The Power of Simplicity: Sea Urchin Embryos as in Vivo Developmental Models for Studying Complex Cell-to-cell Signaling Network Interactions
Summary
This video article details a straightforward in vivo methodology that can be used to systematically and efficiently characterize components of complex signaling pathways and regulatory networks in many invertebrate embryos.
Abstract
Remarkably few cell-to-cell signal transduction pathways are necessary during embryonic development to generate the large variety of cell types and tissues in the adult body form. Yet, each year more components of individual signaling pathways are discovered, and studies indicate that depending on the context there is significant cross-talk among most of these pathways. This complexity makes studying cell-to-cell signaling in any in vivo developmental model system a difficult task. In addition, efficient functional analyses are required to characterize molecules associated with signaling pathways identified from the large data sets generated by next generation differential screens. Here, we illustrate a straightforward method to efficiently identify components of signal transduction pathways governing cell fate and axis specification in sea urchin embryos. The genomic and morphological simplicity of embryos similar to those of the sea urchin make them powerful in vivo developmental models for understanding complex signaling interactions. The methodology described here can be used as a template for identifying novel signal transduction molecules in individual pathways as well as the interactions among the molecules in the various pathways in many other organisms.
Introduction
Gene regulatory networks (GRNs) and signal transduction pathways establish the spatial and temporal expression of genes during embryonic development that are used to build the adult animal body plan. Cell-to-cell signal transduction pathways are essential components of these regulatory networks, providing the means by which cells communicate. These cellular interactions establish and refine the expression of regulatory and differentiation genes in and among the various territories during embryogenesis1,2. Interactions among secreted extracellular modulators (ligands, antagonists), receptors, and co-receptors control the activities of signal transduction pathways. An assortment of intracellular molecules transduces these inputs resulting in altered gene expression, division, and/or shape of a cell. While many of the key molecules used at the extracellular and intracellular levels in the major pathways are known, it is an incomplete knowledge due in large part to the complexity of individual signaling pathways. In addition, different signaling pathways often interact with one another either positively or negatively at the extracellular, intracellular, and transcriptional levels3,4,5,6. Importantly, the core components of signal transduction pathways are highly conserved in all metazoan species, and, remarkably, most of the major signaling pathways often perform similar developmental functions in many species when comparing organisms from closely related phyla in particular 7,8,9,10,11.
The study of signaling during development is a daunting task in any organism, and there are several significant challenges to studying signaling pathways in most deuterostome models (vertebrates, invertebrate chordates, hemichordates, and echinoderms): 1) In vertebrates there are large numbers of possible ligand and receptor/co-modulator interactions, intracellular transduction molecules, as well as potential interactions among different signaling pathways due to the complexity of the genome12,13,14; 2) The complex morphology and morphogenetic movements in vertebrates often make it more difficult to interpret functional interactions in and among signal transduction pathways; 3) Analyses in most non-echinoderm invertebrate deuterostome model species are limited by short windows of gravidity with the exception of some tunicate species15,16.
The sea urchin embryo has few of the above-mentioned limitations and offers many unique qualities for performing a detailed analysis of signal transduction pathways in vivo. These include the following: 1) The relative simplicity of the sea urchin genome significantly reduces the number of possible ligand, receptor/co-receptor and intracellular transduction molecule interactions17; 2) The GRNs controlling the specification and patterning of the germ layers and major embryonic axes are well established in sea urchin embryos, aiding in the understanding of the regulatory context of the cell/territory receiving the signals18,19; 3) Many signal transduction pathways can be studied between early cleavage and gastrula stages when embryos consist of a single layered epithelium whose morphology is easier to analyze; 4) The molecules involved in signaling pathways in the sea urchins are easily manipulated; 5) Many sea urchins are gravid for 10 to 11 months a year (e.g. Strongylocentrotus purpuratus and Lytechinus variegatus).
Here, we present a method to systematically and efficiently characterize components of the signaling pathways that specify and pattern territories in sea urchin embryos to illustrate the advantages that several invertebrate model systems offer in the study of complex molecular mechanisms.
Protocol
1. High Throughput Morpholino Design Strategy
- Identify a gene(s) of interest (e.g. candidate gene approach, cis-regulatory analysis, RNAseq and/or proteomic differential screens).
- Use genomic, transcriptomic, and gene expression data available on frequently updated websites (e.g. SpBase http://www.echinobase.org20 and S. purpuratus Genome Search http:///urchin.nidcr.nih.gov/blast/index.html) to determine that the spatiotemporal expression profile overlaps with the developmental mechanism in question. If no expression data is available, then generate qPCR primers and/or an antisense in situ probe to assess the gene expression pattern.
- After determining the spatial and temporal expression pattern, obtain the DNA sequence from the genomic web sites.
- For generating translation-blocking morpholino oligonucleotides, obtain sequence of the 5' un-translated region (5' UTR) directly upstream from the initiation codon from expressed sequence tag (EST) databases available on SpBase or S. purpuratus Genome Search websites. If this information is unavailable for the gene of interest, then perform 5' RACE.
- To design a splice-blocking morpholino, search for the genomic sequence including the exons and introns using the scaffold blastn tool in SpBase or S. purpuratus Genome Search tool.
- Use the oligonucleotide designing website http://oligodesign.gene-tools.com in order to design the desired 25 base pair morpholino sequence.
- For a translational-blocking morpholino, use the mRNA sequence from 5' to 3' as the source of translational target sequence. Include the 5' UTR sequence (approximately 70 nucleotides upstream of the transcription start site) plus 25 bases of coding region (downstream of the start site) with the start codon marked with parenthesis (ATG).
- For a splice-blocking morpholino, select intron-exon or exon-intron boundary sequence and include 50 bases (25 bases of exon sequence and 25 bases of intron sequence) around the boundary regions. Scan the genome with the morpholino sequence to verify that the sequence is unique.
2. Microinjection of Morpholino Oligonucleotides
- Prepare 3 mM stock solutions of the morpholino oligonucleotides by adding 100 µL of nuclease-free water into the 300 nmol morpholino vial.
NOTE: Do not use DEPC treated water for the re-suspension because diethyl pyrocarbonate can damage the morpholino. - For the first reconstitution spin down the vial containing the stock oligonucleotide solution for 30 s at full speed (14,000 – 16,000 x g), briefly vortex, heat at 65 °C for 5 to 10 min, briefly vortex, and keep the morpholino stock at room temperature for at least 1 h. Morpholino stock solutions are stored from -20 °C to +4 °C.
- Prepare injection solution containing morpholino oligonucleotides at the desired concentration. This solution usually contains 20% glycerol or 125 mM KCl as a carrier and 15% FITC-Dextran (fluorescein isothiocyanate dextran 10,000 MW 2.5 mg/μL stock solution). FITC-Dextran and other fluorescent dextran conjugates are routinely used to identify injected embryos by epifluorescence microscopy. Store injection solutions at -20 °C.
- Heat the morpholino solution at 65 °C in a heat block or water bath for at least 2 – 5 min.
- Briefly spin the morpholino solution for 30 s at full speed (14,000 – 16,000 x g), vortex for 1 min and centrifuge at full speed (14,000 – 16,000 x g) for at least 10 min.
- Load the injection needles with the morpholino solution. For a detailed microinjection protocol please see Stepicheva and Song, 201421 and Cheers and Ettensohn, 200422.
3. Fixation and In Situ Protocol at 24 h Post-fertilization (hpf) in S. purpuratus Embryos
NOTE: This protocol is modified from Arenas-Mena et al., 200023 and Sethi et al., 201424.
- Fixation
- Add several drops of fixative (see below) to wells containing embryos, mix gently by pipetting, and allow them to settle. Remove the fixative solution, and then mix embryos with two additional 180 µL washes of fixative.
NOTE: It is important to thoroughly mix the embryos during the washes by gentle pipetting up and down several times. Failure to do so can lower the signal to noise ratio. - Leave the embryos to fix in the second fixative wash for 50 min to 1 h at room temperature (RT) in 4% electron microscopy grade paraformaldehyde consisting of 10 mM MOPS pH 7.0, 0.1% Tween-20, and artificial seawater (ASW). Make this solution fresh each time for best results. In addition, for ease and practicality embryos are fixed in 96-well plates.
- For 20 mL fixative use 5 mL 16% paraformaldehyde, 15 mL ASW, 200 µL 1 M MOPS pH 7.0, and 20 µL Tween-20.
- Wash 5 times at RT with 180 µL of MOPS wash buffer consisting of 0.1 M MOPS pH 7.0, 0.5 M NaCl and 0.1% Tween-20 for at least 5 min or until embryos drop to the bottom of the well. Again, It is important to thoroughly mix the embryos into the wash buffer by gently pipetting up and down several times. MOPS wash buffer can be used for 2 days if stored at 4 °C.
- For 40 mL MOPS wash buffer use 4 mL 1 M MOPS pH 7.0, 4 mL 5 M NaCl, 32 mL dH2O, and 40 µL Tween-20.
- Fixed embryos can be stored at 4 oC for 2 days.
NOTE: If storing fixed embryos longer, then add 0.2% sodium azide in MOPS wash buffer to prevent bacterial growth.
- Add several drops of fixative (see below) to wells containing embryos, mix gently by pipetting, and allow them to settle. Remove the fixative solution, and then mix embryos with two additional 180 µL washes of fixative.
- Pre-hybridization
- Aspirate the MOPS wash buffer and add 180 μL of a 2:1 ratio of MOPS wash buffer to hybridization buffer, mix embryos into the solution by gentle pipetting several times, and incubate for at least 20 min at RT. Hybridization buffer consists of 70% formamide, 0.1 M MOPS pH 7.0, 0.5 M NaCl, 1 mg/mL BSA and 0.1% Tween-20.
- For 40 mL hybridization buffer use 4 mL 1 M MOPS pH 7.0, 4 mL 5 M NaCl, 4 mL dH2O, 0.04 g BSA, and 40 µL Tween-20. Mix well by vortexing, add 28 mL formamide, and vortex again.
- Remove the 2:1 ratio of MOPS wash and hybridization buffers and add 180 µL of a 1:2 ratio of MOPS wash buffer to hybridization buffer, mix embryos into the solution, and incubate for at least 20 min at RT.
- Before probe hybridization gently mix embryos into 100 – 150 µL of hybridization buffer. Incubate embryos at 50 °C for at least 1 h.
NOTE: Incubating embryos overnight is acceptable. Before incubating, seal the wells with an adhesive sheet in order to prevent evaporation.
- Aspirate the MOPS wash buffer and add 180 μL of a 2:1 ratio of MOPS wash buffer to hybridization buffer, mix embryos into the solution by gentle pipetting several times, and incubate for at least 20 min at RT. Hybridization buffer consists of 70% formamide, 0.1 M MOPS pH 7.0, 0.5 M NaCl, 1 mg/mL BSA and 0.1% Tween-20.
- Hybridization
- In a separate tube add 0.1 – 0.3 ng/µL of probe and 500 µg/mL yeast tRNA into hybridization buffer, then vortex gently to create probe solution. Yeast tRNA is added to the probe solution to decrease non-specific binding of the anti-sense probe. Preheat this solution to 50 °C, and aspirate hybridization buffer.
- Mix pre-hybridized embryos into 100 µL of the probe solution, seal with an adhesive sheet, and hybridize at 50 °C for 2 to 7 days depending on the probe (the foxq2 probe can be incubated for 2-days).
NOTE: Incubation times will vary based off the probe. Some genes expressed at low levels require up to a 7-day incubation. 96-well plates can be placed in a humidity box as insurance against evaporation if the adhesive sheet fails to seal properly. - Wash 5 times at 50 °C with 180 μL of freshly made MOPS wash buffer for a total of 3 h. Then, wash 3 times (15 min) with 180 µL of MOPS wash buffer at RT. Remember to mix embryos into the wash buffer each time by gently pipetting several times.
- Antibody Incubation
- Aspirate the MOPS wash buffer and mix embryos in 180 µL of blocking buffer consisting of 10% Normal Sheep Serum and 5 mg/mL BSA in MOPS wash buffer and incubate for at least 45 min at RT or 4 °C overnight.
- Remove blocking buffer and then mix embryos into blocking buffer containing a 1:1,500 dilution of alkaline phosphatase-conjugated anti-digoxigenin antibody diluted in blocking buffer. Incubate overnight at RT in a sealed plate to avoid evaporation. NOTE: Do not leave antibody on longer than overnight.
- Wash embryos 6 times (5 min or until embryos drop) in MOPS wash buffer at RT. Embryos can be stored overnight at 4 °C.
- In situ development
- Wash embryos 3 times (10 min) at RT with freshly made pH 9.5 buffer consisting of 0.1 M Tris pH 9.5, 100 mM NaCl, 50 mM MgCl2, 1 mM Levamisole, and 0.1% Tween-20.
- For 20 mL pH 9.5 buffer use 2 mL 1 M Tris pH 9.5, 400 µL 5M NaCl, 1 mL 1 M MgCl2, 20 µL Tween-20, 16.6 mL dH2O, and 0.0048 g Levamisole.
- Incubate embryos at 37 °C in staining buffer protected from light. Staining buffer consists of 10% Dimethyl Formamide, 4.5 µL/mL 4-Nitro blue tetrazolium chloride (NBT), and 3.5 µL/mL 5-Bromo-4-chloro-3-indolyl-phosphate (BCIP) in freshly made pH 9.5 buffer.
- For 1 mL of staining buffer use 100 µL Dimethyl Formamide, 4.5 µL NBT, and 3.5 µL BCIP.
- Stop the alkaline phosphatase reaction by washing 3 to 5 times in MOPS wash buffer. The reaction with the foxq2 probe typically takes 30 min to 1 h. Some probes may need overnight incubation at either RT or 4 °C.
- Mix embryos into a solution of 70% MOPS wash and 30% glycerol. The glycerol provides a refractive index necessary for microscopy. Embryos can be stored in this solution for several weeks. Seal the plates with plastic paraffin to prevent evaporation.
- Wash embryos 3 times (10 min) at RT with freshly made pH 9.5 buffer consisting of 0.1 M Tris pH 9.5, 100 mM NaCl, 50 mM MgCl2, 1 mM Levamisole, and 0.1% Tween-20.
- Slide preparation and image capturing
- Prepare a slide by arranging three small strips of double-sided tape into a triangle with small gaps between strips onto a slide.
- Transfer the embryos in the 70% MOPS wash and 30% glycerol solution to the center of the triangle and cover with a coverslip.
- Seal the coverslip by applying a layer of nail polish around the edges of the coverslip.
- Capture images using a compound light microscope with a 20X objective and an attached camera.
Representative Results
In the sea urchin embryo we have shown that 3 different Wnt signaling branches (Wnt/β-catenin, Wnt/JNK, and Wnt/PKC)4,25 interact to form a Wnt signaling network that governs anterior-posterior (AP) patterning. One of the most important consequences of these signaling events is that the initial broadly expressed anterior neuroectoderm (ANE) GRN becomes restricted to a small territory around the anterior pole by the beginning of gastrulation (24 hpf in S. purpuratus). These results indicate that Wnt/β-catenin signaling prevents ANE gene activation in the posterior half of the embryo by the 32-cell stage. Then, this pathway relays a signal to the non-canonical Wnt/JNK signaling pathway that progressively down regulates the ANE GRN in the anterior half of the embryo between the 60-cell stage and early gastrulation. Finally, a third non-canonical Wnt pathway, Wnt/PKC, antagonizes the Wnt/JNK signaling pathway and prevents it from eliminating ANE specification around the anterior pole (Figure 1A)4.
We use the spatiotemporal expression of foxq2 to assay for the activity of each of the Wnt signaling branches during AP patterning because it is one of the first two genes activated in the ANE GRN and it is easily assessed by in situ hybridization because of its robust expression26. If any individual Wnt signaling branch is perturbed, then there are clear expression phenotypes that indicate which pathway is involved: 1) In the absence of Wnt/β-catenin signaling a broad maternal regulatory mechanism (Figure 1A) activates foxq2 expression throughout the entire embryo (Figure 1Bb); 2) In the absence of Wnt/JNK signaling foxq2 is expressed throughout the anterior half of the embryo (Figure 1Bc), but it is still down regulated in the posterior half due to the activity of Wnt/β-catenin signaling (Figure 1A); In the absence of Wnt/PKC signaling foxq2 expression is completely down regulated throughout the embryo (Figure 1Bd), because the Wnt/β-catenin and Wnt/JNK signaling pathways are up regulated4,25. Thus, we have developed an assay that we have termed the foxq2 transcriptional readout system which, when combined with our systematic workflow (Figure 2), allows us to efficiently identify and test whether a gene of interest is involved in one or more of the Wnt signaling branches.
Using the methodology and foxq2 readout system presented here, we have identified several putative extracellular or intracellular molecules likely involved in the Wnt network governing AP axis specification, four of which are presented in Figure 3. Embryos injected with morpholinos designed to knockdown the expression of either the transcription factor, ATF2, or the secreted extracellular Wnt modulator, Wif-1, showed an expansion of foxq2 expression toward the posterior pole of the embryo at early gastrulation stages (Figure 3A – C). These results mimic the phenotypes observed when members of the Wnt/JNK signaling pathway are knocked down4,25, suggesting that they are members of this signaling branch that is necessary to down regulate ANE GRN expression in the anterior half of the embryo. In contrast, foxq2 expression was eliminated when we knocked down the expression of the transcription factor, NFAT (Figure 3D), and the secreted extracellular modulator, sFRP3/4 (Figure 3E), suggesting that these molecules are involved in the Wnt/PKC pathway that is necessary to antagonize the down regulation of the ANE GRN by Wnt/JNK signaling. Based on these rapidly obtained results we are now able to perform more detailed functional analyses that will place these factors within the evolving Wnt signaling network involved in the GRN that governs AP patterning in the sea urchin embryo.
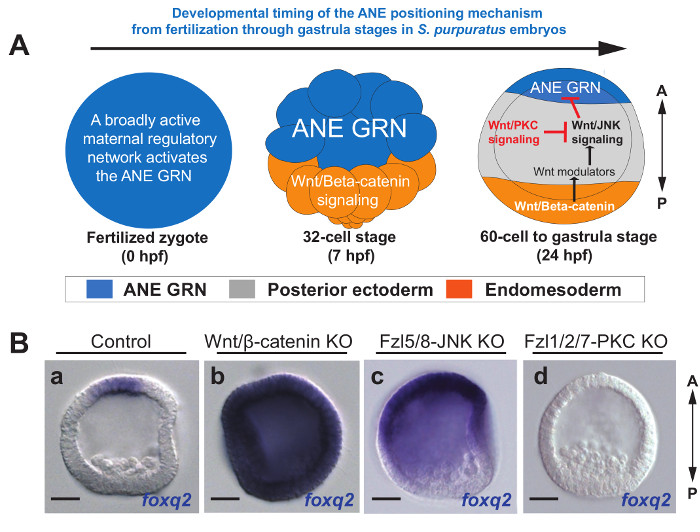
Figure 1. The Model for AP Specification and Patterning in the Sea Urchin and the foxq2 Transcriptional Readout System. (A) The progressive down regulation of the ANE GRN to a territory around the anterior pole by the Wnt signaling network detailed in the text and in4,9. (B) A transcriptional readout system showing foxq2 expression in the indicated Wnt pathway knockdowns (KO). Scale bar = 20 μm. Please click here to view a larger version of this figure.
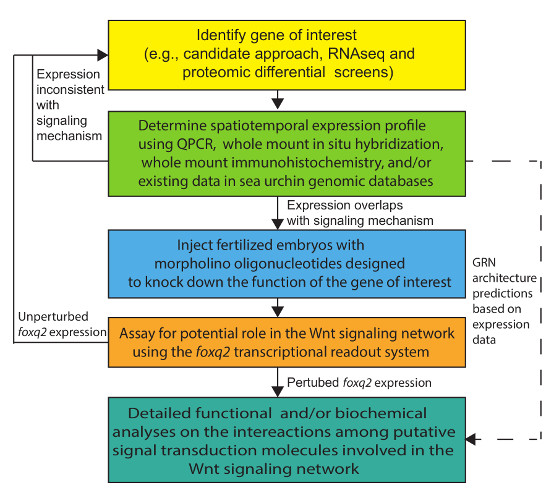
Figure 2. An Experimental Flow Diagram for Efficient Wnt Network Analysis in the Sea Urchin Embryo. Please click here to view a larger version of this figure.
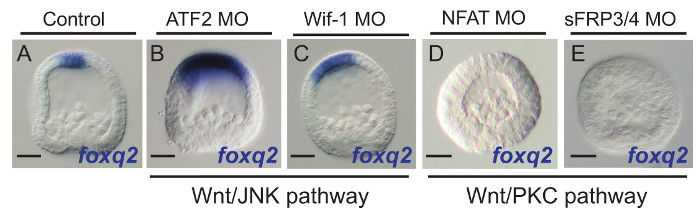
Figure 3. Signal Transduction Molecules Involved in the Wnt Network Governing AP Axis Specification and Patterning Identified Using the foxq2 Transcriptional Readout System. (A, B, C) Morpholino knockdowns suggest that an intracellular signaling modulator, ATF2, and secreted extracellular modulator, Wif-1, are potential players of the Wnt/JNK signaling pathway. (A, D, E) Knockdown experiments indicated that NFAT, an intracellular signaling modulator, and sFRP3/4, a secreted Wnt signaling modulator, are involved in Fzl1/2/7-PKC signaling. Scale bar = 20 μm. Please click here to view a larger version of this figure.<!–
Discussion
The methodology presented here is an example that illustrates the power of using embryos with less genomic and morphological complexity than vertebrates to understand the signaling transduction pathways and GRNs governing fundamental developmental mechanisms.. Many labs are using similar assays during early sea urchin development to dissect the signaling pathways involved in other cell fate specification events (e.g. Notch, Hedgehog, TGF- β, and FGF signaling)27,28,29,30,31. These sea urchin studies have revealed many interesting and novel signaling mechanisms, and many aspects of these signaling mechanisms governing early sea urchin development appear to be conserved among deuterostomes9,27,30,31,32. Importantly, the exploration of the developmental biology of non-traditional metazoan embryos has seen an increase in recent years, and many of these share characteristics with sea urchin embryos during early development9,15,16,33. Thus, the methodology presented here could be widely applied to the study of the signal transduction pathways in many of these organisms during early development.
All techniques used to knock down gene expression can potentially produce off-target knock down effects. Morpholinos have proven to be a remarkably effective gene perturbation method in sea urchin embryos in large part because the community performs rigorous controls to alleviate concerns that the knockdown phenotype is non-specific. These basic control assays can be modified and applied to other knockdown techniques (i.e. CRISPER/Cas9). The controls are: 1) Careful dose response assays are performed with each morpholino until ≥ 80% of injected embryos show defective phenotype and/or marker gene expression; 2) Morpholinos are discarded if they cause severe developmental delays or even death at moderate concentrations. These phenotypes are likely due toxic off-target effects; 3) At least two morpholinos that are designed to target different binding sites are used to confirm knockdown phenotypes; 3) Missense morpholinos are injected; 4) Morpholino phenotypes are rescued by introducing mRNA for the target gene into morpholino knockdown embryos; 5) When available, whole mount antibody staining assays are used to show that morpholino knockdowns prevent translation of the gene of interest. It is important to note that the sea urchin community uses at least four species for functional studies, depending on where scientists are located. In most cases, to our knowledge, the various morpholinos used to knock down the orthologs have generated similar phenotypes in each species (for example see these functional studies on Nodal signaling34,35,36,37). These results argue convincingly that our rigorous control experiments strongly select for those morpholinos that produce on-target knockdown effects.
Another important aspect of this methodology is to carefully identify the transcriptional targets that are most likely directly activated by the signaling pathway under consideration. The example that we provide here is fortuitous, because the spatiotemporal activation of the signaling pathways and the down regulation of a robustly expressed gene, foxq2, occur early in development and the GRNs governing germ layer and axis specification in the sea urchin embryo are well-established. Thus, an obvious limitation of this methodology is that in many cases it may be necessary to knockdown the activity of a particular signaling pathway during later stages of development and within specific territories. There are several technologies now available that can be used to overcome these limitations (e.g. photo-morpholinos, Crisper/Cas938, FACseq) even in non-traditional model embryos that are not amenable to genetic manipulation. In many cases it may not be possible to identify a candidate gene down stream of a signaling pathway of interest whose spatiotemporal expression is as easily assessed as foxq2, which has a remarkably high signal-to-noise ratio. It is common to find genes with much lower signal-to-noise ratios. If another gene is unavailable for a particular assay, then the in situ probe generated for the gene of interest should be as long as possible. In general, using probes longer than 1,000 base pairs significantly increases the signal and reduces the noise in colorimetric and fluorescent in situ assays.
Once the transcriptional assay that indicates where and when the signal transduction pathway of interest is signaling is established, the next important step is to determine both the spatial and the temporal expression of the putative regulatory factors involved in the developmental mechanism under study. Differential screen and/or QPCR data are important tools, but they can be misleading. Confirming with whole mount in situ hybridization that the gene of interest is expressed within the territory where the signaling pathway is active prevents a waste of time and resources. In addition, these data allow predictions to be made about the gene regulatory architecture, which will inform the more detailed analyses that will follow once the players involved are identified with this initial assay (Figure 2). We present a simple colorimetric assay in this protocol; however, fluorescent in situ hybridization (FISH) can also be used to visualize several fluorescent probes at the same time, allowing for enhanced spatial resolution within the territory of interest as well as confocal microscopy24. In addition, FISH can be paired with antibody staining to identify post-translational modifications and/or where the signaling pathway of interest is active24.
There are two critical steps in the protocol that are often overlooked. One is the preparation of the morpholino oligonucleotide solution. It is important to heat the morpholino solution at 65 °C for 2 min to 5 min and to centrifuge it at full speed for at least 10 min before loading the injection needles. These steps are essential when the morpholino stock solution is stored at -20 °C because morpholino oligonucleotides can precipitate from the solution at low temperatures and the spinning of the solution prevents the injection needles from being clogged by particulates. Another critical aspect to consider is that embryos should be thoroughly mixed into the various solutions during every step of the fixation and in situ hybridization protocols. In our hands, the signal is reduced and/or the background is increased if this simple step is not performed.
Perhaps the most important aspect of the methodology presented here is that it allows for efficient functional analyses of large sets of potential signal transduction molecules generated by next generation transcriptomics and proteomics. Once these initial functional analyses confirm a group of regulatory factors are involved in a pathway of interest, the next challenge is to use established assays (e.g. functional knockdowns; detailed multi-colored whole mount in situ; biochemical interactions) to assess how they fit into the extracellular, intracellular, and transcriptional levels of the signal transduction pathways involved in a particular developmental process.
Declarações
The authors have nothing to disclose.
Acknowledgements
We would like to thank Dr. Robert Angerer for his careful reading and editing of the manuscript. NIH R15HD088272-01 as well as the Office of Research and Development, and Department of Biological Sciences at Mississippi State University provided support for this project to RCR.
Materials
| Translational-blocking morpholino and/or splice-blocking morpholino | Gene Tools LLC | Customized | More information at www.gene-tools.com |
| Glycerol | Invitrogen | 15514-011 | |
| FITC (dextran fluorescein isothiocyanate) | Invitrogen, Life Technologies | D1821 | Make 25mg/mL stock solution |
| Paraformaldehyde 16% solution EM Grade | Electron Microscopy Sciences | 15710 | |
| MOPS | Sigma Aldrich | M1254-250G | |
| Tween-20 | Sigma Aldrich | 23336-0010 | |
| Formamide | Sigma Aldrich | 47671-1L-F | |
| Yeast tRNA | Invitrogen | 15401-029 | |
| Normal Goat Serum | Sigma Aldrich | G9023-10mL | |
| Alkaline Phosphatase-conjugated anti-digoxigenin antibody | Roche | 11 093 274 910 | |
| Tetramisole hydrochloride (levamisole) | Sigma Aldrich | L9756-5G | |
| Tris Base UltraPure | Research Products Internationall Corp | 56-40-6 | |
| Sodium Chloride | Fisher Scientific | BP358-10 | |
| Magnesium chloride | Sigma Aldrich | 7786-30-3 | |
| BCIP (5-Bromo-4-Chloro-3-indolyl-phosphate | Roche | 11 383 221 001 | |
| 4 Nitro blue tetrazolium chloride (NBT) | Roche | 11 383 213 001 | |
| Dimethyl Formamide | Sigma Aldrich | D4551-500mL | |
| Potassium Chloride | Sigma Aldrich | P9541-5KG | |
| Sodium Bicarbonate | Sigma Aldrich | S5761-500G | |
| Magnesium Sulfate | Sigma Aldrich | M7506-2KG | |
| Calcium Chloride | Sigma Aldrich | C1016-500G |
Referências
- Erwin, D. H., Davidson, E. H. The evolution of hierarchical gene regulatory networks. Nature reviews. Genetics. 10, 141-148 (2009).
- Peter, I. S., Davidson, E. H. Evolution of gene regulatory networks controlling body plan development. Cell. 144, 970-985 (2011).
- Borggrefe, T., et al. The Notch intracellular domain integrates signals from Wnt, Hedgehog, TGFbeta/BMP and hypoxia pathways. Biochimica et biophysica acta. 1863, 303-313 (2016).
- Range, R. C., Angerer, R. C., Angerer, L. M. Integration of canonical and noncanonical Wnt signaling pathways patterns the neuroectoderm along the anterior-posterior axis of sea urchin embryos. PLoS Biol. 11, e1001467 (2013).
- Cleary, M. A., van Osch, G. J., Brama, P. A., Hellingman, C. A., Narcisi, R. FGF, TGFbeta and Wnt crosstalk: embryonic to in vitro cartilage development from mesenchymal stem cells. Journal of tissue engineering and regenerative medicine. 9, 332-342 (2015).
- Lapraz, F., et al. RTK and TGF-beta signaling pathways genes in the sea urchin genome. Dev Biol. 300, 132-152 (2006).
- Pires-daSilva, A., Sommer, R. J. The evolution of signalling pathways in animal development. Nature reviews. Genetics. 4, 39-49 (2003).
- Sethi, A. J., Wikramanayake, R. M., Angerer, R. C., Range, R. C., Angerer, L. M. Sequential signaling crosstalk regulates endomesoderm segregation in sea urchin embryos. Science. 335, 590-593 (2012).
- Range, R. Specification and positioning of the anterior neuroectoderm in deuterostome embryos. Genesis. 52, 222-234 (2014).
- Petersen, C. P., Reddien, P. W. Wnt signaling and the polarity of the primary body axis. Cell. 139, 1056-1068 (2009).
- Lapraz, F., Haillot, E., Lepage, T. A deuterostome origin of the Spemann organiser suggested by Nodal and ADMPs functions in Echinoderms. Nature communications. 6, 8434 (2015).
- Kikuchi, A., Yamamoto, H., Sato, A. Selective activation mechanisms of Wnt signaling pathways. Trends in cell biology. 19, 119-129 (2009).
- Hogan, B. L. Bone morphogenetic proteins: multifunctional regulators of vertebrate development. Genes Dev. 10, 1580-1594 (1996).
- Houart, C., et al. Establishment of the telencephalon during gastrulation by local antagonism of Wnt signaling. Neuron. 35, 255-265 (2002).
- Bertrand, S., Escriva, H. Evolutionary crossroads in developmental biology: amphioxus. Development. 138, 4819-4830 (2011).
- Rottinger, E., Lowe, C. J. Evolutionary crossroads in developmental biology: hemichordates. Development. 139, 2463-2475 (2012).
- Genome Sequencing Sea Urchin, C., et al. The genome of the sea urchin Strongylocentrotus purpuratus. Science. 314, 941-952 (2006).
- Ben-Tabou de-Leon, S., Su, Y. H., Lin, K. T., Li, E., Davidson, E. H. Gene regulatory control in the sea urchin aboral ectoderm: spatial initiation, signaling inputs, and cell fate lockdown. Dev Biol. 374, 245-254 (2013).
- Saudemont, A., et al. Ancestral regulatory circuits governing ectoderm patterning downstream of Nodal and BMP2/4 revealed by gene regulatory network analysis in an echinoderm. PLoS Genet. 6, e1001259 (2010).
- Cameron, R. A., Samanta, M., Yuan, A., He, D., Davidson, E. SpBase: the sea urchin genome database and web site. Nucleic Acids Res. 37, D750-D754 (2009).
- Stepicheva, N. A., Song, J. L. High throughput microinjections of sea urchin zygotes. Journal of visualized experiments : JoVE. , e50841 (2014).
- Cheers, M. S., Ettensohn, C. A. Rapid microinjection of fertilized eggs. Methods in cell biology. 74, 287-310 (2004).
- Arenas-Mena, C., Cameron, A. R., Davidson, E. H. Spatial expression of Hox cluster genes in the ontogeny of a sea urchin. Development. , 4631-4643 (2000).
- Sethi, A. J., Angerer, R. C., Angerer, L. M. Multicolor labeling in developmental gene regulatory network analysis. Methods in molecular biology. , 249-262 (2014).
- Wikramanayake, A. H., Huang, L., Klein, W. H. beta-Catenin is essential for patterning the maternally specified animal-vegetal axis in the sea urchin embryo. Proc Natl Acad Sci U S A. 95, 9343 (1998).
- Yaguchi, S., Yaguchi, J., Angerer, R. C., Angerer, L. M. A Wnt-FoxQ2-nodal pathway links primary and secondary axis specification in sea urchin embryos. Dev Cell. 14, 97-107 (2008).
- Molina, M. D., de Croze, N., Haillot, E., Lepage, T. Nodal: master and commander of the dorsal-ventral and left-right axes in the sea urchin embryo. Curr Opin Genet Dev. 23, 445-453 (2013).
- Range, R. C., Glenn, T. D., Miranda, E., McClay, D. R. LvNumb works synergistically with Notch signaling to specify non-skeletal mesoderm cells in the sea urchin embryo. Development. 135, 2445-2454 (2008).
- Range, R., et al. Cis-regulatory analysis of nodal and maternal control of dorsal-ventral axis formation by Univin, a TGF-beta related to Vg1. Development. 134, 3649-3664 (2007).
- Warner, J. F., Miranda, E. L., McClay, D. R. Contribution of hedgehog signaling to the establishment of left-right asymmetry in the sea urchin. Dev Biol. 411, 314-324 (2016).
- Rottinger, E., et al. FGF signals guide migration of mesenchymal cells, control skeletal morphogenesis [corrected] and regulate gastrulation during sea urchin development. Development. 135, 353-365 (2008).
- Warner, J. F., McCarthy, A. M., Morris, R. L., McClay, D. R. Hedgehog signaling requires motile cilia in the sea urchin. Mol Biol Evol. 31, 18-22 (2014).
- Technau, U., Steele, R. E. Evolutionary crossroads in developmental biology. Cnidaria. Development. 138, 1447-1458 (2011).
- Yaguchi, J., Takeda, N., Inaba, K., Yaguchi, S. Cooperative Wnt-Nodal Signals Regulate the Patterning of Anterior Neuroectoderm. PLoS Genet. 12, e1006001 (2016).
- Duboc, V., Rottinger, E., Besnardeau, L., Lepage, T. Nodal and BMP2/4 signaling organizes the oral-aboral axis of the sea urchin embryo. Dev Cell. 6, 397-410 (2004).
- Bradham, C. A., et al. Chordin is required for neural but not axial development in sea urchin embryos. Dev Biol. 328, 221-233 (2009).
- Su, Y. H. Gene regulatory networks for ectoderm specification in sea urchin embryos. Biochimica et biophysica acta. 1789, 261-267 (2009).
- Lin, C. Y., Su, Y. H. Genome editing in sea urchin embryos by using a CRISPR/Cas9 system. Dev Biol. 409, 420-428 (2016).

