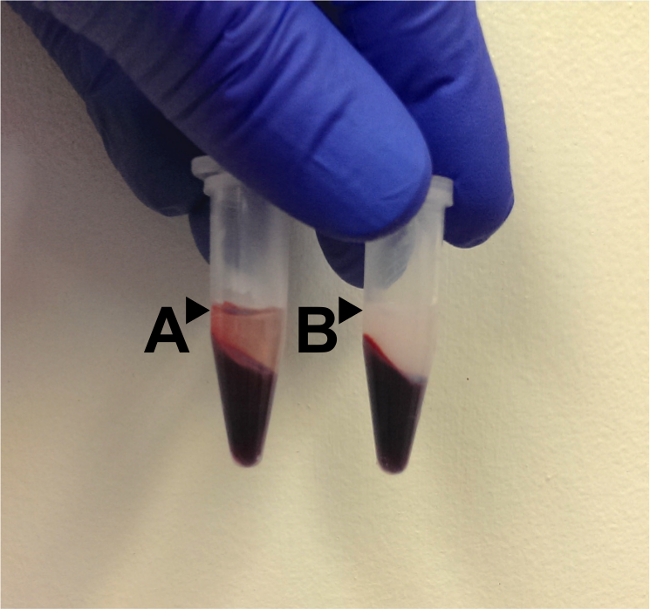
Figure 1: Sample appearance. (A) A hemolyzed sample is shown. After centrifugation, the plasma or serum layer (surface indicated by the black arrow) appears tinged with pink or red. Darker tints indicate greater levels of hemolysis. (B) After centrifugation, a properly collected sample will have a clear, yellowish appearance to the upper band (surface indicated by the black arrow), which corresponds to the non-hemolyzed plasma or serum. When removing this layer, it is important to not disturb the underlying whole blood, either by pushing the pipette tip into the whole blood layer or by aspirating some of the whole blood into the tip. Any plasma or serum contaminated with whole blood should be discarded.
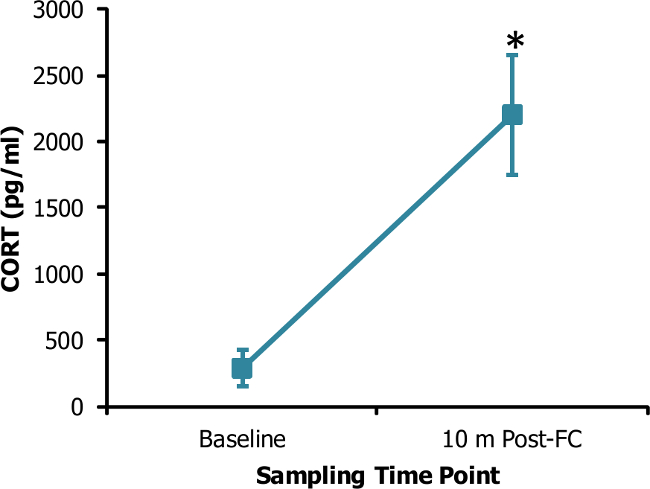
Figure 2: Plasma corticosterone is rapidly elevated following a stressful experience. Blood was obtained from the lateral tail vein of adult female Long-Evans rats before and 10 min following exposure to 4 tones (10 sec, 2 kHz, 85 dB) co-terminating with footshocks (1 sec, 350 µA). Blood plasma corticosterone at baseline (290.4 ± 138.8 pg / ml) was significantly less than the levels observed 10 min following presentation of the footshock stress (2204.8 ± 454.5 pg / ml, p = 0.02, n = 4), as determined by paired t-test. *, p <0.05
Discussion
Here, we describe a quick and simple procedure for obtaining a blood sample from a rat which offers significant advantages over other commonly used techniques. First, it does not require anesthesia, in contrast with sampling from the jugular vein or retroorbital sinus. When blood samples are collected surrounding behavioral procedures, administration of anesthetics is undesirable because it can interfere with learning and memory4,5. Second, it offers the ability to collect larger blood volumes than other venipuncture techniques, such as collection from the saphenous or dorsal pedal veins. Using the technique described here, up to 1.5 ml of blood may be collected from a rat at a single time point, a volume which readily allows multiple assays to be run in parallel. Finally, this procedure minimizes the potential for tissue damage compared to tail tip amputation or retroorbital bleeding. The use of this procedure facilitates compliance with the Animal Welfare Act and the Guide for the Care and Use of Laboratory Animals, which require minimizing the pain and distress that result from laboratory procedures performed on animals.
It is recommended that investigators new to this method practice the restraint and tail bleeding techniques in order to minimize the time that experimental animals are restrained. Blood collected in less than 3 min from the initiation of restraint provides optimal results.
The protocol described here may be used for sampling 1 to 4 times per week, but no more than twice per day. While repeated blood collections may be performed, different sampling sites moving upwards from the base of the tail should be used, and the left and right tail veins should be alternated as sampling sites. The total blood volume of rodents is 6-7% of their body weight, and no more than 15% of the total blood volume should be collected within a 2 week period. Serum or plasma comprises approximately 40-60% of the collected sample volume.
Blood sampling via the lateral tail veins may also be performed in the mouse as described here with a few minor modifications. First, only small gauge (27 G) catheters may be used. Second, it is recommended to use a tube restrainer, rather than a wrap, to immobilize the mice. The volume of blood that may be obtained from the mouse using venipuncture of the submandibular vascular bundle (200-500 µl) is greater than can be safely collected from the tail vein (200 µl maximum). Because sampling blood from the submandibular vascular bundle requires minimal restraint and may yield more blood, this is the preferred route for sampling in the mouse.
The rapidity with which this procedure may be performed, along with its minimally invasive nature, also minimizes the potential perturbation of blood-based measures by the acute stress response6. The acute stress response can alter circulating levels of many molecules, including interleukins and other immune-active factors7, hormones of the hypothalamic-pituitary-adrenal axis8, hormones in the sympathetic nervous system9, ghrelin10, endogenous opioids11, dopamine, and serotonin12. If resting circulating measures of these molecules or others regulated by these molecules are desired, it is important to minimize the stress response, which is triggered within as little as a minute of the start of stressor exposure.
Stress responses not only alter the composition of the blood, but also represent a technical obstacle for blood sampling because of the constriction of vasculature via increased drive from the sympathetic nervous system. It becomes increasing difficult to obtain steady blood flow from a rat that is mounting an acute stress response. Therefore, the animal's distress must be minimized in order to rapidly obtain samples that reflect the physiological state of interest.
Declarações
The authors have nothing to disclose.
Acknowledgements
We thank Virginia Doherty and Junmei Yao for technical assistance. This research was funded by NIMH (R01 MH084966), and the U.S. Army Research Office and the Defense Advanced Research Projects Agency (grant W911NF-10-1-0059) to KAG.
Materials
| Sodium heparin (1000 USP units/ml) | Patternson Veterinary Supply | 25021040010 | |
| Ethylenediaminetetraacetic acid (EDTA) | JT Taylor | JT2020-01 | |
| Dermachlor Rinse-Chlorhexadine | Butler Schein | 6356 | Topical antiseptic solution, 2% chlorhexidine gluconate |
| SURFLO Winged Infusion Sets, Terumo, butterfly catheters | VWR Scientific | TESV25BLK | |
| BD Tuberculin 1cc syringes | VWR Scientific | BD309659 | |
| 1.5 ml microcentrifuge tubes | VWR Scientific | 89202-682 | |
| 500 μl microcentrifuge tubes | VWR Scientific | 21150-330 | |
| Scissors, stainless steel, 5" | VWR Scientific | 82027-586 | |
| 500ml plastic beaker | VWR Scientific | 414004-149 | |
| Clean cloth wrap | Butler Schein | 2993 | |
| Velcro tape, .75" width | Monoprice | B004AF9II6 | Hook and loop tape |
| Timer | VWR Scientific | 62344-641 |
Referências
- Vausort, M., Wagner, D. R., Devaux, Y. Long Noncoding RNAs in Patients with Acute Myocardial Infarction. Circ Res. 115 (7), 668-677 (2014).
- Shah, R., et al. Biomarkers for Early Detection of Colorectal Cancer and Polyps: Systematic Review. Cancer Epidemiol Biomarkers Prev. 23 (9), 1712-1728 (2014).
- Chan, M. K., et al. Applications of blood-based protein biomarker strategies in the study of psychiatric disorders. Prog Neurobiol. , (2014).
- Cao, L., Li, L., Lin, D., Zuo, Z. Isoflurane induces learning impairment that is mediated by interleukin 1beta in rodents. PLoS One. 7 (12), e51431 (2012).
- Culley, D. J., Baxter, M. G., Yukhananov, R., Crosby, G. Long-term impairment of acquisition of a spatial memory task following isoflurane-nitrous oxide anesthesia in rats. Anesthesiology. 100 (2), 309-314 (2004).
- Vahl, T. P., et al. Comparative analysis of ACTH and corticosterone sampling methods in rats. Am J Physiol Endocrinol Metab. 289 (5), E823-E828 (2005).
- Kalinichenko, L. S., Koplik, E. V., Pertsov, S. S. Cytokine profile of peripheral blood in rats with various behavioral characteristics during acute emotional stress. Bull Exp Biol Med. 156 (4), 441-444 (2014).
- McEwen, B. S. Central effects of stress hormones in health and disease: Understanding the protective and damaging effects of stress and stress mediators. Eur J Pharmacol. 583 (2-3), 174-185 (2008).
- Sanchez, A., Toledo-Pinto, E. A., Menezes, M. L., Pereira, O. C. Changes in norepinephrine and epinephrine concentrations in adrenal gland of the rats submitted to acute immobilization stress. Pharmacol Res. 48 (6), 607-613 (2003).
- Meyer, R. M., Burgos-Robles, A., Liu, E., Correia, S. S., Goosens, K. A. A ghrelin-growth hormone axis drives stress-induced vulnerability to enhanced fear. Mol Psychiatry. , (2013).
- Knoll, A. T., Carlezon, W. A. Dynorphin, stress, and depression. Brain Res. 1314 (56-73), (2010).
- Harvey, B. H., Brand, L., Jeeva, Z., Stein, D. J. Cortical/hippocampal monoamines, HPA-axis changes and aversive behavior following stress and restress in an animal model of post-traumatic stress disorder. Physiol Behav. 87 (5), 881-890 (2006).

