Preparation of Plasma Membrane Vesicles from Bone Marrow Mesenchymal Stem Cells for Potential Cytoplasm Replacement Therapy
Summary
Age-related diseases are associated with multiple defects in components of the cytoplasm. Here, we present a protocol to prepare plasma membrane vesicles from bone marrow mesenchymal stem cells. This technique could potentially be used as a means of cytoplasm replacement therapy to ameliorate or even reverse age-associated phenotypes.
Abstract
We have previously reported on the generation of plasma membrane vesicles (PMVs) through the mechanical extrusion of mammalian cells. The fusion of PMVs with mitochondrial deficient Rho0 cells restored mitotic activity under normal culture conditions. Atherosclerosis, type 2 diabetes, Alzheimer's disease, and cancer are age-related diseases that have been reported to be associated with multiple mechanical and functional defects in the cytosol and organelles of a variety of cell types. Bone marrow mesenchymal stem cells (BMSCs) represent a unique cell population from the bone marrow that possess self-renewal capabilities while maintaining their multipotency. The supplementation of senescence cells with young cytoplasm from autologous BMSCs via the fusion of PMVs provides a promising approach to ameliorate or even reverse age-associated phenotypes. This protocol describes how to prepare PMVs from BMSCs via extrusion through a polycarbonate membrane with 3 µm pores, determine the existence of mitochondria and examine the maintenance of membrane potential within PMVs using a confocal microscope, concentrate PMVs by centrifugation, and carry out the in vivo injection of PMVs into the gastrocnemius muscle of mice.
Introduction
A tremendous amount of effort has been devoted to establishing approaches for gene, enzyme, and cell replacement therapies. This has resulted in great breakthroughs and even clinical applications1,2,3. Recently, a controversial mitochondria replacement therapy based on nucleus transfer technology was applied to in vitro fertilization for women of old age or carrying a lethal mitochondrial DNA mutation4. Defects found in age-related diseases, including atherosclerosis, type 2 diabetes, Alzheimer's disease, and cancer, are usually multi-faceted. It has been documented that the accumulation of lipid droplets; the deposition of amyloid protein; the retention of unfolded proteins in the endoplasmic reticulum; and defective proteasome, autophagosome, and mitochondria contribute to the development or aggravation of these diseases5,6,7,8,9,10,11. Presently, there is no available mechanism aimed at direct remediation of malfunction in the cytosol and organelles, which causes senescence and ageing phenotypes.
We have previously reported on the generation of plasma membrane vesicles (PMVs) through the mechanical extrusion of mammalian cells12. With the exception of the nucleus, components in the membrane or cytosol, including proteins and RNA, as well as the organelles, such as mitochondria, were found in PMVs. Essentially, a PMV can be regarded as a miniature enucleated cell. More importantly, the fusion of PMVs with mitochondria-deficient Rho0 cells restored mitotic activity under normal culture conditions. This is the first report on establishing a potentially efficient approach for cytoplasm replacement therapy.
Bone marrow mesenchymal stem cells (BMSCs) are multipotent progenitor cells that are routinely generated from the bone marrow and are readily expanded in culture. Embryonic stem cell markers Oct4, Nanog, and SOX2 have been detected at low levels in MSCs13. Telomerase activity is also measurable. In addition, the absence of co-stimulatory molecules and human leukocyte antigen (HLA) Class II molecules, as well as low HLA Class I expression on MSCs, make them ideal cells for allogeneic, or "off-the-shelf," use in both regenerative medicine and immunomodulatory applications14.
Here, we describe how to prepare PMVs from mouse BMSCs via extrusion through a polycarbonate membrane with 3-µm pores, determine the existence of mitochondria and examine the maintenance of membrane potential in PMVs using confocal microscopy, prepare concentrated but not aggregated PMVs by centrifugation, and carry out the in vivo injection of PMVs into the gastrocnemius muscle of mice.
Protocol
8 to 12 week-old BALB/c mice were purchased from Shanghai Experimental Animal Center (Shanghai, China) and raised in a specific pathogen-free and air-conditioned animal facility. Animal care and experimental procedures were in compliance with the guidelines for the use and care of laboratory animals established by Shantou University.
1. Assembly of the Apparatus
- To ensure sterility, turn on the UV light of a tissue culture hood for 30 min before use.
- Unscrew a disposable 25 mm filter unit and submerge the cap and bottom of the unit into a 200 mL glass cup filled with 75% ethanol for 30 min. The unit is made of medical-grade polypropylene.
- Pick up the cap and bottom of the unit using forceps, shake off the remaining ethanol by hand, and allow the parts to air dry for 10 min in the hood.
- Wet a 19 mm polycarbonate membrane in PBS and place it carefully on top of the supporting matrix of the bottom of the unit. The polymer film has a smooth, flat surface and track-etched 3 µm pores.
- Reassemble the unit by screwing the cap tightly against the bottom. Make sure that the membrane is not dislodged from the center.
- Remove the needle of a 1 mL insulin syringe and draw 1 mL of PBS. Attach the syringe to the filter unit and then push PBS through the unit to wet the assembly and to test if there is any leakage.
2. Generation of Plasma Membrane Vesicles (PMVs)
- Establish a BMSC culture, as reported by Nemeth et al.15, in DMEM culture medium containing 15% FBS and 1% penicillin/streptomycin. Cultivate the cells in a 6-well plate in a humidified incubator containing 5% CO2 at 37 °C.
- To propagate the cells, remove the culture medium and rinse the well with 0.5 mL of calcium-free PBS.
- Add 0.5 mL of 0.25% trypsin-EDTA and incubate the cells at 37 °C for 3 min. Tap the plate against the palm of the hand a couple times to facilitate cell detachment. Stop digestion by adding 1 mL of culture medium.
- Collect the cells in a 15 mL conical tube and spin at 200 x g for 2 min in a bench top centrifuge. Resuspend the cells in 4 mL of culture medium and aliquot the cells into two wells of a 6-well plate.
NOTE: Cells usually reach confluence at around 24 h. - To generate PMVs, harvest the cells from one well (about 5 x 105 cells) of a 6-well plate by trypsin digestion and monodisperse the cells in 0.5 mL of culture medium.
- Load the cells into the insulin syringe, attach it to the filter unit, and push the plunger quickly to squeeze the cells through the filter. Discard the extruded medium because there should only be a few PMVs inside it.
- Load an additional 0.5 mL of medium into the syringe and quickly push it through the filter. Collected the medium of the second extrusion, which contains PMVs of various size.
- Load 150 µL of the collected medium into a 35-mm glass-bottom dish and allow the PMVs to settle to the bottom for about 10 min. Examine the PMVs under an inverted phase contrast microscope equipped with a CCD camera using the 20X objective lens.
3. Examination of the Content within the PMVs Using Confocal Microscopy
- To perform transfection in the BMSCs, dilute 2 µg of supercoiled plasmid DNA and 6 µL of cationic transfection reagent (e.g., PolyJet) in 50 µL of serum-free DMEM. Vortex to mix well and spin briefly to collect all liquid from the sides of the tube.
- Add the diluted solution (from step 3.1) dropwise into diluted DNA solution, vortex strongly for 1 min, spin briefly, and incubate at room temperature for 10 min.
- Replace the old medium on the BMSCs, which have been seeded at about 30% confluence overnight, with 2 mL of fresh culture medium and add DNA transfection mixture dropwise.
- Replace with 2 mL of fresh medium after 12 h of transfection.
- To trace cytoplasmically localized proteins, transfect the cells with the plasmid containing an EGFP expression cassette (pEGFP-N1), as described above. Harvest the cells by trypsin digestion 48 h after transfection for PMV generation, as described above.
- To trace the mitochondria, stain the cells with mitochondrial dye (1 µM, MitoTracker) in fresh culture medium for 30 min at 37 °C.
- To detect the membrane potential of the mitochondria, stain the cells with the cyanine dye JC-1 (5,5',6,6'-tetrachloro-1,1',3,3'-tetraethylbenzimi-dazolylcarbocyanine iodide) (10 µg/mL) in fresh culture medium for 30 min at 37 °C.
- Wash the cells with 0.5 mL of PBS three times and then harvest the cells by trypsin digestion for PMV generation, as described above.
- Load 150 µL of the collected medium into a 35-mm glass-bottom dish and allow the PMVs to settle to the bottom for about 10 min. Examine the PMVs under a confocal microscope using the 100X oil objective lens.
- To detect EGFP or the mitochondrial dye, turn on the 488-nm laser and collect signal at 505-530 nm. To detect JC-1, turn on the 488-nm and 543-nm lasers and collect signals at 505-530 nm and 560-615 nm. Set the pinhole value to around 1.4.
4. Preparation of Concentrated PMVs by Centrifugation
- Harvest the cells by trypsin digestion and generate PMVs, as described above, in 0.5 mL of culture medium.
- Spin the PMVs at 1,200 x g in a benchtop centrifuge for 5 min at room temperature. Carefully aspirate the supernatant and resuspend the pellet in 100 µL of culture medium with gentle swirling.
- Load 50 µL of PMVs into a 35 mm glass-bottom dish and allow them to settle at room temperature for about 10 min. Examine the PMVs under a confocal microscope using the 40X oil objective lens.
- Add the series of diluted polyethylenimine (PEI) solution to the BMSC culture for 1 h and check for signs of cytotoxicity.
NOTE: Here, the PEI at 2 µg/mL was chosen since no sign of toxicity was detected in BMSCs. - Harvest the cells by trypsin digestion and add PEI at 2 µg/mL for 1 h to charge the cell membrane as a means of preventing aggregation. Generate PMVs, as described above, in 0.5 mL of culture medium. Concentrate the PMVs and examine them under a confocal microscope, as described above.
5. Injection of PMVs into the Gastrocnemius Muscle
- To stain the membrane, add CM-DiI (chloromethyl dialkyl-indocarbocyanine) (10 μM) dye in 1 mL of fresh culture medium to the BMSCs for 30 min.
- Harvest the cells (about 5 x 105) by trypsin digestion and resuspend them in 0.5 mL of culture medium. Add PEI (2 µg/mL) for 1 h. Generate and concentrate the PMVs in 100 µL of culture medium, as described above.
- Anaesthetize a BALB/c mouse by injecting sodium barbital (10 mg/kg) after 12 h of fasting.
- Clean the outside of the gastrocnemius muscle with 75% ethanol. Slowly inject the PMVs (100 µL) into the gastrocnemius muscle using a 30 G insulin syringe.
- After administering the anesthesia, place a wet gauze over the eyes for the duration of the experiment and warm the mouse with an incandescent light until it wakes up. House the mouse alone in an individual ventilation cage.
- To harvest the gastrocnemius muscle 12 h post-operation, kill the mouse by cervical dislocation.
- Sprinkle 75% ethanol over the gastrocnemius muscle, open the skin with scissors, and cut out the entire gastrocnemius muscle at both ends.
- Rinse the muscle with PBS and examine it under a fluorescence microscope using the 20X objective lens.
- Trim the muscle with a sharp razor blade to remove of the parts with no fluorescence. Cut the parts with strong red fluorescence into about 9 mm3 cubes.
- Embed each cube in optimal cutting temperature medium (OCT), submerge it in liquid nitrogen for 5 min, and store it in a -20 °C freezer until use.
- Cut the frozen sections into pieces 20 µm thick using a cryostat and adhere each section to a glass slide. Rinse the section once with PBS.
- Draw a circle around the section with a stain circle pen and add 100 µL of DAPI (1 µg/mL). Aspirate the DAPI solution after 10 min of incubation at room temperature in the dark and wash three times with PBS.
- Drop mounting oil over the specimen, cover it with a glass slip, and seal the slide with nail polish. Examine the section under a confocal microscope using the 100X oil objective lens.
- To detect CM-DiI, turn on the 543 nm laser and collect signal at 560-615 nm. To detect DAPI, turn on the 405 nm laser and collect signal at 420-480 nm. Set the pinhole value to about 1.4.
Representative Results
The key to a successful preparation of PMVs depends heavily on the correct assembly of the filter unit (Figure 1), which can be tested by pushing 1 mL of PBS through the membrane. If leakage occurs, reassemble the filter unit and test again. However, the leakage can only be tested reliably when cells are pushed through the membrane. If only a few PMVs are detected under a regular microscope using the 10X objective, or if the size of the PMVs is mostly around 1 µm, that would indicate that most of the cells are trapped inside the unit due to incorrect assembly. PMVs with a size in the range of about 3 µm in diameter were predominant when examined under a phase contrast microscope using the 20X objective (Figure 2). Since PMVs of nanometer-size were not easily visualized, the percentage of large PMVs in terms of number was low. It was found to be helpful to press the syringe quickly to reduce the amount of membrane debris, which apparently fails to form spherical PMVs. Furthermore, PMVs of smaller sizes (less than 1 µm) were found in the first extrusion medium, with larger PMVs recovered in the second extrusion.
The most unique aspect of the PMVs was the enclosing of cellular components aside from the nucleus12. BMSCs were transfected with an expression cassette of EGFP for 48 h before being used for PMV preparation. The results show that cytoplasmically localized EGFP protein can be encapsulated in PMVs (Figure 3A). The enclosure of mitochondria was evidenced by the staining of mitochondrial dye, showing that a large fraction of, but not all, PMVs contain green fluorescent dots, indicative of mitochondria (Figure 3). Some of the PMVs, even those with a diameter as large as 3 µm, did not show evidence of containing mitochondria, suggesting that some mechanical constraints might prevent the encapsulation of mitochondria into PMVs. The membrane potential of mitochondria was detected by JC-1 staining, which emits red fluorescence when mitochondria have a normal potential. The result show that red fluorescence indeed was detected in PMVs (Figure 3), while the green fluorescence was not detectable (data not shown), suggesting that the mitochondria within the PMVs were functional.
A concentration process for PMVs is needed, especially for in vivo application to reduce the volume as well as to remove the cellular components released during extrusion. After centrifugation at 1,200 x g for 5 min at room temperature, very few PMVs of smaller size were found in the supernatant. To facilitate the resuspension of the pellet, a 2-mL round-bottom tube was used for the centrifugation. However, most of the PMVs were aggregated in the pellet, especially PMVs of smaller sizes (Figure 4). This is probably due to adhesion molecules on the membrane. The medium was therefore supplemented with PEI (2 µg/mL), whose concentration was tailored to have no noticeable cytotoxicity. The positive charge of PEI attached to the cell membrane prevented the aggregation of PMVs during centrifugation (Figure 4).
Finally, the membranes of BMSCs were labeled with CM-DiI dye before the preparation of the PMVs (Figure 5). After concentration, PMVs were injected into the gastrocnemius muscles of mice, which were harvested 12 h post-operation and examined under a confocal microscope. Frozen sections of the gastrocnemius muscle were examined for the presence of red fluorescence, which was detected within the myofiber (Figure 5), demonstrating that PMVs were delivered to the cytoplasm, probably via endocytosis. Of note, red fluorescence was found around the periphery of the myofiber (data not shown), indicating that a large fraction of PMVs were either not endocytosed or were just at the beginning of endocytosis at 12 h post-injection.
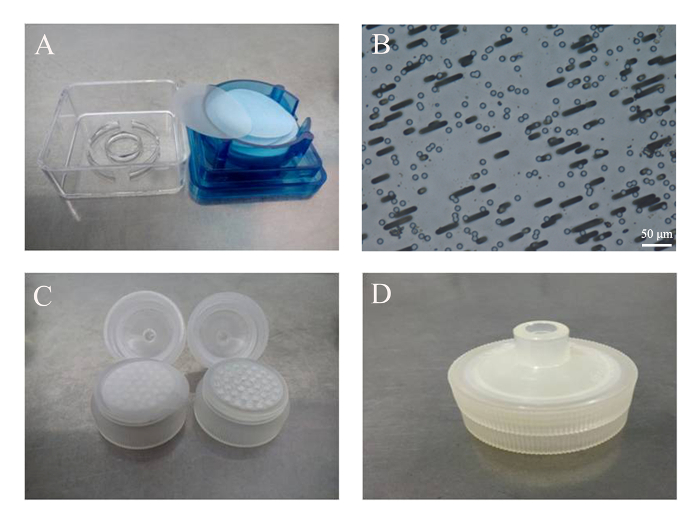
Figure 1: Assembly of the filter unit. (A) Commercially available polycarbonate membranes. (B) Track-etched pores of 3 µm in diameter, viewed under an optical microscope with a 20X objective. Scale bar = 50 µm. (C) The membrane was placed on top of the supporting matrix of a disposable filter unit. (D) The assembled filter unit with a membrane inside. Please click here to view a larger version of this figure.
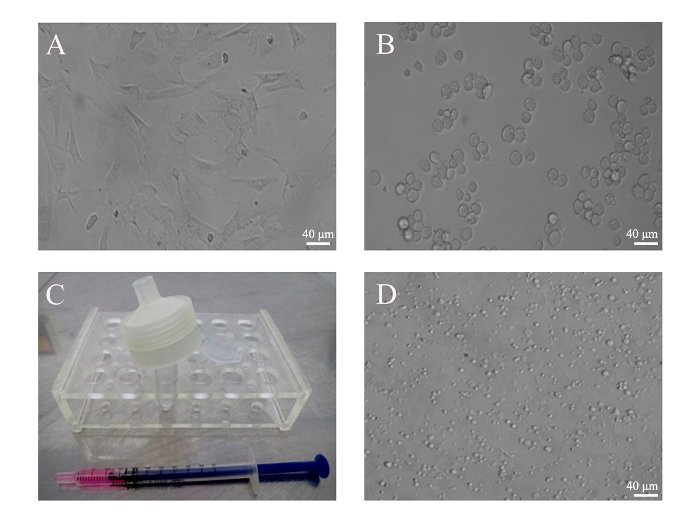
Figure 2: Generation of PMVs from BMSCs. (A) BMSCs from mice were cultivated in a 6-well plate. (B) BMSCs (5 x 105) were harvested by trypsin digestion and monodispersed in 500 µL of culture medium. Images of attached and resuspended BMSCs were taken under an optical microscope with a 20X objective. (C) Cells were loaded into an insulin syringe before being attached to the filter unit. (D) PMVs generated from BMSCs were loaded into a 35 mm glass-bottom dish and examined under an inverted phase contrast microscope using a 20X objective lens. Scale bars = 40 µm. Please click here to view a larger version of this figure.
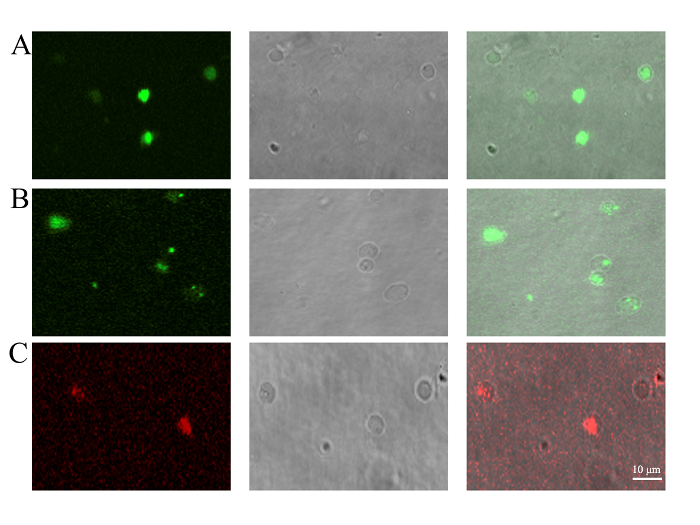
Figure 3: Detection of PMV content by confocal microscopy. PMVs were generated from (A) BMSCs transfected with pEGFP-N1 for 48 h to trace the cytoplasmically localized EGFP protein, (B) BMSCs stained with mitochondrial dye (1 µM) for 30 min to trace the existence of mitochondria, or (C) BMSCs staining with JC-1 (10 µg/mL) for 30 min to confirm the membrane potential of mitochondria. PMVs generated from BMSCs were loaded onto a 35 mm glass-bottom dish and examined by confocal microscopy using a 100X oil objective lens. Scale bars = 10 µm. Please click here to view a larger version of this figure.
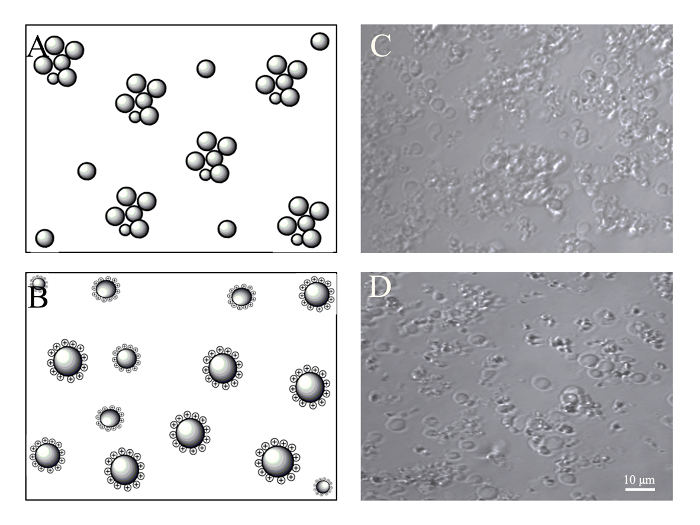
Figure 4: Concentration of PMVs by centrifugation. BMSCs (5 x 105) were harvested by trypsin digestion and resuspended in 0.5 mL of culture medium, which was supplemented with or without 2 µg/mL of PEI. Generated PMVs were spun at 1,200 g for 5 min, resuspended in 100 µL of culture medium, and examined under a confocal microscope with a 40X oil objective. (A) Schematic of PMV aggregation probably caused by the interaction of adhesion molecules after centrifugation. (B) Schematic of PMVs maintaining monodispersion after being charged with PEI. (C) Aggregated PMVs after centrifugation. (D) PMVs show less aggregation after centrifugation due to positive charging with PEI. Scale bars = 10 µm. Please click here to view a larger version of this figure.
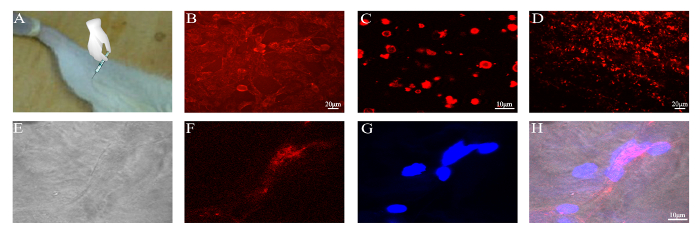
Figure 5: Delivery of CM-DiI-labeled PMVs to the gastrocnemius muscle. PMVs were generated from 5 x 105 BMSCs, concentrated by centrifugation into a volume of 100 µL, and injected slowly into the gastrocnemius muscle of a BALB/c mouse. (A) Schematic of PMV injection. (B) BMSCs labeled with CM-DiI; Scale bar = 20 µm. (C) PMVs generated from CM-DiI-labeled BMSCs and examined by confocal microscopy; Scale bar = 10 µm. (D) Gastrocnemius muscle harvested 12 h post-operation; Scale bar = 20 µm. (E–H) Frozen section of the gastrocnemius muscle examined by confocal microscopy; Scale bar = 10 µm. Please click here to view a larger version of this figure.
Discussion
Cytoplasm replacement therapy as proposed in this manuscript has unique advantages over other reported approaches such as gene, molecular, and cell therapy. PMVs generated from BMSCs encapsulate not only the products of stemness genes but also intact cellular organelles, which are essential to remedy the ageing phenotypes associated with senescence. When young cytoplasm is delivered to senescent cells, the malfunctioning mechanisms may gain a brief relief; at the same time, the epigenome could be reprogrammed and invigorated to provide a long-lasting effect. Additionally, exogenous mitochondria might have a faster proliferation rate, as indicated in our previous study12. The replacement of damaged mitochondria is essential because mitochondrial dysfunction has been linked to many senescent diseases11. In addition, unlike stem cells, the enucleated PMVs are not be able to proliferate; thus, it is unlikely to raise the concern of cancer development. Lastly, the lack of nuclear DNA in PMVs also reduces the risk of genome damage by DNA random integration.
Cultivated cells produce a variety of membranous extracellular vesicles (EV) under normal or stress conditions. Exosomes originate from late endosomes and are released by most mammalian cells as extracellular vesicles of 30-120 nm in diameter16. Larger EVs, called microvesicles, have a size ranging from 0.1-1 µm and are directly shed from the plasma membranes of cells17. Microvesicles and exosomes may have overlapping cellular constituents and functionalities in tissue repair. On the other hand, giant plasma membrane vesicles18,19 and similarly, plasma membrane spheres20 about 30 µm in size can be generated from multiple cell types by chemical induction or cell swelling. However, the application of these cell-size vesicles in therapeutics is still largely undeveloped. In any event, the production of EVs are time consuming, and, unlike PMVs, the contents within EVs are limited by the obviously absent cellular organelles.
This manuscript describes the use of PEI to prevent the aggregation of PMVs after centrifugation. PEI is a potent non-viral gene delivery carrier due to its capability of condensing DNA using multiple positively charged amino groups. A concentration of 2 µg/mL of PEI, which showed no sign of cytotoxic effect, was chosen to positively charge the cell surface and consequently resulted in the superior dispersion of PMVs. Moreover, the binding of PEI to the surface might help with the endocytosis of PMVs. Once inside of the cells, the further protonation of the amino groups in low pH can lead to endosomal swelling and bursting21.
However, the PMVs generated here are far from perfect. The 10-fold reduction in diameter, for example, from 30 µm (BMSCs) to 3 µm (PMVs) could theoretically lead to a 90% loss of cellular content. Even worse, a large fraction of PMVs have a diameter smaller than 3 µm. Furthermore, substantial cellular debris was detected during the squeezing process, probably due to the stiffness of the cell membrane, which was caused by the presence of integrated membrane proteins and lipids such as cholesterol. It is possible, however, that the flexibility of the cell membranes could be improved by incorporating fatty acids with short side chains, such as Octyl-beta-Glucoside.
Another reason for the generation of cellular debris is the presence of the cytoskeleton, which may also hinder the inclusion of organelles into the PMVs. This might explain that some of the PMVs, even those that have a diameter of 3 µm or larger, have no detectable mitochondria. Since most of the organelles are attached to the polymerized cytoskeleton, it would be interesting to test if blocking the polymerization of the cytoskeleton could improve the encapsulation of organelles.
In addition, the in vivo application of PMVs must overcome a myriad of hurdles. For example, to target hepatocytes, PMVs must avoid being captured, not only by the scavenger system in the circulation, but also by Kupffer cells that line the sinus of the liver. A method to facilitate the migration of PMVs from the circulation to target tissues may also be required. Nonetheless, in the face of these many caveats, the advantage of using PMVs for therapy is outstanding. We showed that PMVs were readily endocytosed into the myofiber of the gastrocnemius muscle after an intramuscular injection. We are currently testing to see if a dystrophic gastrocnemius muscle induced by hydrogen peroxide can be recused by PMV medication.
Declarações
The authors have nothing to disclose.
Acknowledgements
This research was supported by the Li Ka Shing Foundation, the Guangdong High-Level University Project "Green Technologies for Marine Industries," the Natural Science Foundation of China (http://www.nsfc.gov.cn/ Grant No. 30971665, 81172894, 81370925), and the Education Department of Guangdong (http://www.gdhed.edu.cn/ Grant No.cxzd1123).
Materials
| IsoporeTM membranes | Millipore | TSTP04700 | 3 mm pore |
| Disposable filter unit | Xinya, Shanghai, China | 25 mm | Medical grade polypropylene |
| Insulin syringe | BD | 328446 | 1 ml |
| pN1-EGFP | Clontech | 6085-1 | |
| MitoTracker | Molecular Probes | M7514 | Green FM, 1 μM |
| JC-1 | Beyotime, Haimen, China | C2006 | 10 mg/ml |
| CM-DiI | Beyotime, Haimen, China | C1036 | 10 mM |
| PEI | Sigma | P3143 | Mn = 75000 |
| Fluorescence Microscope | Nikon | Eclipse TE 2000 | With CCD camera |
| Confocol Microscope | Carl Zeiss | LSM 510 Meta | |
| PolyJet | SigaGen | SL100688 | For cell transfection |
Referências
- Abe, A., Miyanohara, A., Friedmann, T. Enhanced gene transfer with fusogenic liposomes containing vesicular stomatitis virus G glycoprotein. J Virol. 72 (7), 6159-6163 (1998).
- Dolatabadi, J., Valizadeh, H., Hamishehkar, H. Solid lipid nanoparticles as efficient drug and gene delivery systems: recent breakthroughs. Adv Pharm Bull. 5 (2), 151-159 (2015).
- Torchilin, V. P. Multifunctional, stimuli-sensitive nanoparticulate systems for drug delivery. Nat Rev Drug Discov. 13 (11), 813-827 (2014).
- Wolf, D. P., Mitalipov, N., Mitalipov, S. Mitochondrial replacement therapy in reproductive medicine. Trends Mol Med. 21 (2), 68-76 (2015).
- López-Otin, C., Blasco, M. A., Partridge, L., Serrano, M., Kroemer, G. The hallmarks of aging. Cell. 153 (6), 1194-1217 (2013).
- Plakkal, J., Paul, A. A., Goo, Y. H. Lipid droplet-associated proteins in atherosclerosis. Mol Med Rep. 13 (6), 4527-4534 (2016).
- Hoppener, J. W. M., Ahren, B., Lips, C. J. M. Islet amyloid and type 2 diabetes mellitus. N Engl J Med. 343 (6), 411-419 (2000).
- Jagust, W. Is amyloid-β harmful to the brain? Insights from human imaging studies. Brain. 139 (Pt 1), 23-30 (2016).
- Naidoo, N. The endoplasmic reticulum stress response and aging. Rev Neurosci. 20 (1), 23-37 (2009).
- Cuervo, A. M. Autophagy and aging: keeping that old broom working. Trends Genet. 24 (12), 604-612 (2008).
- Bratic, A., Larsson, N. G. The role of mitochondria in aging. J Clin Invest. 123 (3), 951-957 (2013).
- Lin, H. P., et al. Incorporation of VSV-G produces fusogenic plasma membrane vesicles capable of efficient transfer of bioactive macromolecules and mitochondria. Biomed Microdevices. 18 (3), 41 (2016).
- Riekstina, U., et al. Embryonic stem cell marker expression pattern in human mesenchymal stem cells derived from bone marrow, adipose tissue, heart and dermis. Stem Cell Rev. 5 (4), 378-386 (2009).
- Purandare, B., Teklemariam, T., Zhao, L. M., Hantash, B. M. Temporal HLA profiling and immunomodulatory effects of human adult bone marrow- and adipose-derived mesenchymal stem cells. Regen Med. 9 (1), 67-79 (2014).
- Nemeth, K., Mayer, B., Sworder, B. J., Kuznetsov, S. A., Mezey, E. A practical guide to culturing mouse and human bone marrow stromal cells. Curr Protoc Immunol. 102, (2013).
- Shahabipour, F., et al. Exosomes: Nanoparticulate tools for RNA interference and drug delivery. J Cell Physiol. , (2017).
- Lamichhane, T. N., et al. Emerging roles for extracellular vesicles in tissue engineering and regenerative medicine. Tissue Eng B. 21 (1), 45-54 (2015).
- Baumgart, T., et al. Large-scale fluid/fluid phase separation of proteins and lipids in giant plasma membrane vesicles. Proc Natl Acad Sci. 104 (9), 3165-3170 (2007).
- Sezgin, E., et al. Elucidating membrane structure and protein behavior using giant plasma membrane vesicles. Nat Protoc. 7 (6), 1042-1051 (2012).
- Lingwood, D., Ries, J., Schwille, P., Simons, K. Plasma membranes are poised for activation of raft phase coalescence at physiological temperature. Proc Natl Acad Sci. 105 (29), 10005-10010 (2008).
- Pandey, A. P., Sawant, K. K. Polyethylenimine: A versatile, multifunctional non-viral vector for nucleic acid delivery. Mater Sci Eng C Mater Biol Appl. 68, 904-918 (2016).

