Palladium N-Heterocyclic Carbene Complexes: Synthesis from Benzimidazolium Salts and Catalytic Activity in Carbon-carbon Bond-forming Reactions
Summary
Detailed and generalized protocols are presented for the synthesis and subsequent purification of four palladium N-heterocyclic carbene complexes from benzimidazolium salts. The complexes were tested for catalytic activity in arylation and Suzuki-Miyaura reactions. For each reaction investigated, at least one of the four complexes successfully catalyzed the reaction.
Abstract
Detailed and generalized protocols are presented for the synthesis and subsequent purification of four palladium N-heterocyclic carbene complexes from benzimidazolium salts. Detailed and generalized protocols are also presented for testing the catalytic activity of such complexes in arylation and Suzuki-Miyaura cross-coupling reactions. Representative results are shown for the catalytic activity of the four complexes in arylation and Suzuki-Miyaura type reactions. For each of the reactions investigated, at least one of the four complexes successfully catalyzed the reaction, qualifying them as promising candidates for catalysis of many carbon-carbon bond-forming reactions. The protocols presented are general enough to be adapted for the synthesis, purification and catalytic activity testing of new palladium N-heterocyclic carbene complexes.
Introduction
N-heterocyclic carbenes (NHCs) have attracted much attention, particularly for their ability to catalyze various important reactions such as metathesis, creation of furan, polymerization, hydrosilylation, hydrogenation, arylation, Suzuki-Miyaura cross-coupling and Mizoroki-Heck cross-coupling1,2,3,4,5,6,7,8,9,10,11. NHCs can be coupled with metals; such metal-NHC complexes have been extensively used in transition metal-catalyzed reactions as ancillary ligands and organocatalysts12,13,14,15,16. Generally, they are extraordinarily stable against air, moisture and heat as a consequence of the high dissociation energies of metal-carbon coordination bonds17.
Here, the protocols for the previously-shown synthesis and purification of four benzimidazolium salts (compounds 1–4) and their palladium NHC complexes (compounds 5–8, respectively) are detailed18. The salts and complexes were previously characterized using various techniques18. Since similar compounds are used for catalysis of arylation and Suzuki-Miyaura cross-coupling reactions9,10,11, the protocols for testing the catalytic activity of the complexes in arylation and Suzuki-Miyaura reactions are also detailed. Importantly, the protocols for synthesizing, purifying and testing the catalytic activity of the complexes are presented general enough to allow easy adaptation to new palladium NHC complexes.
Protocol
Caution: Many volatile solvents are used as part of the protocols detailed below so carry out all experiments in a working fume hood. Wear appropriate personal protective equipment throughout and consult the MSDS of each reagent before use; herein, brief information has been provided about the hazardous reagents and steps.
1. Synthesis and purification of benzimidazolium salts (compounds 1-4)
- Clamp a 100 mL Schlenk tube upright and put a stirrer bar, 1 mmol of benzimidazole, 1 mmol of potassium hydroxide and 60 mL of ethyl alcohol as the solvent into it.
Caution: Potassium hydroxide can be harmful. Avoid breathing its dust and keep it away from water.
Caution: Ethyl alcohol is volatile and flammable. Keep it away from open flames or ignition sources.
Note: Sodium hydroxide can be used if potassium hydroxide is not available. Consult the MSDS of sodium hydroxide before proceeding with this suggested modification. - Place the Schlenk tube into an oil bath for even and safe heating of the reaction mixture during the stirring steps to come. Attach the tube to a condenser to prevent solvent evaporation during stirring. Make sure that the glass fitting parts are sufficiently greased and well-fitted.
- Stir the reaction mixture at 25 °C for 1 h to allow for complete dissolution of all the solids as well as the breaking of the nitrogen-hydrogen bond in benzimidazole molecules.
Note: Using a condenser for this stirring step is not essential but since a condenser must be used for refluxing in step 1.5 below, it can be convenient to set up the condenser at this step and use it for both steps. Otherwise, this step can be carried out by sealing the Schlenk tube with a greased stopper. - After 1 h, detach the Schlenk tube from the condenser and slowly add 1 mmol of the chosen aryl halide to the mixture.
Caution: Aryl halides are irritants and can be harmful. Consult the relevant MSDSs before proceeding. - Re-attach the Schlenk tube to the condenser and reflux the mixture at 78 °C (close to ethyl alcohol's boiling point) for 6 h to allow the reaction to reach completion. Let the mixture cool down to 25 °C after refluxing is finished.
- Detach the Schlenk tube from the condenser and use some paper towels to wipe the grease off the tube's mouth. Then, filter the reaction mixture using a funnel and filter paper to remove the potassium chloride precipitate that formed during the reaction. Collect the filtrate in a beaker.
- Transfer the filtrate, which contains the N-alkylbenzimidazole product, to a clean Schlenk tube. Seal the tube with a greased stopper and remove the ethyl alcohol solvent in the filtrate with vacuum.
Note: For all the steps in the protocol involving vacuum, use a vacuum of moderate strength as well as slight and continuous shaking of the tube attached to vacuum. - Once all of the solvent is removed, unseal the Schlenk tube and add 5 mL of diethyl ether to wash the N-alkylbenzimidazole product left behind. Gently shake the tube to perform washing.
- After washing is done, use some paper towels to wipe the grease off the tube's mouth and decant the ether into a beaker. Repeat this washing step a few times, adding 5 mL of diethyl ether and decanting it each time.
Caution: Diethyl ether is volatile and flammable. Keep it away from open flames or ignition sources.
Note: For all the washing steps in the protocol, another solvent can be used if it: 1) does not react with the substance being washed, 2) does not dissolve the substance being washed, and, 3) easily evaporates.
- After washing is done, use some paper towels to wipe the grease off the tube's mouth and decant the ether into a beaker. Repeat this washing step a few times, adding 5 mL of diethyl ether and decanting it each time.
- After the final washing step, seal the Schlenk tube with a greased stopper and dry the washed N-alkylbenzimidazole product with vacuum. After drying, use some paper towels to wipe the grease off the tube's mouth and then transfer the product to a small vial for use in the next reaction.
Note: The protocol can be paused here and resumed at a later time. - Clamp a clean Schlenk tube upright and expel the air inside it by purging it with argon gas. Introduce the gas from the sidearm of the tube and keep the mouth of the tube unsealed during this process. Argon is heavier than air so it will expel the air by filling the tube from the bottom up. Keep purging the tube with argon while adding the reagents in the next step.
- Slowly add a stirrer bar, 1 mmol of the N-alkylbenzimidazole, 1 mmol of the chosen alkyl halide and 4 mL of anhydrous N,N-dimethylformamide (DMF) as the solvent to the Schlenk tube. Once all the reagents are added, quickly seal the mouth of the tube with a greased stopper, then seal its sidearm by turning the stopcock and then turn off the argon gas.
Caution: Alkyl halides are irritants and can be harmful. Consult the relevant MSDS before proceeding.
Caution: DMF is flammable. Keep it away from open flames or ignition sources. - Place the sealed Schlenk tube into an oil bath and stir the reaction mixture at 80 °C for 24 h to allow the reaction to reach completion.
Note: This reaction needs to be carried out in an inert atmosphere so the abovementioned purging steps involving argon need to be carefully followed. - After 24 h, remove part of the DMF solvent in the mixture with vacuum; approximately 1-2 min of vacuuming should be sufficient.
Note: If wanted, remove all of the DMF solvent from the grease-like mixture but this is not necessary. - Unseal the Schlenk tube and add 15 mL of diethyl ether. Stir the mixture until the benzimidazolium salt product precipitates out.
Note: Petroleum ether can be used if diethyl ether is not available. Consult the MSDS of petroleum ether before proceeding with this suggested modification. - After precipitation occurs, remove the diethyl ether using an appropriate filtering method.
NOTE: We have used a special glass tube with a sidearm, an internal filter and two open ends to which Schlenk tubes can be attached; since the sidearm on this tube and those on the Schlenk tubes can be attached to vacuum, this filtering tube provides immense convenience for: 1) filtering after the precipitation step as well as the washing steps to come, and, 2) drying after the washing steps.- If using something similar, attach the filled Schlenk tube to one end of the filtering tube and an empty Schlenk tube to the other end. Then attach the empty Schlenk tube to vacuum, and, carefully and gradually invert the apparatus so that the diethyl ether passes through the filter to this empty Schlenk tube. If, however, such a tube cannot be found, use other methods such as filtration with a funnel and filter paper.
- Wash the salt product with 15 mL of diethyl ether and remove the diethyl ether using the same filtration method used in step 1.15. Repeat this washing step a few times, using 15 mL of diethyl ether and filtering it each time.
- After the final washing step, dry the washed salt product (here, dry inside the filter tube with vacuum) and then collect it for further purification through recrystallization.
Note: The protocol can be paused here and resumed at a later time. - Add the salt and an ethyl alcohol-diethyl ether mixture (12 mL:4 mL) to a clean Schlenk tube. Heat the mixture using a heat gun until the salt dissolves completely.
- Afterwards, seal the tube with a greased stopper and clamp it in an almost horizontal position. Leave the salt to recrystallize at room temperature.
- Once the salt is recrystallized, use some paper towels to wipe the grease off the tube's mouth and then filter the mixture using a funnel and filter paper to separate the salt crystals.
- Wash the salt crystals, while they are still on the filter paper in the funnel, with 15 mL of diethyl ether. Repeat this washing step a few times.
- After the final washing step, allow the crystals to dry in air on the filter paper. Collect the purified salt for characterization and the synthesis of the palladium NHC complex.
Note: The protocol can be paused here and resumed at a later time. - Characterize the salt as reported previously18.
2. Synthesis and purification of palladium NHC complexes (compounds 5-8)
- Clamp a 75 mL Schlenk tube upright and add a stirrer bar, 1 mmol of the chosen benzimidazolium salt, 1 mmol of palladium chloride, 5 mmol of potassium carbonate as a base and 3 mL of 3-chloropyridine into it.
Caution: Palladium chloride is toxic and can be an irritant.
Caution: Potassium carbonate can be harmful. Avoid breathing its dust and keep it away from water.
Caution: 3-chloropyridine is extremely harmful. It is toxic and corrosive. Avoid skin contact and breathing its fumes. - Seal the tube with a greased stopper and place it into an oil bath. Stir the reaction mixture at 80 °C for 16 h to allow the synthesis of the palladium NHC complex to reach completion.
- After 16 h, allow the mixture to cool down to room temperature and unseal the tube. Add 10 mL of dichloromethane to the mixture to improve the efficiency of the filtering described in steps 2.4 and 2.5 below; this is optional and can be skipped if desired.
Caution: Dichloromethane is toxic, an irritant and a suspected carcinogen. Avoid skin contact and breathing its fumes. - Assemble the following filtering apparatus to remove the unreacted palladium chloride and benzimidazolium salt from the reaction mixture: Use a glass filtering tube without a tap.
- First, add four spatulas of the filter agent (e.g., Celite) into the tube to make a filter agent layer above the filter that is in the middle of the tube. Then, add four spatulas of silica gel above the filter agent layer. Finally, squeeze a small cotton wad above the silica gel layer such that the filter agent and silica layers are fixed in place between the filter and the cotton wad.
- Filter the reaction mixture through the pad of filter agent and silica gel as follows: Attach the Schlenk tube containing the reaction mixture to the glass filtering tube such that the Schlenk tube faces the end of the filtering tube with the cotton wad. Then, attach an empty Schlenk tube to the other end of the filtering tube.
- Connect the empty Schlenk tube to vacuum, and, carefully and gradually invert the apparatus so the reaction mixture gets filtered through (in order) the cotton, silica, filter agent and filter layers. The unreacted palladium chloride and benzimidazolium salt will be retained in the layers while the filtrate containing the palladium NHC complex will enter the empty Schlenk tube.
Note: If dichloromethane is added to the reaction mixture (step 2.3), it can contribute some pressure inside the filtering tube and this may cause liquid to seep out from the connecting part between the filled Schlenk tube and the filtering tube upon inversion. To prevent this, it is important to connect the empty Schlenk tube to vacuum before inversion of the apparatus (as described above) so that upon inversion, the reaction mixture does not have enough time to seep out from the abovementioned connecting part.
- Connect the empty Schlenk tube to vacuum, and, carefully and gradually invert the apparatus so the reaction mixture gets filtered through (in order) the cotton, silica, filter agent and filter layers. The unreacted palladium chloride and benzimidazolium salt will be retained in the layers while the filtrate containing the palladium NHC complex will enter the empty Schlenk tube.
- Detach the Schlenk tube containing the filtrate from the filtering apparatus above and seal it with a greased stopper. Remove the solvent in the filtrate with vacuum.
- Once all of the solvent is removed, unseal the Schlenk tube and add 5 mL of diethyl ether to wash the palladium NHC complex product left behind. Gently shake the tube to perform washing. After washing is done, use some paper towels to wipe the grease off the tube's mouth and decant the ether into a beaker. Repeat this washing step a few times, adding 5 mL of diethyl ether and decanting it each time.
- After the final washing step, seal the Schlenk tube with a greased stopper and dry the washed palladium NHC complex product with vacuum. After drying, use some paper towels to wipe the grease off the tube's mouth and then collect the product for further purification through recrystallization.
Note: The protocol can be paused here and resumed at a later time. - For recrystallization, find an appropriate solvent for the specific palladium NHC complex (i.e. one that the complex does not readily dissolve in at room temperature but does so upon heating) and follow the same steps detailed above for the salts (steps 1.18 to 1.22). Afterwards, collect the purified complex for characterization.
Note: The protocol can be paused here and resumed at a later time. - Characterize the complex as reported previously18.
3. Catalytic activity of the complexes (5-8) in arylation reactions
- Carry out all catalytic reactions under air in a fume hood.
- Use the purchased reagents without further purification for carbon-carbon bond-forming reactions.
- Clamp a 25 mL Schlenk tube upright and add a stirrer bar, 2 mmol of 2-n-butylthiophene or 2-n-butylfuran and 1 mmol of the chosen aryl bromide into it.
Caution: 2-n-butylfuran and 2-n-butylthiophene are both acutely toxic. Avoid skin contact and breathing their fumes. - Then add 1 mmol of potassium acetate, 0.01 mmol of the chosen palladium NHC complex and 2 mL of N,N-dimethylacetamide (DMA) into the tube.
Caution: DMA is toxic. Avoid skin contact and breathing its fumes. - Seal the tube with a greased stopper and place it into an oil bath. Stir the reaction mixture for various times and at various temperatures to find the time and temperature conditions leading to maximum product yield for the given reaction.
Note: The progress of the reaction can be followed by thin layer chromatography (TLC) but if only comparing the effect of different reaction conditions on yield (including the palladium NHC complex used for catalysis), then running the reaction to completion is not necessary. In these cases, run the reaction for a constant amount of time less than the time required for completion and vary the reaction condition tested. Once the reaction has run for the amount of time wanted, stop it by removing the solvent from the reaction mixture as described in the next step.- To follow the progress of the reaction with TLC, compare the movement of the reaction mixture through a TLC plate with those of the reactants; if the mixture still produces the spots for the reactants, this means the reaction has not gone to completion yet. To get a sample of the reaction mixture after a given time, unseal the Schlenk tube while the reaction is still running and use a capillary tube to quickly obtain a drop for the TLC test. To run the mixture and reactants through the TLC plate, find an appropriate solvent (mobile phase) for the specific case.
- Once the reaction is complete or has run for the desired amount of time, remove the solvent in the reaction mixture with vacuum.
- Unseal the Schlenk tube and add a hexane-diethyl ether mixture (10 mL:2 mL) into it. This solvent mixture will be the mobile phase for flash column chromatography in steps 3.8 and 3.9 below. Shake the mixture vigorously to ensure that the product dissolves in the mobile phase and is not left behind in the tube.
Caution: Hexane is volatile and flammable. Avoid breathing its fumes and keep it away from open flames or ignition sources. - Assemble a flash chromatography column as follows to purify the product: Use a glass dropper. First, insert a small wad of cotton into the dropper and push it in until it rests firmly just where the glass chamber starts to thin out. Then, add silica gel on top of the cotton wad such that two thirds of the dropper's thick section is filled.
- Clamp the silica gel column upright and use a glass dropper to gradually transfer the reaction mixture into it. Elute the mixture through the column and collect the eluent containing the purified product in a clean beaker or test tube.
Note: The protocol can be paused here and resumed at a later time. - Transfer the eluent to a clean tube that can be attached to vacuum and seal the tube with a greased stopper. Remove the solvent in the eluent with vacuum.
Note: The protocol can be paused here and resumed at a later time. - Once all of the solvent is removed, unseal the tube and add 1.5 mL of dichloromethane. Gently shake the tube to dissolve the product and thereby allow its analysis with GC or GC/MS. Calculate the yield using GC or GC/MS19,20,21,22,23.
Note: Chloroform can be used if dichloromethane is not available. Consult the MSDS of chloroform before proceeding with this suggested modification.
4. Catalytic activity of the complexes (5-8) in Suzuki-Miyaura cross-coupling reactions
- Conduct all catalytic reactions according to the previously reported protocols18,24.
- Clamp a 25 mL Schlenk tube upright and add a stirrer bar, 1.5 mmol of phenylboronic acid or the chosen boronic acid derivative, 1 mmol of the chosen aryl chloride and 2 mmol of sodium tert-butoxide as a base into it.
Caution: Phenylboronic acid and its derivatives are irritants and can be toxic. Avoid skin contact. Consult the relevant MSDSs before proceeding.
Caution: Aryl chlorides are harmful and, depending on the specific chemical, can be toxic and flammable. Consult the relevant MSDSs before proceeding.
Caution: Sodium tert-butoxide is a flammable solid. It is highly reactive with water and caustic when in solution. Keep it away from open flames or ignition sources and avoid skin contact.
Note: Potassium hydroxide, sodium hydroxide, potassium carbonate, sodium carbonate, potassium acetate, sodium acetate or potassium tert-butoxide can be used if sodium tert-butoxide is not available. Consult the MSDSs of these bases before proceeding with these suggested modifications. - Add 0.01 mmol of the chosen palladium NHC complex to the tube.
- Add a DMF-water mixture (2 mL:2 mL) into the tube.
Note: If needed, use a higher ratio of DMF to water or use DMF on its own. - Seal the tube with a greased stopper and place it into an oil bath. Stir the reaction mixture for various times and at various temperatures to find the time and temperature conditions leading to maximum product yield for the given reaction.
Note: The progress of the reaction can be followed by TLC but if only comparing the effect of different reaction conditions on yield (including the palladium NHC complex used for catalysis), then running the reaction to completion is not necessary. In these cases, run the reaction for a constant amount of time less than the time required for completion and vary the reaction condition tested. Once the reaction has run for the amount of time wanted, stop it and proceed to the next step. To follow the progress of the reaction with TLC, please see step 3.5.1 for some details. - Once the reaction is complete or has run for the desired amount of time, allow the mixture to cool down to room temperature. Unseal the Schlenk tube and add a hexane-ethyl acetate mixture (5 mL:1 mL) to the reaction mixture. Re-seal the tube and shake the new mixture vigorously for a few minutes to allow the migration of the synthesized product to the hexane-ethyl acetate phase.
Caution: Ethyl acetate is volatile and flammable and can cause serious eye damage. Avoid breathing its fumes and keep it away from open flames or ignition sources. - Clamp the Schlenk tube upright and let the mixture settle into two distinct phases over the course of a few minutes.
- Use a glass dropper to carefully extract the top, organic phase and transfer it to a clean beaker containing 1 g of anhydrous magnesium sulfate. The magnesium sulfate powder will help remove any residual water from the extracted organic phase.
- Repeat steps 4.6 to 4.8 at least once to maximize extraction of the synthesized product.
- Follow steps 3.8 and 3.9 to purify the product with flash column chromatography. The hexane-ethyl acetate mixture present in the extracted organic phase will serve as the mobile phase for this purification step. Collect the eluent containing the purified product in a clean beaker or test tube.
Note: The protocol can be paused here and resumed at a later time. - Analyze the product and calculate the yield using GC or GC/MS19,20,21,22,23.
Representative Results
Benzimidazolium salts (1–4) (Figure 1) were synthesized in anhydrous DMF using N-alkylbenzimidazoles and various alkyl halides, then purified and characterized as reported before18,24. They were white or cream-colored solids and had yields ranging from 62% to 97%. Palladium NHC complexes (5–8) (Figure 2) were then synthesized from the salts, purified and characterized, also as reported before18,24. They were yellow or cream-colored solids and had lower yields than the salts, ranging from 25% to 60%. The four palladium complexes were tested for catalytic activity in arylation and Suzuki-Miyaura cross-coupling reactions.
Table 1 shows representative results regarding the catalytic effect of the palladium NHC complexes on the arylation reactions studied. The reaction between 2-n-butylthiophene and 4-bromoacetophenone (Table 1, entry 1) was given as an example to highlight the poor results obtained in arylation reactions in the absence of an appropriate catalyst; this particular reaction gave only a 1% yield after 1 h at 110 °C, in the absence of a catalyzing complex. For the reaction of 2-n-butylfuran with 4-bromoacetophenone, the complexes 5-8 led to yields of 14, 49, 83 and 89% respectively, after 1 h at 110 °C (Table 1, entries 2-5). Entries 6-8 in Table 1 show the reaction between 2-n-butylfuran and bromobenzene in the presence of complex 7; quite good yields of 71, 84 and 98% were achieved after 21 h at 80, 90 and 110 °C, respectively. The remaining 2 entries in Table 1 (entries 9 and 10) show the reaction of 2-n-butylthiophene with bromobenzene and 4-bromoanisole, respectively. The first of these reactions was catalyzed by complex 8, which allowed a yield of 97% to be achieved after 1 h at 110 °C (Table 1, entry 9). The second reaction was catalyzed by complex 5 to give a yield of 79% after 1 h at 130 °C (Table 1, entry 10).
The catalytic effect of the complexes on the studied Suzuki-Miyaura reactions between boronic acid derivatives and aryl chlorides was variable (Table 2). Here, the aim was to compare the performance of the four complexes in catalysis of these reactions, so for each of the reactions studied, the other reaction conditions were kept constant: a 2 mL:2 mL DMF-water mixture was used as the solvent, sodium tert-butoxide was used as the base, reactions were run for 2 h and reaction temperature was kept at 80 °C. Under these conditions, the complexes 5-8 respectively resulted in conversions of 67, 55, 77 and 25%, and, yields of 56, 51, 59 and 9% for the reaction of 2,5-dimethoxyphenylboronic acid with 4-methoxy-1-chlorobenzene (Table 2, entries 1-4). For the reaction of 4-tert-butylphenylboronic acid with 4-chlorotoluene under these conditions, all four complexes 5-8 proved to be excellent catalysts, resulting in conversions of 99, 99, 98 and 100%, and, yields of 92, 95, 93 and 99.9%, respectively (Table 2, entries 5-8). Finally, for the reaction of thianaphthene-2-boronic acid with 1-chloro-4-nitrobenzene under these conditions, complexes 5-8 respectively resulted in conversions of 5, 9, 55 and 30%, and, yields of 3, 1, 35 and 14% (Table 2, entries 9-12).
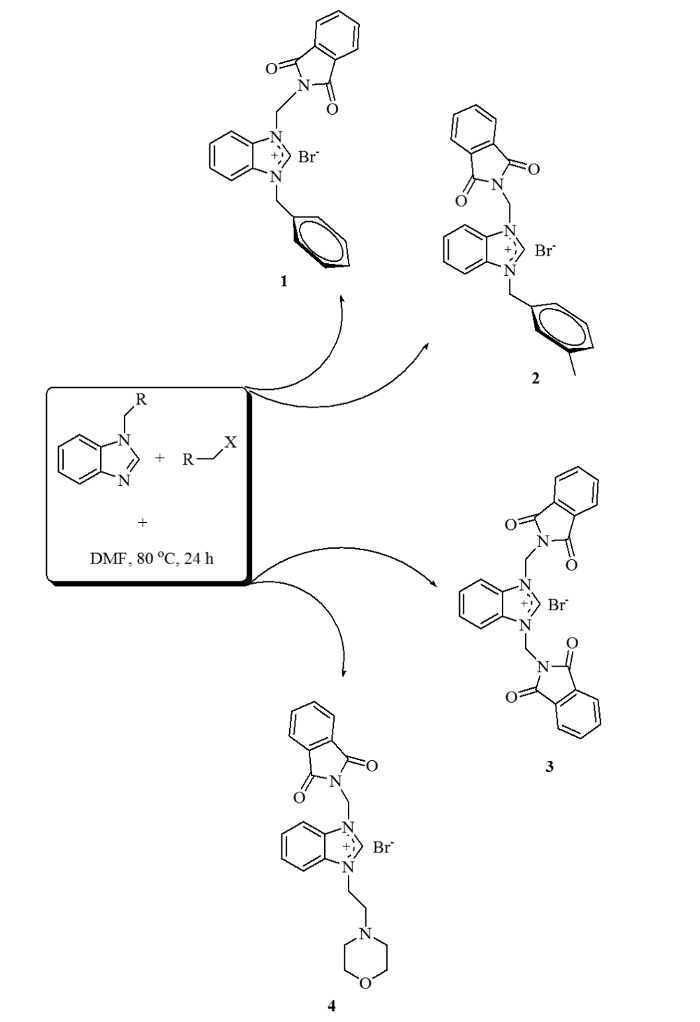
Figure 1: Synthesis of the benzimidazolium salts.
Schematic of the reactions between 1-alkylbenzimidazole and various alkyl halides to form benzimidazolium salts 1-4. Please click here to view a larger version of this figure.
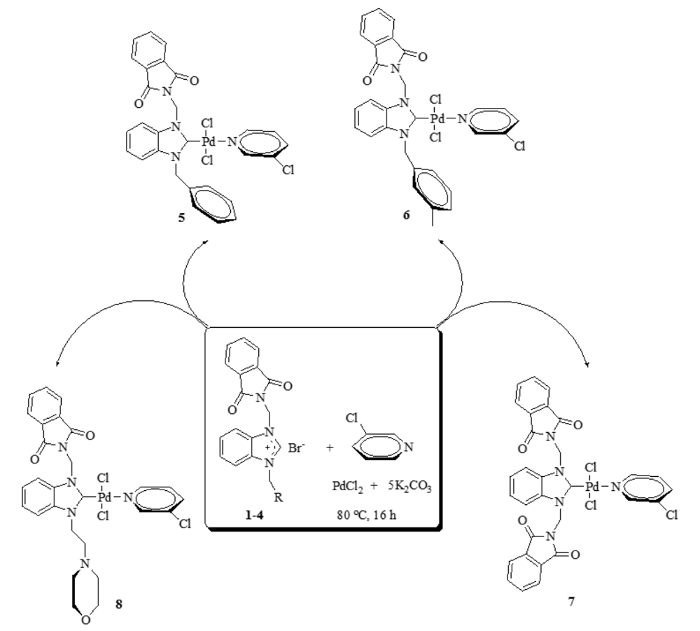
Figure 2: Synthesis of the palladium NHC complexes.
Schematic of the reactions between benzimidazolium salts 1-4, palladium chloride, potassium carbonate and 3-chloropyridine to form palladium NHC complexes 5-8. Please click here to view a larger version of this figure.
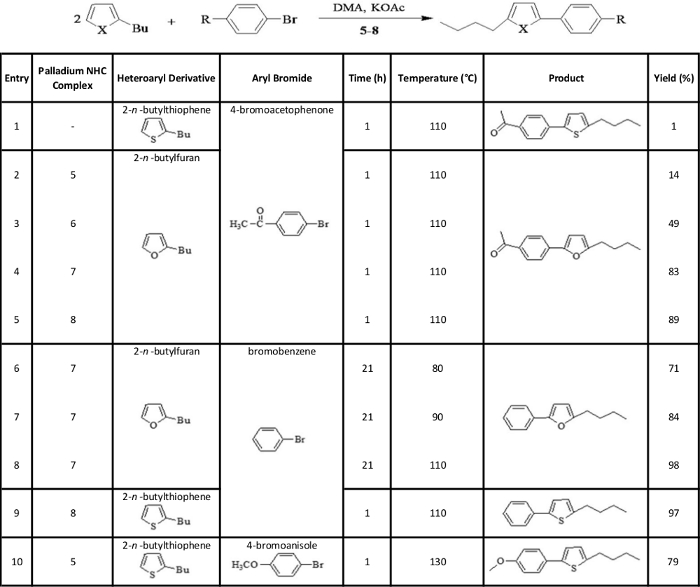
Table 1: Catalyzed arylation reactions – representative results.
Arylation of heteroaryl derivatives with various aryl bromides in the presence of the synthesized palladium NHC complexes. Reaction conditions: 2-n-butylthiophene or 2-n-butylfuran (2 mmol), aryl bromide (4-bromoacetophenone, bromobenzene or 4-bromoanisole) (1 mmol), palladium NHC complex (5-8) (0.01 mmol), potassium acetate (1 mmol), DMA (2 mL), 80-130 °C, 1-21 h. Please click here to view a larger version of this table.
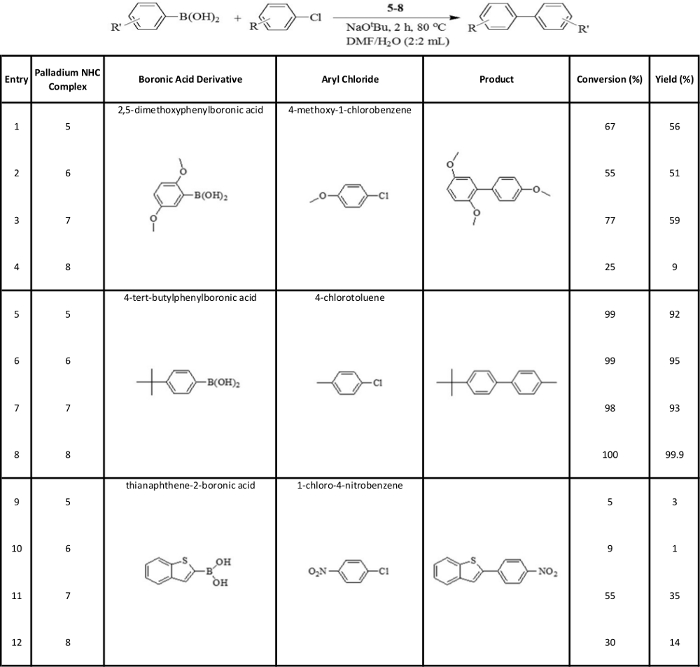
Table 2: Catalyzed Suzuki-Miyaura reactions – representative results.
Suzuki-Miyaura cross-coupling reactions of boronic acid derivatives with aryl chlorides in the presence of the synthesized palladium NHC complexes. Reaction conditions: boronic acid derivative (1.5 mmol), aryl chloride (1 mmol), sodium tert-butoxide (2 mmol), palladium NHC complex (5-8) (0.01 mmol), DMF-water (2 mL:2 mL), 80 °C, 2 h. Please click here to view a larger version of this table.
Discussion
The protocols for the synthesis and purification of four benzimidazolium salts and subsequently their palladium NHC complexes were deliberately presented in utmost detail to help young scientists or those new to the field master them. With this same goal in mind, the protocols for testing the catalytic activity of the four complexes in arylation and Suzuki-Miyaura reactions were also presented in utmost detail. Furthermore, we have attempted to present the protocols in as general a form as possible to allow others to easily adapt them for the synthesis, purification and testing of the catalytic activity of many other/new palladium NHC complexes.
If necessary, the protocols are open to some modifications. Suggestions for possible modifications have been given in the Protocol section under the relevant steps. Some of these suggestions are about the omission of certain protocol steps highlighted as optional, while others are about exchanging the equipment or reagents used in certain steps of the protocols. With respect to the modification of the reagents, it is possible, in principle, to replace some of the reagents used in the protocols with others but we have limited our suggestions in this regard to only those examples that we have verified experimentally or through brief surveying of the literature.
Regarding the catalytic activity of the synthesized complexes, their value for the catalysis of arylation reactions can be seen through the representative results in Table 1. For catalysis of the reaction between 2-n-butylfuran and 4-bromoacetophenone, complex 6 was a good candidate while complexes 7 and 8 performed particularly well (Table 1, entries 2-5). Complex 7 was an excellent catalyst for the reaction between 2-n-butylfuran and bromobenzene (Table 1, entries 6-8); the positive effect of increased temperature on yield for this reaction shows that if the reaction is catalyzed by an appropriate complex, modifying other reaction conditions such as temperature can help maximize the yield. For the reaction of 2-n-butylthiophene with bromobenzene, complex 8 was an excellent catalyst (Table 1, entry 9), while for the reaction between 2-n-butylthiophene and 4-bromoanisole, complex 5 performed quite well as a catalyst (Table 1, entry 10). Overall, each of the arylation reactions studied was catalyzed well by at least one of the four complexes synthesized. Further work can be done to potentially increase the yield values for these reactions by modifying reaction conditions such as time and temperature.
For catalysis of the Suzuki-Miyaura reactions between boronic acid derivatives and aryl chlorides, the synthesized complexes showed variable performance under the reaction conditions used in this study (Table 2). Complexes 5-7 proved to be good candidates, while complex 8 did not perform well for catalysis of the reaction between 2,5-dimethoxyphenylboronic acid and 4-methoxy-1-chlorobenzene (Table 2, entries 1-4). All four complexes were excellent catalysts for the reaction between 4-tert-butylphenylboronic acid and 4-chlorotoluene (Table 2, entries 5-8). For the reaction of thianaphthene-2-boronic acid with 1-chloro-4-nitrobenzene, complexes 5 and 6 did not perform well as catalysts, while complexes 7 and 8 showed some promise (Table 2, entries 9-12). Overall, just like the results for the arylation reactions, each of the Suzuki-Miyaura reactions studied was catalyzed well by at least one of the four complexes synthesized. For those cases where the chosen complex performed well in catalyzing the given reaction, further work can be done to potentially increase conversion and yield values by varying reaction conditions like time, temperature, solvent composition and the base used.
In summary, the four palladium NHC complexes can be easily synthesized by following the detailed protocols given and proved to be promising candidates for the catalysis of many carbon-carbon bond-forming reactions.
Declarações
The authors have nothing to disclose.
Acknowledgements
We acknowledge the financial support by Faculty of Pharmacy (The University of Sydney), Erciyes University Research Fund and TUBITAK (1059B141400496). We thank Tim Harland (The University of Sydney) for editing the video.
Materials
| 1-chloro-4-nitrobenzene | Sigma-Aldrich (Interlab A.S., USA) | ||
| 2,5-dimethoxyphenylboronic acid | Sigma-Aldrich (Interlab A.S., USA) | ||
| 2-n-butylfuran | Sigma-Aldrich (Interlab A.S., USA) | ||
| 2-n-butylthiophene | Sigma-Aldrich (Interlab A.S., USA) | ||
| 3-chloropyridine | Merck (Darmstadt, Germany) | ||
| 4-bromoacetophenone | Merck (Darmstadt, Germany) | ||
| 4-bromoanisole | Sigma-Aldrich (Interlab A.S., USA) | ||
| 4-chlorotoluene | Sigma-Aldrich (Interlab A.S., USA) | ||
| 4-methoxy-1-chlorobenzene | Merck (Darmstadt, Germany) | ||
| 4-tert-butylphenylboronic acid | Sigma-Aldrich (Interlab A.S., USA) | ||
| Benzimidazole | Merck (Darmstadt, Germany) | ||
| Bromobenzene | Merck (Darmstadt, Germany) | ||
| Celite | Merck (Darmstadt, Germany) | ||
| Dichloromethane | Merck (Darmstadt, Germany) | ||
| Diethyl ether | Sigma-Aldrich (Interlab A.S., USA) | ||
| Ethyl acetate | Sigma-Aldrich (Interlab A.S., USA) | ||
| Ethyl alcohol | Merck (Darmstadt, Germany) | ||
| Hexane | Merck (Darmstadt, Germany) | ||
| Magnesium sulfate | Scharlau (Barcelona, Spain) | ||
| N,N-dimethylacetamide | Merck (Darmstadt, Germany) | ||
| N,N-dimethylformamide | Merck (Darmstadt, Germany) | ||
| Palladium chloride | Merck (Darmstadt, Germany) | ||
| Phenylboronic acid | Sigma-Aldrich (Interlab A.S., USA) | ||
| Potassium acetate | Merck (Darmstadt, Germany) | ||
| Potassium carbonate | Scharlau (Barcelona, Spain) | ||
| Potassium hydroxide | Merck (Darmstadt, Germany) | ||
| Silica gel | Merck (Darmstadt, Germany) | ||
| Sodium tert-butoxide | Merck (Darmstadt, Germany) | ||
| Thianaphthene-2-boronic acid | Sigma-Aldrich (Interlab A.S., USA) |
Referências
- Akkoc, S., Gok, Y. Synthesis and characterization of 1-phenyl-3-alkylbenzimidazol-2-ylidene salts and their catalytic activities in the Heck and Suzuki cross-coupling reactions. J. Coord. Chem. 66 (8), 1396-1404 (2013).
- Aktas, A., Akkoc, S., Gok, Y. Palladium catalyzed Mizoroki-Heck and Suzuki-Miyaura reactions using naphthalenomethyl-substituted imidazolidin-2-ylidene ligands in aqueous media. J. Coord. Chem. 66 (16), 2901-2909 (2013).
- Cetinkaya, B., Alici, B., Ozdemir, I., Bruneau, C., Dixneuf, P. H. 2-imidazoline and 1,4,5,6-tetrahydropyrimidine-ruthenium(II) complexes and catalytic synthesis of furan. J. Organomet. Chem. 575 (2), 187-192 (1999).
- Chouthaiwale, P. V., Rawat, V., Sudalai, A. Pd-catalyzed selective hydrosilylation of aryl ketones and aldehydes. Tetrahedron Lett. 53 (2), 148-150 (2012).
- Herrmann, W. A. N-heterocyclic carbenes: A new concept in organometallic catalysis. Angew. Chem. Int. Ed. 41 (8), 1290-1309 (2002).
- Jensen, T. R., Schaller, C. P., Hillmyer, M. A., Tolman, W. B. Zinc N-heterocyclic carbene complexes and their polymerization of D,L-lactide. J. Organomet. Chem. 690 (24-25), 5881-5891 (2005).
- Lai, Y. B., Lee, C. S., Lin, W. J., Naziruddin, A. R., Hwang, W. S. Bis-chelate N-heterocyclic tetracarbene Ru(II) complexes: Synthesis, structure, and catalytic activity toward transfer hydrogenation of ketones. Polyhedron. 53, 243-248 (2013).
- Savka, R. D., Plenio, H. A hexahydro-s-indacene based NHC ligand for olefin metathesis catalysts. J. Organomet. Chem. 710, 68-74 (2012).
- Yigit, M., Yigit, B., Gok, Y. Synthesis of novel palladium(II) N-heterocyclic carbene complexes and their catalytic activities in the direct C5 arylation reactions. Inorg. Chim. Acta. 453, 23-28 (2016).
- Yasar, S., Sahin, C., Arslan, M., Ozdemir, I. Synthesis, characterization and the Suzuki-Miyaura coupling reactions of N-heterocyclic carbene-Pd(II)-pyridine (PEPPSI) complexes. J. Organomet. Chem. 776, 107-112 (2015).
- Ozdemir, I., et al. N-Heterocyclic carbenes: Useful ligands for the palladium-catalysed direct C5 arylation of heteroaromatics with aryl bromides or electron-deficient aryl chlorides. Eur. J. Inorg. Chem. 12 (12), 1798-1805 (2010).
- Clavier, H., Nolan, S. P. N-heterocyclic carbene and phosphine ruthenium indenylidene precatalysts: A comparative study in Olefin metathesis. Chem. Eur. J. 13 (28), 8029-8036 (2007).
- Johnson, J. S. Catalyzed reactions of acyl anion equivalents. Angew. Chem. Int. Ed. 43 (11), 1326-1328 (2004).
- Marion, N., Diez-Gonzalez, S., Nolan, S. P. N-heterocyclic carbenes as organocatalysts. Angew. Chem. Int. Ed. 46 (17), 2988-3000 (2007).
- Perry, M. C., Burgess, K. Chiral N-heterocyclic carbene-transition metal complexes in asymmetric catalysis. Tetrahedron: Asymmetry. 14 (8), 951-961 (2003).
- Zeitler, K. Extending mechanistic routes in heterazolium catalysis-promising concepts for versatile synthetic methods. Angew. Chem. Int. Ed. 44 (46), 7506-7510 (2005).
- Schwarz, J., et al. N-Heterocyclic carbenes, part 25 – Polymer-supported carbene complexes of palladium: Well-defined, air-stable, recyclable catalysts for the Heck reaction. Chem. Eur. J. 6 (10), 1773-1780 (2000).
- Akkoc, S., Gok, Y., Ilhan, I. O., Kayser, V. N-Methylphthalimide-substituted benzimidazolium salts and PEPPSI Pd-NHC complexes: synthesis, characterization and catalytic activity in carbon-carbon bond-forming reactions. Beilstein J. Org. Chem. 12, 81-88 (2016).
- Karaca, E. O., et al. Palladium complexes with tetrahydropyrimidin-2-ylidene ligands: Catalytic activity for the direct arylation of furan, thiophene, and thiazole derivatives. Organometallics. 34 (11), 2487-2493 (2015).
- Ozdemir, I., et al. N-Heterocyclic carbene-palladium catalysts for the direct arylation of pyrrole derivatives with aryl chlorides. Beilstein J. Org. Chem. 9, 303-312 (2013).
- Senocak, A., et al. Synthesis, crystal structures, magnetic properties and Suzuki and Heck coupling catalytic activities of new coordination polymers containing tetracyanopalladate(II) anions. Polyhedron. 49 (1), 50-60 (2013).
- Akkoc, S., Gok, Y. Dichlorido(3-chloropyridine-N) 1,3-dialkylbenzimidazol-2-ylidene palladium(II) complexes: Synthesis, characterization and catalytic activity in the arylation reaction. Inorg. Chim. Acta. 429, 34-38 (2015).
- Akkoc, S., Gok, Y. Catalytic activities in direct arylation of novel palladium N-heterocyclic carbene complexes. Appl. Organomet. Chem. 28 (12), 854-860 (2014).
- Akkoc, S., Gok, Y., Ilhan, I. O., Kayser, V. In situ Generation of Efficient Palladium N-heterocyclic Carbene Catalysts Using Benzimidazolium Salts for the Suzuki-Miyaura Cross-coupling Reaction. Curr. Org. Synth. 13 (5), 761-766 (2016).

