Fabrication of White Light-emitting Electrochemical Cells with Stable Emission from Exciplexes
Summary
The authors present a method for fabricating stable white-light-emitting electrochemical cells utilizing emission from exciplexes formed between a blue-emitting fluorene polymer and aromatic amines.
Abstract
The authors present an approach for fabricating stable white light emission from polymer light-emitting electrochemical cells (PLECs) having an active layer which consists of blue-fluorescent poly(9,9-di-n-dodecylfluorenyl-2,7-diyl) (PFD) and π-conjugated triphenylamine molecules. This white light emission originates from exciplexes formed between PFD and amines in electronically excited states. A device containing PFD, 4,4',4''-tris[2-naphthyl(phenyl)amino]triphenylamine (2-TNATA), Poly(ethylene oxide) and K2CF3SO3 showed white light emission with Commission internationale de l'éclairage (CIE) coordinates of (0.33, 0.43) and a Color Rendering Index (CRI) of Ra = 73 at an applied voltage of 3.5 V. Constant voltage measurements showed that the CIE coordinates of (0.27, 0.37), Ra of 67, and the emission color observed immediately after application of a voltage of 5 V were nearly unchanged and stable after 300 sec.
Introduction
Research and development of polymer light-emitting electrochemical cells (PLECs) have expanded in recent years.1-15 PLECs are similar to organic light-emitting diodes (OLEDs) in that both are surface emitting organic devices and are expected to find their way into future lighting applications. OLEDs are already on the market, but the cost is still high, one reason being that OLEDs need a complicated device structure with multiple layers. In contrast, PLECs have a very simple device structure which consists of a single active layer (emitting layer) between a pair of electrodes. This means that PLECs are suited to mass production processes such as roll-to-roll printing and coating.
A PLEC has an active layer consisting of a fluorescent π-conjugated polymer (FCP). The FCP can be electrochemically doped with a polymer electrolyte (a mixture of an ion conducting polymer and a salt). The FCP is p-doped on the anode side and n-doped on the cathode side during operation, and generates excitons which emit light between the p- and n-doped regions. Therefore, the emission color reflects the exciton emission (=fluorescence) wavelength of the FCP.
Stable white light emission is important for lighting applications, and color mixing techniques which employ two or more emitters have been widely used to achieve this.10-14 Recently, we presented a different approach for obtaining stable white light emission, using an active layer which contains blue-fluorescent poly(9,9-di-n-dodecylfluorenyl-2,7-diyl) (PFD) and π-conjugated aromatic amines15. This white light emission comes from exciplexes formed between PFD and amine molecules in excited states. Exciplex emission has a broader spectrum compared to the exciton emission from the PDF and/or amines, which gives it a color close to that of natural light. This translates to a higher color rendering index (CRI), which is preferable for lighting applications.
In this article, the authors describe the procedure used to fabricate the exciplex based LECs and show the stability of their white light emission.
Protocol
1. Preparation of Active Layer Solutions
- Active layer solution for the amine doped PFD devices
NOTE: The PFD, 4,4',4''-tris[2-naphthyl(phenyl)amino]triphenylamine (2-TNATA), 9,9-dimethyl-N,N'-di(1-naphthyl)-N,N'-diphenyl-9H-fluorene-2,7-diamine (DMFL-NPB), Poly(ethylene oxide) (PEO), were used as received. The potassium trifluoromethanesulfonate (K2CF3SO3) was dried under vacuum at 200 °C for 1 hr prior to use.- For the devices having a PFD:amine ratio of 1:0.25, dissolve 10 mg of PFD and 2.5 mg of the aromatic amine in 1 ml of chloroform and stir for 1 hr at 40 °C. For those having a PFD:amine ratio of 1:1, use 10 mg of the aromatic amine.
- Separately, dissolve 10 mg of PEO in 1 ml of cyclohexanone and stir for 1 hr at 60 °C, and dissolve 2.5 mg of potassium trifluoromethanesulfonate (KCF3SO3) in 1 ml of cyclohexanone and stir for 1 hr at 40 °C.
- Add 0.78 ml of the PEO solution and 0.147 ml of the KCF3SO3 solution to the PFD solution using micropipettes. Stir the mixed solution for 4 hr at 40 °C.
- Filter the mixed solution using a membrane filter prior to spin coating.
- Active layer solution for the undoped PFD device
- For the undoped PFD device, dissolve 10 mg of PFD in 1 ml of chloroform and stir for 1 hr at 40 °C. The steps which follow are the same as those described previously for the amine doped PFDs in 1.1.2 – 1.1.4.
2. Fabrication of LEC Devices
NOTE: Fabrication process of LEC devices is summarized in Figure 1.
- Ultrasonically clean patterned indium-tin oxide (ITO) glass substrates with diluted detergent, followed by ionized water, acetone and 2-propanol using a desktop ultrasonic bath (38 kHz) for 3 min for each step. Finally, remove the solvent using an N2 blower.
- Treat the substrates with UV/O3 for 3 min using a UV/O3 treating unit according to manufacturer's protocol. Perform the active layer coating process under an inert atmosphere in a glove box.
- Set a cleaned substrate on the head of a spin coater. Dispense around 100 µl of the active layer solution using a micropipette. Spin the substrate as follows: 800 rpm for 60 sec, increase the rate to 1,000 rpm over 3 sec, then spin at 1,000 rpm for 10 sec. The active layer thickness will be around 150 nm.
- Dry the coated substrates in the glove box overnight.
- Wipe off excess polymer to ensure a proper electrode connection and encapsulation.
- Place the substrates on an evaporation holder for deposition of aluminum. Load the holder in the evaporation chamber, and thermally deposit a 100 nm layer of aluminum at an evaporation rate of 0.4 nm/sec through a stainless steel evaporation mask, which has 3 mm wide openings for depositing the aluminum counter electrodes.
- When deposition is complete, transfer the devices to a glove box under an inert atmosphere. Apply a bead of UV curable epoxy resin in the shape of a rectangle using a dispenser. Place a cover glass (15 mm x 12 mm x 0.7 mm-thick) on the resin to encapsulate the device (see Figure 1).
- Cure the resin using UV radiation (cumulative dose: 6,000 mJ/cm2, wavelength: 365 nm) from a UV-LED light source.
3. Characterization
- J-V-L measurements
NOTE: The current density (J)-voltage (V)-luminance (L) (J-V-L) characteristics and Commission Internationale de l'Eclairage (CIE) coordinates were measured using a spectral photo detector equipped with a DC voltage current source monitor. The measurement system is controlled by a PC with a custom control software for data acquisition. The system was calibrated following manufacturer's protocol and measurements were performed in the dark under a black curtain.- Connect the terminals to the contacts of the device with alligator clips. Place the device on the measurement stage.
- Run the control software for data acquisition. The system controls the applied voltage and current over time and collects the emission spectra by the spectrometer through an optical fiber.
Representative Results
The electroluminescence (EL) spectra were used to calculate the CIE coordinates and CRI values (Figures 2, 4, 5). Photographic images of the emitting devices were collected to verify the whiteness of the emission (Figure 3).
The EL spectra of the amine doped PFD devices and the undoped PFD device are shown in Figure 2. The undoped PFD device showed blue emission that corresponds to PFD exciton emission. Meanwhile, the 2-TNATA and the DMFL-NPB doped devices showed longer wavelength emissions compared to the undoped PFD device. The emissions from the amine doped devices originated from exciplexes formed between the PFD and amines in electronically excited states.
The 2-TNATA and the DMFL-NPB doped devices showed white light emission as seen in the color photographs of the emitting devices (Figure 3). The changes in the CIE coordinates of the amine doped devices (doping ratios of PFD:amine = 1:0.25 and 1:1) are shown in Figure 4. The 2-TNATA doped device (PFD:2-TNATA = 1:0.25) showed CIE coordinates of (0.33, 0.43) and a Color Rendering Index (CRI) of Ra = 73 at Vturn-on = 3.5 V (Vturn-on is defined as the voltage required to produce a luminance of over 1 cd/cm2 during a voltage sweep measurement) and the DMFL-NPB doped device with the same ratio of PFD:DMFL-NPB (1:0.25) showed CIE coordinates of x = 0.23, y = 0.33, and a CRI of Ra = 54 at Vturn-on = 3.5 V. The emission color of the DMFL-NPB doped device was slightly blue shifted compared to that of the 2-TNATA doped device. This is due to a difference in the exciplex forming abilities of the amines with the PFD, with 2-TNATA having a greater ability to form exciplexes than DMFL-NPB.15
Figure 5 shows the changes in current density, luminance and CIE coordinates of the 2-TNATA doped device when a constant voltage of 5 V was applied. Immediately after applying the voltage, the device showed CIE coordinates of (0.27, 0.37) and a Ra of 67, and the emission color was almost unchanged and stable after 300 sec.
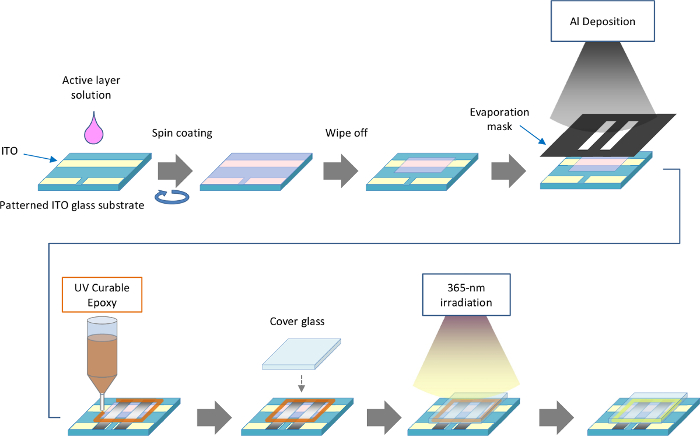
Figure 1. Fabrication process of LEC device. Please click here to view a larger version of this figure.
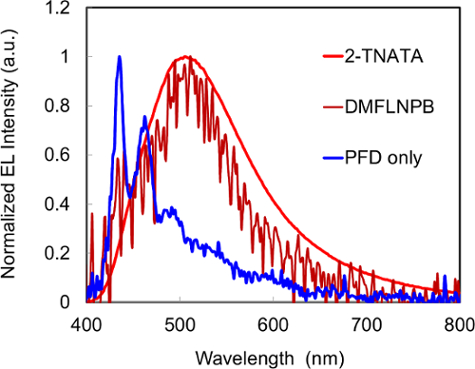
Figure 2. EL emission spectra of PLECs, 2-TNATA doped, DMFL-NPB doped and undoped devices. Please click here to view a larger version of this figure.
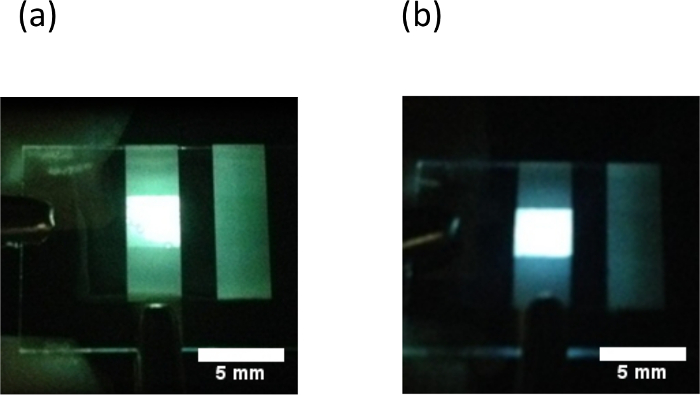
Figure 3. Photograph of light emission from the amine doped devices. Doping ratio of PFD:amine = 1:1. a) 2-TNATA doped device. b) DMFL-NPB doped device (scale bars: 5 mm). Please click here to view a larger version of this figure.
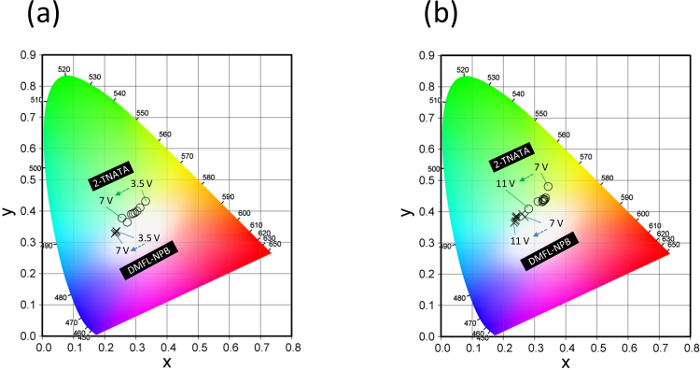
Figure 4. Changes in CIE coordinates of the 2-TNATA and the DMFL-NPD doped devices with increasing voltage. a) Devices with doping ratio of PFD:amine = 1:1. b) Devices with doping ratio of PFD:amine = 1:0.25. Please click here to view a larger version of this figure.
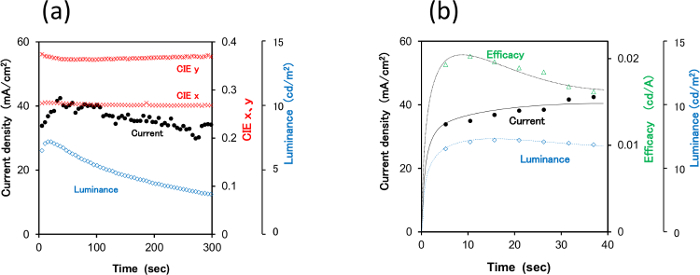
Figure 5. Temporal evolution of a) CIE coordinates, luminance and current, and b) efficacy, luminance, and current of the 2-TNATA doped PLECs. Please click here to view a larger version of this figure.
Discussion
The LEC has an active layer containing hydrophobic PFD and aromatic amines, and hydrophilic polyethylene oxide and KCF3SO3. Because these materials have very different solubilities, careful preparation of the spin coating solution is critical to avoid incomplete solvation. Each must be first dissolved separately and completely in solvents with sufficient solvating ability, then the solutions are mixed together to form a uniform mixture. Balancing the exciton and exciplex emissions is key to obtaining white emission. Therefore, the amounts of PFD and amines must be precisely measured.
In LECs it is also important to control the phase-separation morphology of the active layer. The authors tried using other ion conducting polymers such as trimethylolpropane ethoxylate (TMPE-OH)16 instead of PEO, but the device fabricated with TMPE-OH did not function as an LEC. The hydrophobic materials (PFD and aromatic amines) and the hydrophilic polymer electrolyte tend to phase-separate, meaning that materials must be selected carefully.
The UV-light used to cure the resin may damage the active layer material. Therefore, the UV-light is shined from the aluminum deposited side through a glass cover to avoid unnecessary exposure.
Compared to methods in which multiple light-emitting materials are used,10-14 the method described above has a major advantage in that white light emission can be obtained through just the addition of simple compounds such as aromatic amines. To produce high CRI white light, it will be necessary to obtain broader band emissions with a spectrum closer to sunlight. Because exciplexes generally produce broadband emissions, finding better combinations of blue light-emitting polymers and amines should make it possible to achieve these higher CRIs.
Figure 5 shows time evolution of luminance, current density, CIE coordinates and efficacy of the 2-TNATA-doped LEC applied at a constant voltage of 5 V. Figure 4b shows the typical behavior of an LEC, such as increasing luminance and current density and changes in efficacy during the first 30 seconds of operation.
The authors have thus demonstrated the fabrication procedure for PLECs with white light emission utilizing exciplex emissions originating from PFD and amines. The authors have also shown the stability of this white light emission, a property which is especially important for large area lighting applications.
Declarações
The authors have nothing to disclose.
Acknowledgements
This work was partially supported by a Grant-in-Aid for Scientific Research (No. 24225003). This work was supported financially by the JX Nippon Oil & Energy Corporation.
Materials
| Poly(9,9-di-n-dodecylfluorenyl-2,7-diyl) (PFD) | Aldrich | 571660 | |
| 4,4’,4’’-Tris[2-naphthyl(phenyl)amino]triphenylamine (2-TNATA) | Aldrich | 768669 | |
| 9,9-Dimethyl-N,N’-di(1-naphthyl)-N,N’-diphenyl-9H-fluorene-2,7-diamine (DMFL-NPB) | Aldrich | ||
| Poly(ethylene oxide) (PEO) | Aldrich | 182028 | |
| Potassium tirifluoromethansulfonate (KCF3SO3) | Aldrich | 422843 | dried under vacuum at 200 °C for 2 hr prior to use |
| Chloroform | Kanto Chemical Co. | 08097-25 | dehydrated |
| Cyclohexanone | Kanto Chemical Co. | 07555-00 | |
| SCAT 20-X (detergent) | Daiichi Kogyo Seiyaku | diluted with water | |
| Acetone | Kanto Chemical Co. | 01866-25 | Electronic grage |
| 2-propanol | Kanto Chemical Co. | 32439-75 | Electronic grage |
| 13mm GD/X Disposable Filter Device PVDF Filter Media, Polypropylene Housing | Whatman | 6872-1304 | |
| UV/O3 Treating Unit | SEN Lights Co. | SSP16-110 | |
| Spectral Photo Detector | Otsuka Electronics | MCPD 9800 | |
| Voltage Current Source Monitor | ADCMT | 6241A | |
| Evaporation Mask | Tokyo Process Service Co., Ltd. | NA | The evaporation mask was wet-etched to create openings for patterned deposition of aluminum. The size of the mask is 100 mm x 100 mm x 0.2 mm-thick. |
Referências
- Pei, Q., Yu, G., Zhang, C., Yang, Y., Heeger, A. J. Polymer light-emitting electrochemical cells. Science. 269 (5227), 1086-1088 (1995).
- Sun, Q., Li, Y., Pei, Q. Polymer light-emitting electrochemical cells for high-efficiency low-voltage electroluminescent devices. J. Disp. Technol. 3 (2), 211-224 (2007).
- Meier, S. B., et al. Light-emitting electrochemical cells: recent progress and future prospects. Mater. Today. 17 (5), 217-223 (2014).
- Edman, L., et al. Single-component light-emitting electrochemical cell fabricated from cationic polyfluorene: Effect of film morphology on device performance. J. Appl. Phys. 98 (4), 044502 (2005).
- Fang, J., Matyba, P., Edman, L. The Design and Realization of Flexible, Long-Lived Light-Emitting Electrochemical Cells. Adv. Funct. Mater. 19 (16), 2671-2676 (2009).
- Yu, Z., et al. Stabilizing the Dynamic p− i− n Junction in Polymer Light-Emitting Electrochemical Cells. J. Phys. Chem. Lett. 2 (5), 367-372 (2011).
- Sandström, A., Dam, H. F., Krebs, F. C., Edman, L. Ambient fabrication of flexible and large-area organic light-emitting devices using slot-die coating. Nat. Commun. 3, 1002 (2012).
- Liang, J., Li, L., Niu, X., Yu, Z., Pei, Q. Elastomeric polymer light-emitting devices and displays. Nat. Photonics. 7 (10), 817-824 (2013).
- Yang, Y., Pei, Q. Efficient blue-green and white light-emitting electrochemical cells based on poly 9, 9-bis (3, 6-dioxaheptyl)-fluorene-2, 7-diyl. J. Appl. Phys. 81 (7), 3294-3298 (1997).
- Tang, S., Buchholz, H. A., Edman, L. White Light from a Light-Emitting Electrochemical Cell: Controlling the Energy-Transfer in a Conjugated Polymer/Triplet-Emitter Blend. ACS Appl. Mater. Iterfaces. 7 (46), 25955-25960 (2015).
- Nishikitani, Y., Takizawa, D., Nishide, H., Uchida, S., Nishimura, S. White Polymer Light-Emitting Electrochemical Cells Fabricated Using Energy Donor and Acceptor Fluorescent π-Conjugated Polymers Based on Concepts of Band-Structure Engineering. J. Phys. Chem. C. 119 (52), 28701-28710 (2015).
- Sun, M., Zhong, C., Li, F., Cao, Y., Pei, Q. A Fluorene− Oxadiazole Copolymer for White Light-Emitting Electrochemical Cells. Macromolecules. 43 (4), 1714-1718 (2010).
- Tang, S., Pan, J., Buchholz, H., Edman, L. White Light-Emitting Electrochemical Cell. ACS Appl. Mater. Interfaces. 3 (9), 3384-3388 (2011).
- Tang, S., Pan, J., Buchholz, H. A., Edman, L. White light from a single-emitter light-emitting electrochemical cell. J. Am. Chem. Soc. 135 (9), 3647-3652 (2013).
- Nishikitani, Y., et al. White polymer light-emitting electrochemical cells using emission from exciplexes with long intermolecular distances formed between polyfluorene and π-conjugated amine molecules. J. Appl. Phys. 118 (22), 225501 (2015).
- Tang, S., Mindemark, J., Araujo, C. M. G., Brandell, D., Edman, L. Identifying Key Properties of Electrolytes for Light-Emitting Electrochemical Cells. Chem. Mater. 26 (17), 5083-5088 (2014).

