Detection of microRNA Expression in Peritoneal Membrane of Rats Using Quantitative Real-time PCR
Summary
Here we present a protocol for the detection of microRNA expression in rat peritoneal membrane using quantitative real-time reverse-transcription polymerase chain reaction. This method is suitable for studying the microRNA expression profile in rat peritoneal membrane in several pathological conditions.
Abstract
MicroRNAs (miRNAs) are small noncoding RNAs that regulate messenger RNA expression post-transcriptionally. The miRNA expression profile has been investigated in various organs and tissues in rat. However, standard methods for the purification of miRNAs and detection of their expression in rat peritoneal membrane have not been well established. We have developed an effective and reliable method to purify and quantify miRNAs using quantitative real-time reverse-transcription polymerase chain reaction (qRT-PCR) in rat peritoneal membrane. This protocol consists of four steps: 1) purification of peritoneal membrane sample; 2) purification of total RNA including miRNA from peritoneal membrane sample; 3) reverse transcription of miRNA to produce cDNA; and 4) qRT-PCR to detect miRNA expression. Using this protocol, we successfully determined that the expression of six miRNAs (miRNA-142-3p, miRNA-21-5p, miRNA-221-3p, miRNA-223-3p, miRNA-327, and miRNA-34a-5p) increased significantly in the peritoneal membrane of a rat peritoneal fibrosis model compared with those in control groups. This protocol can be used to study the profile of miRNA expression in the peritoneal membrane of rats in many pathological conditions.
Introduction
MicroRNAs (miRNAs) are short, noncoding RNAs that post-transcriptionally regulate messenger RNA (mRNA) expression1. Changes in the expression of miRNAs regulate the expression of many mRNAs that play pivotal roles in various pathological conditions, including cancer, inflammation, metabolic disorders, and fibrosis2,3,4,5,6,7,8. Therefore, miRNAs have potential as novel biomarkers and therapeutic targets2,3,4,5,6,7,8. The miRNA expression profile has been determined in various rat organs and tissues, including liver, heart, lung, and kidney9. However, standard methods for the purification and detection of miRNAs in rat peritoneal membrane have not been well established.
The overall goal of this protocol is to successfully purify and detect miRNAs in the rat peritoneal membrane. First, the peritoneal membrane sample was homogenized using a glass homogenizer, followed by exposure to a biopolymer-shredding system in a microcentrifuge spin column10. Next, total RNA including miRNA was purified from the peritoneal membrane sample using a silica-membrane-based spin column10. Then, cDNA was synthesized from the purified total RNA using reverse transcriptase, poly(A) polymerase, and oligo-dT primer11. Finally, the expression of miRNA was determined by qRT-PCR using an intercalating dye11. The rationale of this protocol is based on previous studies that showed significant purification and detection of miRNA in tissues by a simple process8,10,11. It has been reported that the use of a biopolymer-shredding system in a microcentrifuge spin column and silica-membrane-based spin column can purify high-quality total RNA from tissues10. The method of synthesizing cDNA from purified total RNA using reverse transcriptase, poly(A) polymerase, and oligo-dT primer, and the method of detecting miRNA expression by qRT-PCR using intercalating dye in this protocol have been reported to show high accuracy and sensitivity11. In addition, this is a simple process, which saves time and prevents technical error. Therefore, this protocol is useful in studies that require highly accurate and sensitive detection of miRNA in rat peritoneal membrane in a wide range of pathological conditions.
Protocol
All animal experimental protocols were approved by the animal ethics committee of Jichi Medical University and were performed in accordance with the Use and Care of Experimental Animals guidelines from the Jichi Medical University Guide for Laboratory Animals.
1. Peritoneum Sample Collection
- Collect the following items: 50 mL centrifuge tube with cotton drenched in isoflurane, cork sheet, Petri dish with phosphate-buffered saline (PBS), surgical scissors, and forceps.
- Euthanize a rat with an overdose of isofluorane, then spray the abdominal skin of the rat with 70% ethanol, and mount the rat on the cork sheet on its back.
- Make a longitudinal incision in the abdominal skin, muscle, and peritoneal membrane using the scissors and forceps.
- Pick up the peritoneal membrane using the forceps, and make horizontal incisions on its upper portion along the lowest rib bone under the diaphragm and on its lower side on the lateral region using the surgical scissors. Next, make lateral longitudinal incisions to remove the peritoneal membrane from the body using the surgical scissors. Then, wash it with PBS in a petri dish.
- Cut out and trim 20 mg (3-5 mm2) of peritoneal membrane samples, which is a suitable size for the following steps, using the surgical scissors and forceps.
2. Purifying total RNAs from Peritoneal Membrane Samples
NOTE: Here, peritoneal membrane samples weighing 20 mg are homogenized using a glass homogenizer and a biopolymer-shredding system in a microcentrifuge spin column. Then, total RNA from the peritoneal membrane sample is isolated using a silica-membrane-based spin column.
- Collect the following items: 1.5 or 2.0 mL microcentrifuge tubes, 100% ethanol, chloroform, glass homogenizer, ice, biopolymer-shredding system in a microcentrifuge spin column10, silica-membrane-based spin column10, phenol/guanidine-based lysis reagent, wash buffer including guanidine and ethanol (wash buffer 1), and wash buffer including ethanol (wash buffer 2).
- Put a 20 mg peritoneal membrane sample into a glass homogenizer and add 700 µL of the phenol/guanidine-based lysis reagent.
- Homogenize the peritoneal membrane sample by slowly pressing the pestle on to the sample with twisting. Repeat this process several dozen times on ice until the peritoneal membrane sample has completely dissolved into the phenol/guanidine-based lysis reagent.
- For further homogenization, transfer homogenized lysate to the biopolymer-shredding system in a microcentrifuge spin column in a 2.0 mL collection tube. Then, centrifuge it at 14,000 x g for 3 min at 4 °C.
- Transfer the homogenized lysate to a new microcentrifuge tube.
- Add 140 µL of chloroform to the homogenized lysate and cap the tube securely. Then, mix the tube by inversion for 15 s.
- Incubate the samples for 2-3 min at room temperature. Then, centrifuge them at 12,000 x g for 15 min at 4 °C.
- Transfer the supernatant (usually 300 µL) to a new microcentrifuge tube without disturbing the precipitate, and add 1.5x its volume (usually 450 µL) of 100% ethanol. Then, mix the sample by vortexing for 5 s.
- Pipette up to 700 µL of the sample into a silica-membrane-based spin column placed in a 2.0 mL collection tube. Close the cap of the column. Then, centrifuge it at 15,000 x g for 15 s. After centrifugation, discard the flow-through in the collection tube.
- Add 700 µL of wash buffer 1 to the silica-membrane-based spin column to stringently wash the sample. Close the cap of the column. Then, centrifuge it at 15,000 x g for 15 s. After centrifugation, discard the flow-through in the collection tube.
- Add 500 µL of wash buffer 2 to the silica-membrane-based spin column to remove any trace of salt. Close the cap of the column. Then, centrifuge it at 15,000 x g for 15 s. After centrifugation, discard the flow-through in the collection tube.
- Repeat 2.11.
- Centrifuge the silica-membrane-based spin column again at 15,000 x g for 1 min. After centrifugation, discard the flow-through in the collection tube.
- Place the silica-membrane-based spin column in a new 1.5 mL collection tube. Then, transfer 25 µL of RNase-free water into the column. Close the cap of the column. Leave the sample at room temperature for 5 min. Then, centrifuge it at 15,000 x g for 1 min.
- Transfer the 25 µL eluate containing total RNA to a new microcentrifuge tube. Then, store it at −80 °C before use.
3. Reverse Transcription of Total RNA
NOTE: Reference and adherence to The Minimum Information for Publication of Quantitative Real-Time PCR Experiments (MIQE) guidelines encourage better practice and help in obtaining reliable and unequivocal results12.
NOTE: Here, a total of 1.0 µg of isolated RNA is reverse-transcribed using the reverse transcriptase, poly(A) polymerase, and oligo-dT primer.
- Collect the following items: 1.5 mL microcentrifuge tubes; eight-well strip tubes; RNase-free water; ice; reverse transcriptase kit (see materials table)11.
- Prepare a master mix solution that contains 2.0 µL of 10x nucleic acid mix, 2.0 µL of reverse transcriptase mix (from the kit), and 4.0 µL of 5x hi-spec buffer for a total of 8.0 µL per tube.
- Dispense 8.0 µL of master mix solution (from the kit) into each tube.
- Measure the quantity of total RNAs using a spectrophotometer. Add 1.0 µg of isolated total RNAs purified from the peritoneal membrane sample to each tube.
NOTE: qRT-PCR may be performed using total RNAs as a template without reverse transcription for the quality control of purified total RNAs. If any DNA is contaminated, unexpected amplification products are observed by qRT-PCR. - Add RNase-free water to each tube to a total of 20 µL. Then, mix thoroughly by pipetting and centrifuge it for 15 s.
- Incubate the samples for 60 min at 37 °C. Immediately incubate for 5 min at 95 °C.
NOTE: This step can be performed in a thermal cycler. - Transfer cDNA to a new microcentrifuge tube and dilute ten times (1:10) with RNase-free water.
- Store the diluted cDNA on ice temporarily, and at −80 °C for long term before use.
4. qRT-PCR of miRNA
NOTE: qRT-PCR of miRNA is performed using intercalating dye. Here, primers for RNA, U6 small nuclear 2 (RNU6-2), miRNA-142-3p, miRNA-21-5p, miRNA-221-3p, miRNA-223-3p, miRNA-327, and miRNA-34a-5p were used.
- Collect the following items: 1.5 mL microcentrifuge tubes, 96-well reaction plate for qRT-PCR, adhesive film for the 96-well reaction plate, miRNA-specific primers, green dye-based PCR kit (see materials table)11, and a real-time PCR Instrument.
- Prepare a master mix containing 12.5 µL of 2x PCR master mix, 2.5 µL of 5 µM each miRNA primer dissolved in nuclease-free water, and 1.25 µL of 10x universal primer for each well.
- Dispense 22.5 µL of master mix into each well of the 96-well plate.
- Add 2.5 µL of template cDNA to each well.
- Seal the plate with adhesive film for the 96-well reaction plate. Then, centrifuge the plate at 1,000 x g for 30 s.
- Run the PCR cycling program with the real-time PCR Instrument and software as follows.
- Set the plate in the real-time PCR instrument. In the real-time PCR software, define experimental properties (input experimental name, choose "96-wells" for the experimental type of instrument, choose "2−ΔΔCT method" for the quantitation method, choose "SYBR green reagents" for the reagent to detect the target sequence, choose "standard ramp speed" for the instrument run).
- Then, assign names to the samples and target miRNAs in each well. Samples are always tested in duplicate or triplicate to obtain enough data for validation of the results. Select reference sample and endogenous control. Select "none" for the dye to use as the passive reference.
- Input a reaction volume of "20 µL" and the following PCR cycling conditions in the real-time PCR software in accordance with the instructions: preincubation at 95 °C for 15 min, and then 40 cycles of denaturation at 94 °C for 15 s, annealing at 55 °C for 30 s, and extension at 70 °C for 30 s.
- Analyze qRT-PCR data using the software of the real-time PCR instrument. Confirm that the threshold and baseline that are automatically determined by software are appropriate in each well.
- Check the threshold cycle of target miRNAs in each sample. The threshold cycle is the intersection between an amplification curve and a threshold line. Then, normalize the expression level of target miRNAs against RNU6-2 as an endogenous control, and calculate the relative expression level of target miRNAs using the 2−ΔΔCT method13.
NOTE: It should be noted that the stability of the expression level of endogenous control miRNA should be verified. In this experiment, RNU6-2 was confirmed to be expressed at high level and relatively invariant across each group.
Representative Results
The results presented here are based on our previously reported study8. We investigated the miRNA expression profile in peritoneal fibrosis. Peritoneal fibrosis is a major complication in peritoneal dialysis. It is characterized by loss of the mesothelial cell monolayer and the excess accumulation of extracellular matrix components, and is associated with peritoneal membrane failure14,15. A peritoneal fibrosis rat model was produced by the intraperitoneal injection of 100 mL/kg peritoneal dialysis fluid (2.5% glucose, 100 mM NaCl, 35 mM sodium lactate, 2 mM CaCl2, and 0.7 mM MgCl2 containing 20 mM methylglyoxal) for 5 days a week for 3 weeks to male rats, aged 12 weeks and with body weights of 230-250 g16. The following groups served as controls: rats without any treatment (mock rats) and rats injected with peritoneal dialysis fluid without methylglyoxal (control rats). Based on miRNA microarray screening, we found that the levels of six miRNAs (miRNA-142-3p, miRNA-21-5p, miRNA-221-3p, miRNA-223-3p, miRNA-327, and miRNA-34a-5p) significantly increased in the peritoneal membrane of peritoneal fibrosis rats compared with those in mock rats and control rats, using qRT-PCR following the protocol presented in this manuscript (Figure 1)8.
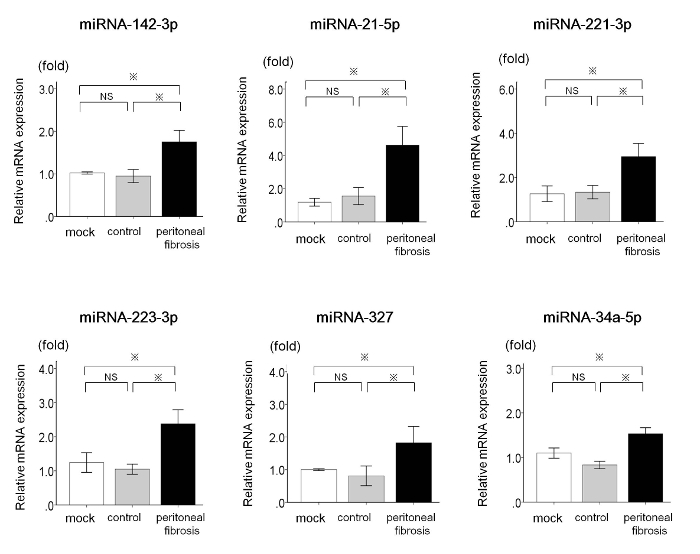
Figure 1: Upregulated miRNAs in the Peritoneal Membrane of Peritoneal Fibrosis Rats. qRT-PCR determination of the expression of miRNA-142-3p, miRNA-21-5p, miRNA-221-3p, miRNA-223-3p, miRNA-327, and miRNA-34a-5p in mock rats (n = 6), control rats (n = 6), and peritoneal fibrosis rats (n = 6). Values are the mean ± standard error (error bars). Analysis of variance (ANOVA) was employed to investigate differences among groups. If statistical significance was detected by ANOVA, Tukey's test was performed as a post hoc analysis to compare the means of two different groups. Differences with a p-value <0.05 were considered significant. Abbreviations: qRT-PCR, quantitative real-time reverse-transcription polymerase chain reaction; miRNAs, microRNAs; NS, not significant; *, p <0.05. This figure has been modified from Morishita et al.8 (with permission). Please click here to view a larger version of this figure.
Discussion
Using the protocol presented in this manuscript, miRNAs in rat peritoneal membrane were successfully purified and detected using qRT-PCR. The reliability of qRT-PCR data analysis depends on the quality of purified miRNAs. Therefore, the purity of miRNAs may be checked before qRT-PCR by the ratio of absorbance at 260 nm to that at 280 nm, which can be measured using a spectrophotometer. When significant amplification of miRNA cannot be obtained using qRT-PCR, the concentration of template cDNA may be increased. The concentration of each miRNA-specific primer may also be increased.
There are several alternative methods to determine the level of miRNA expression, including microarray, Northern blotting, and ribonuclease protection assay. qRT-PCR is a sensitive, high-throughput procedure that requires a minimal amount of sample as compared with Northern blotting or ribonuclease protection assay. Microarrays enable investigation of the expression of numerous miRNAs simultaneously, and the data generally show a strong correlation with the data obtained by qRT-PCR17; however, no consensus has been reached regarding a methodology that can confirm the validity of comparisons of microarray data between research groups18.
This protocol has the following limitations. First, the validation of miRNA detection by this protocol has not been verified in other organs such as liver, lung, and kidney. Second, it also has not been verified in other experimental animals, including mouse, hamster, dog, and pig. The platform of this protocol for the purification of miRNAs using a biopolymer-shredding spin column and a silica-membrane-based spin column, and detection of miRNAs using qRT-PCR has been reported to be able to purify high-quality RNA from tissues and shows high accuracy and sensitivity for the detection of miRNA expression10,11. We also showed that the detection of miRNA expression in rat peritoneal membrane can be successfully conducted using this protocol. This protocol can be used for studies investigating the profile of miRNA expression in the peritoneal membrane of rats in a wide range of pathological conditions. In addition, many samples can be treated together using this protocol because it involves a simple process. Therefore, this protocol is useful for studies that require analysis of the expression profile of many miRNAs in various pathological conditions of the peritoneal membrane.
Several critical points should be kept in mind when following this protocol. First, samples containing purified RNAs should be placed on ice to avoid degradation. In addition, the step of homogenization should be performed on ice to protect RNAs from heat degradation. Furthermore, peritoneal membrane samples should be adequately homogenized until they have completely dissolved in lysis reagent, and the use of a column shredder for further homogenization is recommended because peritoneal membrane samples contain substantial connective tissue that is resistant to dissolution by homogenization. Second, the stability of the expression level of endogenous control miRNA should be verified across the experimental set-up in qRT-PCR. This is necessary because various pathological conditions in which this procedure can be used may affect the expression levels of endogenous control miRNAs, which can compromise the results.
In conclusion, we have described a protocol for the purification and detection of microRNA expression in rat peritoneal membrane using qRT-PCR.
Declarações
The authors have nothing to disclose.
Acknowledgements
We would like to thank Miyako Shigeta for her excellent technical support. This work was partially supported by JSPS KAKENHI (grant number 25461252).
Materials
| QIA shredder | Qiagen | 79654 | biopolymer-shredding system in a micro centrifuge spin-column |
| miRNeasy Mini kit | Qiagen | 217004 | silica-membrane based spin column |
| QIAzol Lysis Reagent | Qiagen | 79306 | phenol/guanidine-based lysis reagent |
| Buffer RLT | Qiagen | 79216 | wash buffer 1 |
| Buffer RWT | Qiagen | 1067933 | wash buffer 2 |
| miScript II RT kit | Qiagen | 218161 | includes 10× Nucleics Mix containing deoxynucleotides, ribonucleotide triphosphates, and oligo-dT primers; miScript Reverse Transcriptase Mix containing poly(A) polymerase and reverse transcriptase and; miScript HiSpec buffer |
| miScript SYBR Green PCR kit | Qiagen | 218073 | includes QuantiTect SYBR Green PCR Master Mix and miScript Universal Primer |
| RNU6-2 primer | Qiagen | MS00033740 | not disclosed |
| miRNA-142-3p primer | Qiagen | MS00031451 | 5'-UGUAGUGUUUCC UACUUUAUGGA-3' |
| miRNA-21-5p primer | Qiagen | MS00009079 | 5'-UAGCUUAUCAG ACUGAUGUUGA-3' |
| miRNA-221-3p primer | Qiagen | MS00003857 | 5'-AGCUACAUUGU CUGCUGGGUUUC-3' |
| miRNA-223-3p primer | Qiagen | MS00033320 | 5'-UGUCAGUUUG UCAAAUACCCC-3' |
| miRNA-34a-5p primer | Qiagen | MS00003318 | 5'-UGGCAGUGUCU UAGCUGGUUGU-3' |
| miRNA-327 primer | Qiagen | MS00000805 | 5'-CCUUGAGGGG CAUGAGGGU-3' |
| MicroAmp Optical 96 well reaction plate for qRT-PCR | Thermo Fisher Scientific | 4316813 | 96-well reaction plate |
| MicroAmp Optical Adhesive Film | Thermo Fisher Scientific | 4311971 | adhesive film for 96-well reaction plate |
| QuantStudio 12K Flex Flex Real-Time PCR system | Thermo Fisher Scientific | 4472380 | real-time PCR instrument |
| QuantStudio 12K Flex Software version 1.2.1. | Thermo Fisher Scientific | 4472380 | real-time PCR instrument software |
| methylglyoxal | Sigma-Aldrich | M0252 | |
| Midperic | Terumo | not assign | peritoneal dialysis fluid |
| Sprague–Dawley rats | SLC | not assign |
Referências
- Krol, J., Loedige, I., Filipowicz, W. The widespread regulation of microRNA biogenesis, function and decay. Nat Rev Genet. 11 (9), 597-610 (2010).
- Beermann, J., Piccoli, M. T., Viereck, J., Thum, T. Non-coding RNAs in Development and Disease: Background, Mechanisms, and Therapeutic Approaches. Physiol Rev. 96 (4), 1297-1325 (2016).
- Saikumar, J., Ramachandran, K., Vaidya, V. S. Noninvasive micromarkers. Clin Chem. 60 (9), 1158-1173 (2014).
- Yang, G., Yang, L., Wang, W., Wang, J., Xu, Z. Discovery and validation of extracellular/circulating microRNAs during idiopathic pulmonary fibrosis disease progression. Gene. 562 (1), 138-144 (2015).
- Zhang, T., et al. Circulating miR-126 is a potential biomarker to predict the onset of type 2 diabetes mellitus in susceptible individuals. Biochem Biophys Res Commun. , (2015).
- Zhong, X., et al. miR-21 is a key therapeutic target for renal injury in a mouse model of type 2 diabetes. Diabetologia. 56 (3), 663-674 (2013).
- Wang, J., et al. Downregulation of urinary cell-free microRNA-214 as a diagnostic and prognostic biomarker in bladder cancer. J Surg Oncol. , (2015).
- Morishita, Y., et al. MicroRNA expression profiling in peritoneal fibrosis. Transl Res. 169, 47-66 (2016).
- Minami, K., et al. miRNA expression atlas in male rat. Sci Data. 1, 140005 (2014).
- Morse, S. M., Shaw, G., Larner, S. F. Concurrent mRNA and protein extraction from the same experimental sample using a commercially available column-based RNA preparation kit. Biotechniques. 40 (1), 54-58 (2006).
- Mestdagh, P., et al. Evaluation of quantitative miRNA expression platforms in the microRNA quality control (miRQC) study. Nat Methods. 11 (8), 809-815 (2014).
- Bustin, S. A., et al. The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clin Chem. 55 (4), 611-622 (2009).
- Schmittgen, T. D., Livak, K. J. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 3 (6), 1101-1108 (2008).
- Krediet, R. T., Lindholm, B., Rippe, B. Pathophysiology of peritoneal membrane failure. Perit Dial Int. 20, S22-S42 (2000).
- Devuyst, O., Margetts, P. J., Topley, N. The pathophysiology of the peritoneal membrane. J Am Soc Nephrol. 21 (7), 1077-1085 (2010).
- Hirahara, I., Ishibashi, Y., Kaname, S., Kusano, E., Fujita, T. Methylglyoxal induces peritoneal thickening by mesenchymal-like mesothelial cells in rats. Nephrol Dial Transplant. 24 (2), 437-447 (2009).
- Dallas, P. B., et al. Gene expression levels assessed by oligonucleotide microarray analysis and quantitative real-time RT-PCR — how well do they correlate?. BMC Genomics. 6, 59 (2005).
- Rockett, J. C., Hellmann, G. M. Confirming microarray data–is it really necessary?. Genomics. 83 (4), 541-549 (2004).

