Noninvasive Sampling of Mucosal Lining Fluid for the Quantification of In Vivo Upper Airway Immune-mediator Levels
Summary
This protocol describes a noninvasive technique for the sampling of undisturbed mucosal lining fluid from the upper airways. It can be used to perform the quantification of in vivo levels of protein mediators, such as cytokines and chemokines, in subjects of all ages.
Abstract
This protocol describes noninvasive sampling of undisturbed upper airway mucosal lining fluid. It also details the extraction procedure used prior to the analysis of immune mediators in fluid eluates for the study of the airway topical immune signature, without the need for stimulation procedures (often used by other techniques). The mucosal lining fluid is sampled on a strip of filter paper placed at the anterior part of the inferior turbinate and left for 2 min of absorption. Analytes are eluted from the filter papers, and the extracted protein-based eluates are analyzed by an electrochemiluminescence-based immunoassay, allowing for the high-sensitivity quantification of low- and high-level analytes in the same sample. We measured the in vivo levels of 20 preselected immune mediators related to specific immune signaling pathways in the upper airway mucosa, but the technique is not limited to that specific panel or sampling site. The technique was first implemented in 7-year-old children from the Copenhagen Prospective Studies on Asthma in Childhood2000 (COPSAC2000) cohort with allergic rhinitis. It was thereafter used in the longitudinal COPSAC2010 birth cohort, sampled at 1 month, 2 years, and 6 years of age and at instances of acute respiratory symptoms. We successfully obtained and analyzed samples from 620 (89%) of 700 1-month-old children; a few samples were below the assay detection limit (reported as the median (Inter-Quartile Range (IQR)). The number of samples below the detection limit (i.e. from 0 to the set point for the lower limit of detection) for each mediator was 29 (7.25 – 119.5). This technique enables the quantification of the in vivo airway mucosal immune profile from birth, can be applied longitudinally, and can be applied to studies on the effect of genetics and early-life environmental exposures, pathophysiology, endotyping, and monitoring of respiratory diseases, and development and evaluation of novel therapeutics.
Introduction
The mucosal lining fluid of the nose makes up the liquid part of the upper-airway system. It consists of a complex matrix of mediators derived from the interplay between the epithelium and the immune cells that make up the first line of defense against invading microorganisms. The nasal mucosa is easily accessible, and there is a strong functional and immunological relationship between the nose and the bronchi1. This compartment is of special interest in relation to airway diseases that are common in childhood, such as asthma and allergic rhinitis, but also to a range of other respiratory disorders more prevalent later in life.
Here, we describe the implementation of a method to sample undisturbed mucosal lining fluid from the nasal cavity using a filter paper-based, noninvasive technique, as well as a subsequent extraction procedure, used to elute protein-based analytes from the filter papers prior to their quantification. This technique can, for example, be used to obtain in vivo immune signatures of both healthy individuals and individuals with various respiratory diseases. Furthermore, it is possible to examine exposures of importance for a specific immune signature and to evaluate if it is a predictor or mediator of later disease development.
Mucosal lining fluid has previously been obtained by nasal lavage2, which is often preceded by a nasal challenge test, where an allergen is introduced in high levels to stimulate an inflammatory response3,4. However, the nasal lavage technique is not feasible in young children and introduces an unknown dilution factor, which confounds the outcomes, as the diluted mediator levels can fall below the detection limit of the assay5. Moreover, due to the unknown dilution factor, the measured analyte responses from the nasal challenge tests are not comparable between individuals, thereby limiting the usefulness of the nasal lavage technique in a cohort setting. Finally, allergen challenge is only applicable in sensitized subjects, and other challenges, such as the histamine challenge, are not physiologically relevant, potentially causing a ceiling effect on mediator release. These problems are circumvented in the presented filter paper-based technique for mucosal lining fluid collection, where the individual secretion of fluids and analyte levels are the only factors that influence inter-individual variance.
During the extraction procedure, analytes are eluted from the filter papers after the addition of identical volumes of buffer to all samples. This favors similar ex vivo dilution of all samples. An extraction buffer of albumin-based isotonic salt solution is used for the extraction step; it enables the extraction of protein-based mediators and stabilizes proteins to limit denaturation during the subsequent freezing of eluted proteins prior to quantification. To avoid protein degradation during the extraction phase, a cocktail of protease inhibitors is added to the extraction buffer.
The implementation of techniques that allow for the quantification of undisturbed, in vivo-generated immune mediators at mucosal sites is of the utmost importance. First, the mucosal site makes up the largest immunological organ in the body. Second, the nasal location is the primary site of airborne exposure and is tightly connected to the respiratory immunological compartment of the lungs1. Third, the possibility of surveying this important organ with a noninvasive technique opens the possibility to provide a plethora of information on the important microbe-immune interaction axis in relation to health and disease in the airways. Fourth, there are many other possible applications of this technique, such as studying local immunological alterations in randomized, controlled trials of drugs and micronutrients.
We initially implemented the technique in the Copenhagen Prospective Studies on Asthma in Childhood2000 (COPSAC2000) cohort, where we determined the immune profile of the mucosal lining fluid in 7-year-old children with allergic rhinitis versus healthy controls13. Subsequently, we successfully applied this technique to the longitudinal COPSAC2010 cohort and assessed airway immune profiles at 1 month, 2 years, and 6 years of age and at instances of acute respiratory symptoms. Results from the 1-month-old neonates have demonstrated important associations between the immune signature and early-life environmental exposure7,8,9,10,11,12.
Protocol
The studies were conducted in accordance with the guiding principles of the Declaration of Helsinki. Approvals from the Ethics Committee for Copenhagen (KF 01-289/96 for COPSAC2000 and H-B-2008-093 for COPSAC2010) and the Danish Data Protection Agency were obtained, and written informed consent was obtained from both parents of each subject before enrollment.
1. Experimental Setup
- Use sheets of filter paper (fibrous hydroxylated-polyester sheets, see the Table of Materials)6,7.
- Cut out strips 3 x 15 mm in size for a 1-month-old child and in an L-shape for older children and adults (5 x 20 x 10 mm (short arm of the L)).
- Insert one piece of filter paper into each nostril of an infant using forceps. Place the filter paper at the anterior part of the inferior turbinate (approximately 1 cm inside the nostril for neonates and 1.5 cm for all other ages).
- Place neonates on a bed and give them sugar water for comfort.
NOTE: For children of all other ages, the sampling is performed most easily with the subject seated on a chair. - For older children, insert the long arm of the L-shaped filter paper into each nostril.
- Place neonates on a bed and give them sugar water for comfort.
- Apply a nose clip around the nostrils to minimize discomfort and to avoid the accidental loss of the filter paper.
- Remove the filter papers after 2 min of absorption.
- Place the filter papers in labeled tubes and freeze them immediately at -80 °C.
- Make a note of any symptoms of airway infection shown by the child on the day of sampling.
- Record any sneezing, persistent crying, or epistaxis that occurs during the 2 min of sampling.
2. Quantification of Airway Immune-mediators
- Take up to 10 random samples from the freezer. Record the identification numbers. Keep the samples on ice during the whole working process.
- After thawing on ice, immerse the filter papers (per subject) from both nostrils in 300 µL of freshly prepared buffer (see the Table of Materials) containing one complete protease inhibitor tablet (see the Table of Materials) per 25 mL of buffer.
- Adjust the volume of buffer as per the size of the filter paper.
- Use 300 µL of buffer for filter papers 3 x 15 mm in size.
- If only one filter paper is available, use half the buffer volume (150 µL).
- Adjust the volume of buffer as per the size of the filter paper.
- Transfer the samples to a plate shaker (400 rpm) for 5 min. Use a timer.
- Transfer the moist filter papers and assay buffer into the cup of a cellulose acetate tube filter (0.22 µm pore size) placed within a microcentrifuge tube (see the Table of Materials).
- Centrifuge at 16,000 x g for 5 min in a cooled centrifuge (at 4 °C) to obtain filtered nasal extract in the tube.
- Remove the cup and keep the tubes on ice while aliquoting the nasal extract into the wells of low-protein binding storage plates (see the Table of Materials).
- Store at -80 °C until analysis.
- Determine the concentrations of cytokines and chemokines in the nasal extracts using a high-sensitivity, electrochemiluminesce-based multiplexed array system (see the Table of Materials).
NOTE: A human 10-plex TH1/TH2 cytokine assay, 9-plex chemokine assay, and single-plex IL-17A, TGF-β1, and TSLP were performed here.- For the assays, incubate the nasal extracts overnight at 4 °C. Conduct the measurements as per the standard manufacturer protocol (see the Table of Materials).
NOTE: The immunoassays (see the Table of Materials) typically have a high dynamic range for measurement ranging between 1 & 10,000 pg/mL, but for some assays, it can be 100,000 pg/mL. This means that all samples can be run at the same dilution, thus limiting the influence of dissimilar dilutions in healthy and diseased subjects, as is the case in other similar immunoassays. The lower limit of detection for all cytokines was 1 pg/mL or less, and for chemokines, it ranged between 1 & 50 pg/mL. TSLP was not detectable in 98% of samples at 1 month of age.
- For the assays, incubate the nasal extracts overnight at 4 °C. Conduct the measurements as per the standard manufacturer protocol (see the Table of Materials).
3. Statistics
- Log-transform the data prior to analysis to obtain normally distributed residuals of the cytokine and chemokine levels.
- If the samples are analyzed in more than one batch, correct the cytokine and chemokine data for batch-wise centering on the log-transformed data.
- Incorporate the overall mean and anti-log transformation to obtain data in the original measurement units.
NOTE: For every set of analyses done, consider adjusting variables that affect the level of cytokines and chemokines. This could be maternal influences (e.g., alcohol consumption and smoking during pregnancy), breastfeeding, adverse events during sampling, siblings, pets in the home, etc.
- Incorporate the overall mean and anti-log transformation to obtain data in the original measurement units.
Representative Results
Baseline Characteristics of the Airway Immune Profiles:
Complete data on upper airway mucosal immune mediator levels at 1 month of age was obtained in 620 (89%) of the 700 children enrolled in the COPSAC2010 cohort. Ten neonates were enrolled before the technique was established, and 19 did not attend the 1-month visit. Additionally, 47 samples were excluded because they were extracted and measured in another laboratory used in a pilot study, and 4 samples were lost in transportation (See the study flowchart, Figure 1). Baseline characteristics for the cohort are shown in Table 1.
Most of the mediators had a low level of detection <10 pg/mL (IL-1β, IL-2, IL-4, IL-5, IL-10, IL-12p70, IL-13, IL-17A, TGF-β1, IFN-γ, TNF-α, CXCL8, CCL13, CCL4, and CCL17), whereas 5 had a detection level >10 pg/mL (CXCL10, CCL2, CCL11, CCL26, and CCL22) (Table 2). The median (IQR) number of samples below the detection limit (i.e. from 0 to the lower limit of detection) for each mediator was 29 (7.25 – 119.5) (Table 2). IFN-γ levels were below the lower detection limit in almost half (46%) of the samples, whereas CXCL8 and IL-1β levels were detectable in all samples, and TGF-β1, CCL4, and TNF-α levels were below the lower detection limit in <1% of the samples. One of the mediators, TSLP, was only detected in 2% of samples, and TSLP data was therefore not used for consecutive data analyses. A strong multicollinearity was evident for the 20 detected immune mediator levels, which is visualized in a heatmap (Figure 2).
The Influence of Airway Bacteria and Viruses in Neonates:
We previously found an association between the airway immune profile and colonization in specific airway bacteria sampled concomitantly with mucosal lining fluid at 1 month of age. An upregulated Type 1- and Type 17-based airway immune profile was observed in neonates colonized with intracellular bacteria, whereas the presence of extracellular bacteria was associated with a Type 17-based profile7. We have also shown previously that the presence of picornavirus was associated with an upregulated profile comprised mainly of Type 1 immune mediators (Figure 3)11. These findings were obtained in children without respiratory symptoms, (i.e. asymptomatic) on the day of sampling, suggesting an immune-triggering role of colonizing bacteria and viruses in the earliest part of life.
The Influence of Pre- and Perinatal Exposure:
We have further observed an association between the level of immune mediators at 1 month of age and a maternal history of atopy8, where children born to atopic mothers displayed an overall lower level of immune mediators than children of non-atopic mothers. We subsequently identified an association between the presence of siblings in the household at birth and a specific Type 1/Type 17-directed mucosal immune response, with evidence of this being related to an in utero immune priming effect, as the immune response was inversely correlated to the time since last childbirth (Figure 4)12. Further evidence for in utero immune priming effects was determined from an association between maternal H1N1 influenza vaccination status and the upregulation of TGF-β1 levels in the neonatal airway10.
Evidence for Micronutrient Mechanisms of Action:
In COPSAC2010, we performed a high-dose versus standard-dose vitamin D intervention trial during pregnancy, which showed an almost one-fourth reduction in the risk of asthma/recurrent wheeze in the offspring at 3 years of age9. Utilizing the measured neonatal airway immune profile, we observed that children born to mothers receiving high-dose vitamin D, compared to standard-dose, were characterized by an upregulation of specific mediators (IL-12p70, IL-13, CCL17, TGF-β1, and IL-2), suggesting that vitamin D promotes an anti-bacterial defense system different from that induced by bacteria, virus, siblings, and maternal atopy9 (Figure 5).
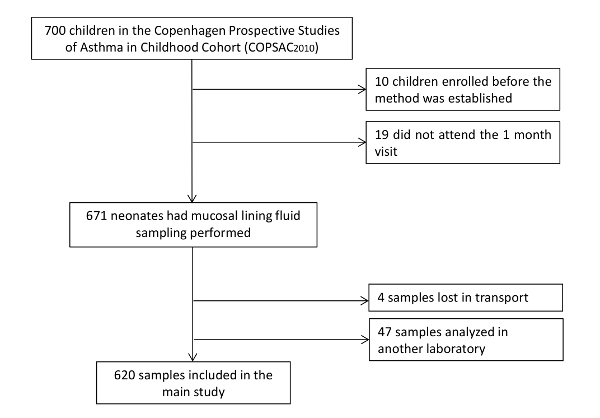
Figure 1: Study Group Flowchart. Complete data on upper airway mucosal immune mediator levels at 1 month of age was obtained in 620 (89%) of the 700 children enrolled in the COPSAC2010 cohort. Ten neonates were enrolled before the technique was established, and 19 did not attend the 1-month visit. Additionally, 47 samples were excluded because they were extracted and measured in another laboratory used in a pilot study, and 4 samples were lost in transportation. Please click here to view a larger version of this figure.
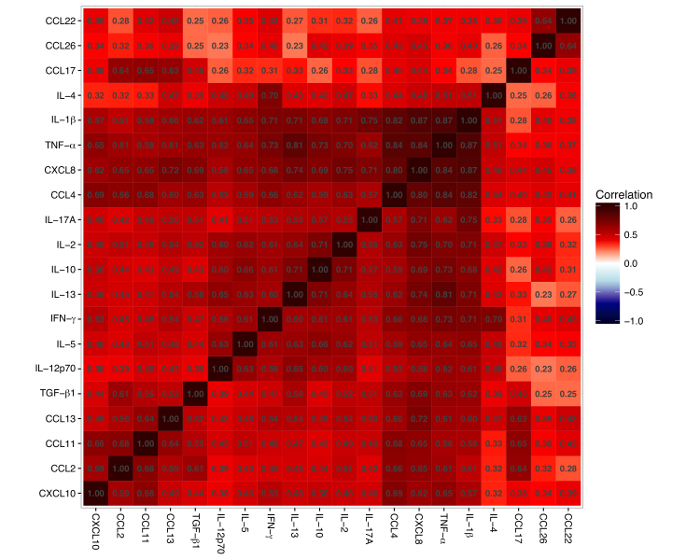
Figure 2: Heatmap of Pairwise Correlations between the 20 Cytokines and Chemokines. The red color represents a positive correlation and the blue color represents a negative correlation. The number inside every square denotes the correlation between two mediators. The figure shows that all mediators are positively correlated. Please click here to view a larger version of this figure.
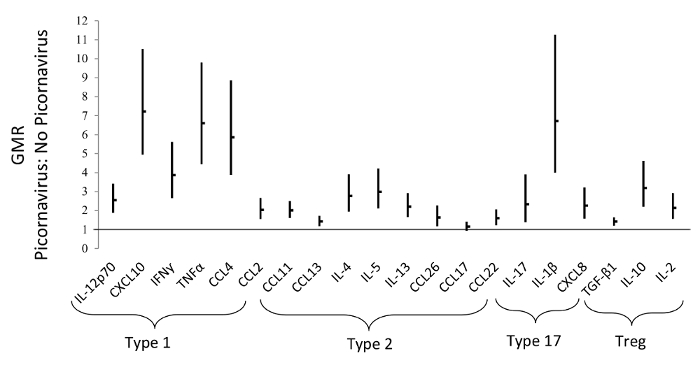
Figure 3: The Presence of Picornavirus is Associated with an Upregulated Profile Comprised Mainly of Type 1 Immune-mediators. This figures shows the Geometric Mean Ratios (GMR), with 95% Confidence Intervals (CI), of airway mediators in neonates with/without the concomitant presence of picornavirus, measured at 1 month of age. This figure has been modified from Wolsk et al.11, with permission. Please click here to view a larger version of this figure.
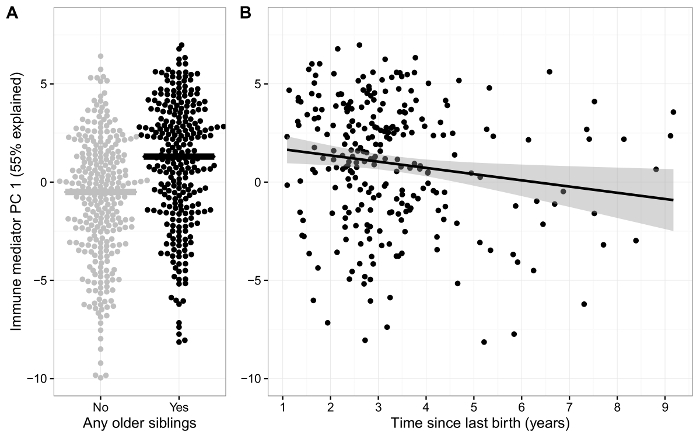
Figure 4: Effects of the Presence of Siblings in the Household at Birth. A) Airway immune signature (PC1) in neonates with and without siblings at birth. B) Scatterplot showing an inverse association between the airway immune signature (PC1) and time since previous birth for children with siblings. This figure has been modified from Wolsk et al.12, with permission. Please click here to view a larger version of this figure.
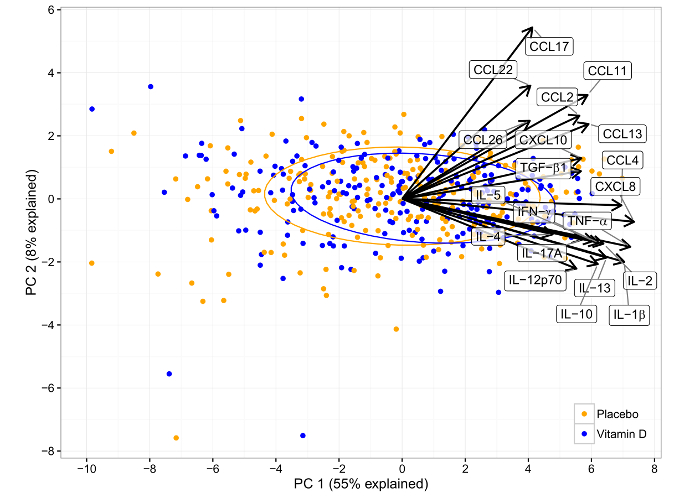
Figure 5: Principal Component Analysis Biplot of the Effect of Vitamin D Intervention in the COPSAC2010 Cohort on the Airway Immune Mediator Level at 1 Month of Age. Each point represents a sample, colored according to the intervention allocation of the child. The loadings (i.e. impact) of the original variables on the principal components are shown as arrows. The intervention resulted in a significantly upregulated immune profile in PC1, which is positively correlated with all immune mediators. This figure has been modified from Chawes et al.9, with permission. Please click here to view a larger version of this figure.
| N = 620 (%) | |
| Maternal atopy | 334 (54) |
| Non Caucasian | 29 (5) |
| Cesarean section | 134 (22) |
| Antibiotics in 3rd trimester | 126 (20) |
| Siblings in the home at birth | 358 (58) |
| Pets (cat and/or dog) in the home | 216 (35) |
| Fully-breastfed at 1 month | 570 (92) |
| No smoking in 3rd trimester | 597 (96) |
| No alcohol consumption in 3rd trimester | 591 (95) |
| Family income >100.000 euro/year | 89 (14) |
| Gestational age >37 weeks | 601 (97) |
| Apgar score >7 at 5 min | 591 (95) |
Table 1: Baseline Characteristics of the COPSAC2010 Cohort.
| Mediator | Detection limit pg/ml | < Detection limit N (%) | Median pg/ml | IQR pg/ml |
| IL-12p70 | 1.4 | 102 (16%) | 10.80 | 4.99 – 22.22 |
| CXCL10 | 31 | 8 (1%) | 1787.78 | 722.8 – 5423 |
| IFN-γ | 4.1 | 285 (46%) | 20.14 | 7.63 – 48.51 |
| TNF-α | 0.6 | 5 (<1%) | 28.33 | 10.06 – 88.55 |
| CCL4 | 4.3 | 4 (<1%) | 194.90 | 71.22 – 655.8 |
| CCL2 | 16.0 | 9 (1%) | 256.11 | 134.4 – 468.5 |
| CCL13 | 2.8 | 13 (2%) | 15.17 | 10.35 – 22.80 |
| IL-4 | 1.1 | 196 (32%) | 1.37 | 0.42 – 2.86 |
| IL-5 | 1.2 | 172 (28%) | 3.72 | 1.42 – 8.10 |
| IL-13 | 2.7 | 48 (8%) | 14.49 | 6.84 – 27.51 |
| CCL11 | 22.0 | 39 (6%) | 139.54 | 85.00 – 236.0 |
| CCL26 | 45.0 | 252 (41%) | 164.93 | 56.33 – 350.02 |
| CCL17 | 7.2 | 90 (15%) | 41.30 | 26.83 – 60.68 |
| CCL22 | 25.0 | 78 (13%) | 398.31 | 243.4 – 575.7 |
| IL-1β | 0.4 | 0 (0%) | 225.49 | 50.07 – 1169 |
| IL-17A | 1.5 | 285 (46%) | 1,480 | 0.43 – 4.79 |
| CXCL8 | 3 | 0 (0%) | 19465 | 6321 – 38274 |
| TGF-β1 | 5.2 | 1 (<1%) | 28.66 | 20.76 – 39.50 |
| IL-10 | 1 | 13 (2%) | 22.32 | 9.28 – 49.16 |
| IL-2 | 1.5 | 19 (3%) | 18.72 | 8.29 – 37.33 |
Table 2: Mucosal Lining Fluid Samples from 1-month-old Neonates from the COPSAC2010 Cohort. Most of the mediators had a low level of detection <10 pg/mL (IL-1β, IL-2, IL-4, IL-5, IL-10, IL-12p70, IL-13, IL-17A, TGF-β1, IFN-γ, TNF-α, CXCL8, CCL13, CCL4, and CCL17), whereas 5 had a detection level >10 pg/mL (CXCL10, CCL2, CCL11, CCL26, and CCL22).
Discussion
With the technique presented here, we were able to determine the in vivo upper-airway mucosal immune profile in children from as early as 1 month of age, which has not been done previously. We observed that presence of specific airway bacteria and picornaviruses7,11, as well as other pre and perinatal exposures, were mirrored in the airway immune profile of the neonates. Furthermore, we used these airway immune profile data to study the mechanism of action of a randomized micronutrient trial of high-dose vitamin D. These results underline the validity and utility of the mucosal lining fluid technique as a noninvasive sampling technique for the subsequent in vivo quantification of airway immune mediators.
The following points are worth considering before method implementation: In order to measure cytokines in the mucosal lining fluid matrix, it is important to use high-sensitivity-based immunoassay methodologies, such as that provided by electrochemiluminescence protocols. The use of enzyme-linked immunoassays can lead to inference in the absorbance readout. Furthermore, although there is a strong relationship between the nose and the bronchi, the measurement of the immune profile in the upper airways is still a limitation. Sampling was performed on the upper part of the mucosal layer; thus, we expect that the fluid mainly represents the composition of the gel layer, not the sol layer. We did not measure mucous layer markers to confirm this, which is a limitation. Individual variations in nasal fluid secretion may in theory influence the detected levels of cytokines and chemokines. However, we have been able to identify clear and varying immunological phenotypes in response to different exposures7,8,9,10,11,12, indicating that inter-individual variations in nasal secretion dynamics may not be a major obstacle for this technique. While performing the technique, it is important to perform all procedures at low temperatures (ice-cooled) to avoid protein degradation during the processing. Protein degradation can be further reduced by adding the recommended protein protease inhibitors to the protein extraction buffer.
Nevertheless, the strength of this technique is that it is noninvasive and easy to perform, enabling longitudinal assessments of the mucosal airway immune signature from the neonatal period and onwards. Such information could potentially provide knowledge on how the local airway immune system matures and develops throughout childhood and into adulthood. Furthermore, the sampling technique is performed undisturbed and in an in vivo-relevant, undiluted manner, which enabled the detection of immune mediators that would have been undetectable with the nasal lavage technique, where an unknown dilution factor reduces the validity of findings13. We used a synthetic, fibrous, hydroxylated-polyester medium that is commercially available (see the Table of Materials) and is designed for sample collection, storage, and conjugate release. The material is hydrophilic, with low biomolecular binding, and protein-containing samples are stable during storage at -80 °C. The material is highly absorbent, and the fiber surfaces have been modified to enhance water wettability.
In the COPSAC2010 study, we selected a panel of 21 cytokines and chemokines that were chosen a priori to represent major phenotypical pathways of the immune system acting locally in the airways. Only one of these mediators, TSLP, was not detectable at one month of age.
An important future application would be to collect mucosal lining fluid from the lower airways (e.g., by using the bronchoscopic microsampling technique) to assess lower-airway immunology. Bronchoscopic microsampling is a new procedure for bronchoscopic diagnosis, capable of collecting local bronchial epithelial lining fluid; it utilizes a sheathed polyester fiber probe14. When the distal airways are reached, the probe is pushed out of the sheath tip to absorb the bronchial epithelial fluid present in the bronchial lumen. Bronchoscopic microsampling has been used in acute respiratory distress syndrome14,15,16 and chronic obstructive pulmonary disease17. However, in asthma, an existing problem is that the probe may cause bleeding due to disease-induced epithelial fragility and increased vascularization. Applying the mucosal lining fluid technique to the bronchoscopic microsampling procedure could have important clinical implications in relation to our understanding of the pathophysiology, as well as to monitoring, treating, and predicting the outcome of several acute and chronic pulmonary diseases in both children and adults.
All types of mediators can theoretically be measured using the multiplex immunoassay platform of the commercial system used here (see the Table of Materials). The platform allows for the high-sensitivity and accurate quantification of any mediator for which a pair of monoclonal antibodies applicable to the development of an immunoassay can be obtained. This platform also brings with it high precision and less sensitivity to pH, temperature, and viscosity variations in the fluids to be analyzed.
In future studies, it would be of great value to expand the current panel of immune mediators to the measurement of, for example: antimicrobial peptides; total and specific IgA; acute-phase reactants, such as C-reactive protein; lipid mediators, such as prostaglandins; and neurological mediators, such as brain-derived neurotrophic factor (BDNF). This could provide a more detailed view of the nature of the mucosal response system at a body site with extensive airborne exposure. The suggested mediators may either be measured by means of targeted quantitative high-sensitivity immunoassays, such as in the current setup, or by targeted or untargeted Nuclear Magnetic Resonance (NMR) spectroscopy- or Liquid Chromatography-Mass Spectrometry (LC-MS)-based metabolomics techniques. The latter approach has already been implemented to some extent with the exhaled breath condensate method, which has some methodological drawbacks with respect to the sampling of the biofluid18 that are not apparent for the mucosal lining fluid technique.
Longitudinal sampling from the neonatal period into adulthood, as performed in the COPSAC2010 cohort, will greatly increase our understanding of underlying immunological factors preceding or acting concomitantly with airway diseases such as asthma and allergies. Such findings may be important to the development of novel therapeutics targeting specific disease endotypes and to the investigation of micronutrient and drug mechanisms of action in randomized clinical trials.
Declarações
The authors have nothing to disclose.
Acknowledgements
We express our gratitude to the children and families of the COPSAC2010 cohort study for all their support and commitment. We acknowledge and appreciate the unique efforts of the COPSAC research team and the technical help from Technician Lisbeth Buus Rosholm, Technical University of Denmark, for the measurement of cytokines and chemokines.
Materials
| Fibrous hydroxylatedpolyester sheets | Accuwik Ultra | SPR0730 | Filter paper |
| Milliplex Assay Buffer | Millipore | L-AB | Buffer |
| low-protein binding storage plates | Thermo Scientific | CLS8161 | Plates |
| Protease Inhibitor | Roche | 11873580001 | complete EDTA-free Protease Inhibitor Cocktail |
| Reader of multi-spot plates | Mesoscale | NA | Sector imager 6000 |
| Assays | Mesoscale | Human 10-plex TH1/TH2 cytokine assay and 9-plex chemokine assay, and singleplex IL-17A, TGF-β1and TSLP. A description can be found online on www.mesoscale.com |
Referências
- Bousquet, J., van Cauwenberge, P., Khaltaev, N. Allergic Rhinitis and Its Impact on Asthma. J Allergy Clin Immunol. 108 (5), 147-334 (2001).
- Howarth, P. H., Persson, C. G. A., Meltzer, E. O., Jacobson, M. R., Durham, S. R., Silkoff, P. E. Objective monitoring of nasal airway inflammation in rhinitis. J Allergy Clin Immunol. 115 (3), 414-441 (2005).
- Bensch, G. W., Nelson, H. S., Borish, L. C. Evaluation of cytokines in nasal secretions after nasal antigen challenge: lack of influence of antihistamines. Ann Allergy Asthma Immunol. 88 (5), 457-462 (2002).
- Sussman, G. L., Mason, J., Compton, D., Stewart, J., Ricard, N. The efficacy and safety of fexofenadine HCl and pseudoephedrine, alone and in combination, in seasonal allergic rhinitis. J Allergy Clin Immunol. 104 (1), 100-106 (1999).
- Bisgaard, H., Robinson, C., Rømeling, F., Mygind, N., Church, M., Holgate, S. T. Leukotriene C4 and histamine in early allergic reaction in the nose. Allergy. 43 (3), 219-227 (1988).
- Chawes, B. L. K., et al. A novel method for assessing unchallenged levels of mediators in nasal epithelial lining fluid. J Allergy Clin Immunol. 125 (6), 1387-1389 (2010).
- Følsgaard, N. V., et al. Pathogenic bacteria colonizing the airways in asymptomatic neonates stimulates topical inflammatory mediator release. Am J Respir Crit Care Med. 187 (6), 589-595 (2013).
- Følsgaard, N. V., et al. Neonatal Cytokine Profile in the Airway Mucosal Lining Fluid Is Skewed by Maternal Atopy. Am J Respir Crit Care Med. 185 (3), 275-280 (2012).
- Chawes, B. L., et al. Effect of Vitamin D3 Supplementation During Pregnancy on Risk of Persistent Wheeze in the Offspring: A Randomized Clinical Trial. JAMA. 315 (4), 353-361 (2016).
- Bischoff, A. L., et al. Altered Response to A(H1N1)pnd09 Vaccination in Pregnant Women: A Single Blinded Randomized Controlled Trial. PloS One. 8 (4), 56700 (2013).
- Wolsk, H. M., et al. Picornavirus-Induced Airway Mucosa Immune Profile in Asymptomatic Neonates. J Infect Dis. 213 (8), 1262-1270 (2016).
- Wolsk, H. M., Chawes, B. L., Følsgaard, N. V., Rasmussen, M. A., Brix, S., Bisgaard, H. Siblings Promote a Type 1/Type 17-oriented immune response in the airways of asymptomatic neonates. Allergy. 71 (6), 820-828 (2016).
- Alam, R., Sim, T. C., Hilsmeier, K., Grant, J. A. Development of a new technique for recovery of cytokines from inflammatory sites in situ. J Immunol Methods. 155 (1), 25-29 (1992).
- Ishizaka, A., et al. New bronchoscopic microsample probe to measure the biochemical constituents in epithelial lining fluid of patients with acute respiratory distress syndrome. Crit Care Med. 29 (4), 896-898 (2001).
- Ishizaka, A., et al. Elevation of KL-6, a lung epithelial cell marker, in plasma and epithelial lining fluid in acute respiratory distress syndrome. Am J Physiol Lung Cell Mol Physiol. 286 (6), 1088-1094 (2004).
- Nakano, Y., et al. Endothelin-1 level in epithelial lining fluid of patients with acute respiratory distress syndrome. Respirology. 12 (5), 740-743 (2007).
- Komaki, Y., et al. Cytokine-mediated xanthine oxidase upregulation in chronic obstructive pulmonary disease’s airways. Pulm Pharmacol Ther. 18 (4), 297-302 (2005).
- Moeller, A., Franklin, P., Hall, G. L., Horak, F., Wildhaber, J. H., Stick, S. M. Measuring exhaled breath condensates in infants. Pediatr Pulmonol. 41 (2), 184-187 (2006).

