A Standardized Approach for Multispecies Purification of Mammalian Male Germ Cells by Mechanical Tissue Dissociation and Flow Cytometry
Summary
This work describes the standardization of a method to obtain purified germ cell populations from testicular tissue of different mammalian species. It is a straightforward protocol that combines mechanical testis dissociation, staining with Hoechst-33342 and propidium iodide, and FACS sorting, with wide applications in comparative studies of male reproductive biology.
Abstract
Fluorescence-activated cell sorting (FACS) has been one of the methods of choice to isolate enriched populations of mammalian testicular germ cells. Currently, it allows the discrimination of up to 9 murine germ cell populations with high yield and purity. This high-resolution in discrimination and purification is possible due to unique changes in chromatin structure and quantity throughout spermatogenesis. These patterns can be captured by flow cytometry of male germ cells stained with fluorescent DNA-binding dyes such as Hoechst-33342 (Hoechst). Herein is a detailed description of a recently developed protocol to isolate mammalian testicular germ cells. Briefly, single cell suspensions are generated from testicular tissue by mechanical dissociation, double stained with Hoechst and propidium iodide (PI) and processed by flow cytometry. A serial gating strategy, including the selection of live cells (PI negative) with different DNA content (Hoechst intensity), is used during FACS sorting to discriminate up to 5 germ cell types. These include, with corresponding average purities (determined by microscopy evaluation): spermatogonia (66%), primary (71%) and secondary (85%) spermatocytes, and spermatids (90%), further separated into round (93%) and elongating (87%) subpopulations. Execution of the entire workflow is straightforward, allows the isolation of 4 cell types simultaneously with the appropriate FACS machine, and can be performed in less than 2 h. As reduced processing time is crucial to preserve the physiology of ex vivo cells, this method is ideal for downstream high-throughput studies of male germ cell biology. Moreover, a standardized protocol for multispecies purification of mammalian germ cells eliminates methodological sources of variables and allows a single set of reagents to be used for different animal models.
Introduction
Given the lack of an in vitro system representative of spermatogenesis progression, and the presence of great cellular heterogeneity in testis, studies of male germ cell biology require robust techniques to isolate enriched populations of specific cell types. Fluorescence-activated cell sorting (FACS) has been widely used for this purpose 1,2,3,4,5, as it provides high yield and purity, and surpasses other isolation methods in the number of germ cell types that it can identify and select 6,7,8. The principle of flow cytometry analysis is based on the detection of differential light patterns following laser beam excitation of single cells. As a cell passes through the laser it reflects/scatters light at all angles, proportional to cell size (forward scatter; FSC) and to intracellular complexity (side scatter; SSC). See Ormerod 9 for detailed information on flow cytometry.
Male germ cells undergo specific modifications in DNA content, chromatin structure, size and shape throughout different stages of spermatogenesis. Thus, distinct cell populations can be identified and separated by combining light scattering and DNA staining with fluorescent dyes 10,11. Several dyes can be used for this purpose (reviewed in Geisinger and Rodriguez-Casuriaga 3), such as Hoechst-33342 (Hoechst) which has been frequently used in flow cytometry analysis of testicular cells for the past decade 1,2,4,10,12. Upon excitation with UV-light, Hoechst emits blue fluorescence proportional to the cellular DNA content whereas far red fluorescence reflects variability in chromatin structure and compaction 1,13,14. As a result, male germ cells in different stages of differentiation exhibit specific patterns during FACS of Hoechst-stained single cell suspensions (Ho-FACS; 1,12). Interestingly, due to a mechanism of dye efflux that is only active during the spermatogonial stage, intensity of Hoechst blue fluorescence is not proportional to chromatin content in these cells, and they cluster as a side population during Ho-FACS 15. Additionally, combining Hoechst staining with the non-permeant dye propidium iodide (PI) allows users to discriminate live (PI negative) from dead (PI positive) cells during FACS 1,2,10,12. This strategy has been previously used in flow cytometric analyses of testicular germ cells and optimized extensively in the mouse to discriminate up to 9 germ cell types, including cells in 4 different stages of meiosis I 1,2,4,16. For the purpose of this work, Hoechst staining has three main advantages. First, Ho-FACS has been successfully applied to the isolation of male germ cells in the mouse model 1,2,12, and other rodents such as rat and guinea pig 17,18,19. Second, Hoechst is a cell-permeant dye and does not require membrane permeabilization, so it preserves cell integrity. Finally, no RNase treatment is required since Hoechst binds preferentially to poly(d[AT]) DNA sequences 1,20, which means that RNA is preserved and, in addition to DNA and proteins, can be used for further downstream molecular studies of germ cell differentiation.
Despite the similarity in DNA ploidy and/or stainability observed in flow cytometry analyses of mammalian species (reviewed in Geisinger and Rodriguez-Casuriaga 3), there has been a good deal of variability in the protocols described for male germ cell isolation by flow cytometry. Different studies have employed specific protocols for tissue dissociation, and used distinct DNA-binding dyes (alone or in combination) and FACS gating strategies in different model organisms, mainly the mouse, rat and guinea pig. Hence, direct comparison of data collected for different species can be affected by unaccounted technical artifacts resulting from variability between methodologies. Importantly, the striking conservation of chromatin dynamics throughout mammalian spermatogenesis (2N-4N-2N-1N) suggests that a standardized protocol could be transversely applied to a variety of mammalian species.
The goal of this study was to develop a single workflow that is applicable to different mammalian species, by combining and adapting previously published techniques 2,20. Standardization of a method for tissue processing was achieved by performing mechanical dissociation to overcome the need for species-specific adjustments required for enzymatic digestion 5. It is noteworthy that mechanical dissociation of rodent testicular tissue has been shown to perform better than enzymatic tissue digestion 20 and Ho-FACS of single cell suspensions generated by both methods exhibit comparable results 5. As proof of principle, this protocol describes the settings used to isolate up to 5 germ cell populations: spermatogonia (SPG); primary (SPC I) and secondary spermatocytes (SPC II), and spermatids (SPD) – round (rSPD) and elongating (eSPD). Importantly, it is easy to implement in the lab, with the main requirements being the system for tissue dissociation and access to a cell sorter equipped with a UV laser. This workflow (Figure 1) is fast and straightforward and allows the simultaneous isolation of 4 germ cell populations from fresh testicular tissue in less than 2 h. The reduced processing time is crucial to maintain cellular integrity for further downstream procedures. Moreover, its successful performance in 5 different species suggests it could be broadly applied within the mammalian clade, making it the ideal method to isolate germ cells for comparative studies of mammalian male reproductive biology.
This protocol is composed of three major sections, aside from preparatory steps: (1) the mechanical dissociation of testicular tissue and (2) staining of testicular cells with Hoechst and PI, followed by (3) FACS sorting of relevant spermatogenic cells. Once collected, these enriched populations of different mammalian testicular germ cells can be used for a wide range of applications. This protocol describes a "one-size fits all" dissociation method to purify male germ cells from many different mammalian species. Depending on the type of study users wish to conduct with the isolated germ cells, other media or buffers can be used. The following protocol steps are for generating single-cell suspensions from one whole murine testis.
Protocol
All procedures described below complied with regulations of the Animal Studies Committee at Washington University in St. Louis.
1. Preparations for Mechanical Dissociation Protocol
- Pre-wet a 50 µm disposable tissue disaggregation cartridge for dissociation of a whole murine testis.
NOTE: This disposable tissue disaggregation cartridge contains microblades designed for cutting of tissues and an immobile steel mesh with approximately 100 hexagonal holes. Different sizes of mesh for tissue disaggregation cartridge are available to adapt this protocol based on species and desired cell types. 50 µm cartridges were used for all mammalian species mentioned in this paper.- Load 1 mL of ice cold phenol red free 1x Dulbecco's Modified Eagle Medium (DMEM) onto the 50 µm disposable tissue disaggregation cartridge.
NOTE: Phenol red may interfere with detection of red fluorescence during FACS. - Aspirate 1x DMEM from the tissue disaggregation cartridge with a disposable 3 mL needle-less syringe from the syringe port.
- Load 1 mL of ice cold phenol red free 1x Dulbecco's Modified Eagle Medium (DMEM) onto the 50 µm disposable tissue disaggregation cartridge.
- Turn on tissue disaggregation system by pressing "ON" button. This system does not require "warm up" time.
- Pre-wet three 40 µm disposable filters with ice cold 1x DMEM. Place each 40 µm disposable filter on a 50 mL conical tube. Pipette 1 mL of 1x DMEM and allow pass through of the liquid.
- Prepare a 100 mm x 15 mm Petri dish for collection of testicular tissues. Pipette 500 µL of 1x DMEM onto the Petri dish.
2. Preparation of Testicular Tissue for Mechanical Dissociation Protocol
- Dissect a male mouse to carefully remove fresh whole testes or testicular fragments (if dealing with other mammalian species) with surgical scissors and forceps.
NOTE: Ensure tissues are free of fat and necrosis. Fresh testicular tissues generate superior single-cell suspension quality than frozen tissues.- Transfer collected testes/fragments to the prepared 100 mm x 15 mm Petri dish containing 500 µL of 1x DMEM.
- Gently rinse the tissue in 1x DMEM to remove red blood cells (if present).
- Carefully remove tunica albuginea. Thickness of tunica albuginea will vary in different species.
- Grab one end of testis with forceps.
- Puncture the other end of testis with a scalpel while still holding the one end of testis with forceps from previous step.
- Scrape testicular tubules using a scalpel while still holding the one end of testis with forceps from Step 2.2.1.
- Cut testicular tubules into pieces of ~2-3 mm3 using a scalpel.
3. Obtaining Single Cell Suspensions by Mechanical Dissociation of Testicular Tissue
NOTE: The dissociation steps described below are for one adult mouse testis. Volumes of testicular tissues from juvenile mice or non-murine species should be adjusted accordingly. Juvenile animals may not contain all the stages of germ cells. Some mammalian species have testicular tissue composition changes during breeding seasons in comparison to non-mating season.
- Transfer the small testicular tubule pieces to the pre-wetted 50 µm tissue disaggregation cartridge using forceps and add 1 mL of 1x DMEM.
- Load the tissue disaggregation cartridge to tissue disaggregation system and process for 5 min by turning the knob from "standby" to "run" mode.
- Remove the tissue disaggregation cartridge from tissue disaggregation system and aspirate cell suspension with a disposable 3 mL needle-less syringe from the syringe port.
- Aspirate a few times to remove all liquid from tissue disaggregation cartridge. Not all of the 1 mL may be recovered from the tissue disaggregation cartridge.
- Pass the aspirated cell suspension through two disposable pre-wetted 40 µm filters. Filter twice to remove any cell aggregates.
- Place one pre-wetted 40 µm filter in a 50 mL conical tube. Directly add aspirated cells in the syringe onto the 40 µm filter. Pipette pass-through single-cell suspension with a disposable pipette.
- Remove the used pre-wetted 40 µm filter and place another pre-wetted 40 µm filter in the 50-mL conical tube. Pipette the collected single-cell suspension onto the clean 40 µm filter.
- Collect filtered single-cell suspension with a clean disposable pipette.
- Pipette filtered cell suspension back to the 50 µm tissue disaggregation cartridge and process for another 5 min in a tissue disaggregation system.
- Recover all the liquid from the tissue disaggregation cartridge.
4. Staining with Hoechst and Propidium Iodide (PI)
- Transfer recovered cell suspension from the 50 µm tissue disaggregation cartridge to a clean 1.5 mL tube . Approximately 1-1.5 mL of single-cell suspension will be recovered.
- Divide the recovered single-cell suspension into four 1.5 mL or 5 mL tubes.
- For first three tubes, pipette 150 µL of single-cell suspension and pipette rest of single-cell suspension into the last of the four tubes (approximately 550 µL of single-cell suspension).
NOTE: Amount of single-cell suspension in the last tube may vary based on the efficiency of cell suspension recovery at step 3.6.
- For first three tubes, pipette 150 µL of single-cell suspension and pipette rest of single-cell suspension into the last of the four tubes (approximately 550 µL of single-cell suspension).
- Set first tube of the four tubes as an unstained control for FACS session.
- Prepare single dye stained (PI or Hoechst) cell suspensions as controls. Add 2.5 µL of Hoechst or 1 µL of PI to each tube (second and third).
- Prepare a double-stained tube. This will be used for collecting germ cell subpopulations. Add 2.5 µL of Hoechst and 1 µL of PI to the last tube.
NOTE: Hoechst has a shelf-life of 2-3 months. Using older stocks of dye will cause significant alterations during FACS session. - Incubate at room temperature for 30 min in the dark. Place samples in a tube rotator or invert the 1.5 mL tubes every 5-10 minutes.
- Filter cell suspension with a pre-wetted 40 µm strainer and keep the filtered solution on ice and in the dark until the FACS session.
NOTE: The filtration step is necessary for preventing cell clumps for FACS. Additionally, treatment with DNase (see Figure 1) or 2-Naphthol-6,8-disulfonic acid dipotassium salt (NDA20) can be used to limit cell clumping. Prolonged waiting periods will affect the fluorescent signal detected during FACS and may result increased cell death.
5. Fluorescence Activated Cell Sorting (FACS) Setup and Purification of Testicular Cells
- Prepare FACS collecting tubes.
- Coat 5 mL polypropylene round-bottom tubes or 1.5 mL tubes with 400 µL of FBS for cell collection. Decant excess FBS after coating.
- Add FACS collecting medium (1x DMEM + 10% Fetal Bovine Serum, FBS) to the tubes: 1 mL for 5 mL tubes or 100 µL for 1.5 mL tubes.
- Set up appropriate sorting conditions in cell sorter and software.
NOTE: Other cell sorting machines and analysis software can be used with the similar setup and gating strategies described below. The conditions described here were adapted from Gaysinskaya and Bortvin 2, Geisinger and Rodriguez-Casuriaga 3, and Getun, et al.4- Load an ultraviolet laser with 463/25 nm band pass filter to detect Hoechst blue and 680 nm LP band pass filter to detect Hoechst red and to detect PI.
- Use a 555DLP dichroic mirror to distinguish blue from red fluorescence.
- Use a 70 µm nozzle and sort cells at a rate of 1000-2000 cells/second.
NOTE: Sorting efficiency is directly influenced by the flow rate. High flow rates (>3500 events/s) increase the speed of sorting but result in contamination of populations. See reference 12 for further information.
- Set gates using control samples.
- Load and run unstained sample.
- Exclude cell debris based on FSC vs SSC plot pattern. Cell debris will pop up in lower left quadrant of the plot. Arbitrarily set the threshold for cell debris by the user or the cell sorter technician.
- Gate on single cells by adjusting threshold for FSC and pulse width. Different cell sorters have different ways to distinguish singlets. As pulse width reflects the time cells take to cross the laser, single cells have lower values when compared to multicellular aggregates. Adjusting threshold on a plot for FSC vs pulse width can be used to select for singlets.
- Set optimal photomultiplier tube (PMT) voltages.
- Use both unstained and single-dye stained cells to establish the threshold of PI or Hoechst fluorescence signal.
NOTE: It is important to optimize the baseline PTM voltages for the cells of interest in order to establish the proper fluorescence range for each dye. This is critical for optimal signal detection and sensitivity. The unstained and single stained control cells are used to establish a range of negatively and positively stained cells with PI and/or Hoechst dyes and minimize noise in signals. For further explanations on optimization of PMT voltage using control cells, please refer to Gaysinskaya and Bortvin 2 - Gate on live cells based on PI staining (PI negative) and FSC plot. Dead cells will be positive for PI fluorescence.
- Set DNA content gate by plotting a histogram of cell counts based on Hoechst blue fluorescence. 3 peaks with increasing concentrations of Hoechst blue fluorescence should appear, representing haploid (1C), diploid (2C), and tetraploid (4C) cells. Set 3 gates, one for each peak.
- Observe at least 500,000 events on FSC vs SSC plot before proceeding to gating on germ cell populations.
- Gate different germ cell populations.
- Load Hoechst/PI stained sample.
- Set the first 3 parent gates that are common to all populations.
- Exclude cell debris based on the FSC vs SSC plot. Select singlets based on the FSC vs pulse-width plot. Select live cells by gating PI negative cells.
- Define spermatogonia gate.
- Plot PI negative cells based on Hoechst blue and red fluorescence intensities. Spermatogonia appear as a side population (See Figure 1).
- Define gates for the remaining germ cell populations. NOTE: Refer to figure 1 and supplementary figure 2 for visual details.
- Plot PI negative cells in DNA content gate.
- For spermatids gate the peak with lowest Hoechst fluorescence (1C).
- For spermatocytes II, gate the peak with intermediate Hoechst fluorescence (2C).
- For spermatocytes I, gate the peak with highest Hoechst fluorescence (4C).
- Plot Hoechst blue and red fluorescence intensity to refine spermatocytes and spermatids populations from DNA content gates.
- Collect the selected subpopulation gates into the collection tubes previously prepared. Each testis will take an average of 45 min to 1.5 h to collect approximately 0.5-6.0 x 106 cells for each subpopulation.
NOTE: Prolonged cell exposure to Hoechst may slightly shift the location of populations with time. Refresh the settings after 20-30 min of FACS. Maximum yield for each subpopulation will be contingent on efficiency of dissociation step.
6. Microscopic Evaluation of Purified Cells
- Spin collected cells at 500-600 x g at 4 °C for 10 min.
- Re-suspend cell pellet with 1 mL of ice cold 1x phosphate buffer solution (PBS) or 1x DMEM.
NOTE: The re-suspension volume can be adjusted according to the number of cells sorted and the desired final concentration. - Pipette 40 µL of washed cells onto a clean glass slide and place a coverslip.
- Fix the remaining cells with 4% paraformaldehyde (PFA) and store fixed cells at 4 °C in dark for future reference.
- Pipette 100 µL 4% PFA directly in the collection tubes.
- Briefly vortex the tubes or pipette few times to ensure well-mixing of the cell suspension.
- Visualize the prepared slides in a microscope equipped with a UV lamp to detect Hoechst fluorescence under a 63X objective. Refer to the Results section – Morphologic evaluation of sorted germ cell populations – for further details on how to identify specific germ cell types.
Representative Results
Single cell suspensions from mechanical dissociation of testicular tissue
Figure 2 compares single cell suspensions obtained by mechanical dissociation of mouse testicular tissue under different conditions. Samples obtained by processing fresh tissue, unstained (Figure 2A) or stained with Hoechst (Figure 2B), show the presence of single cells in various stages of differentiation, and importantly, cellular structure appears to be preserved, including flagella of spermatozoa. Although some clumping and debris was observed in the stained samples, which was reduced by adding DNase after dissociation (Figure 2D), these results indicate that Hoechst staining does not significantly alter the quality of the single cell suspensions. Interestingly, single cell suspensions can also be obtained from frozen tissue for the mouse (Figure 2C) and other mammalian species – rat, dog, guinea-pig and mini-pig (Supplementary Figure 1) by mechanical dissociation. Various cell types are visible and identifiable in those samples; however, processing of frozen tissue appears to lead to increased cell death and clumping, and an overall lower cellular yield. As such, it is highly recommended to prepare single cell suspensions from fresh tissue for further downstream applications.
The quality of single cell suspensions prepared from fresh testicular tissue of mouse, rat, dog, guinea pig and mini pig, was assessed during Ho-FACS (Table 1). After exclusion of cellular debris, 95.7-98.4% of cells are singlets, and from those 86.5-93.8% are alive, as shown by quantification of PI negative cells (See Protocol). These results indicate that mechanical dissociation is a reliable method to prepare single cell suspensions for flow cytometry from testicular tissue of different mammalian species.
Ho-FACS to isolate germ cells from testicular tissue of different mammalian species
After mechanical dissociation, single cell suspensions are stained with Hoechst and PI and processed by FACS. As mentioned above, Hoechst staining allows discrimination of cells in different stages of differentiation based on chromatin quantity and structure, whereas PI staining of non-permeabilized cells separates live from dead cells. Hence, after filtering out cell debris and multicellular aggregates, the PI gate selects cells with intact membranes (PI negative; Figure 1). Live cells are then analyzed based on Hoechst fluorescence: blue is proportional to DNA content and increasing red fluorescence reflects less condensed chromatin and structural variations. As such, male germ cells of different stages are expected to cluster in specific regions of cytograms plotting the function of blue/red Hoechst fluorescence (Figure 3A).
Indeed, the Ho-FACS plots generated for the different species show the presence of distinct cell populations based on Hoechst fluorescence (Figure 3B-3C). Although these plots are sufficient to discriminate germ cell populations, a DNA content (C) gate can be defined2 to limit cross contamination. This gate is generated by a histogram of cell counts in function of Hoechst blue fluorescence intensity. For all species, three peaks can be detected and represent cells with 1C, 2C and 4C (Figure 1 and Supplementary Figures 2-5). Notably, as previously mentioned, spermatogonia are a side population and cannot be reliably gated based on histograms of DNA content. Hence, this population is gated after exclusion of dead cells, based on Hoechst blue/ red fluorescence plots (Figure 1). This strategy is represented in Figure 1 for the rat and supplementary Figures 2-5 for the remaining species. It is important to note that since Hoechst fluorescence alone can discriminate the different germ cell types, both the PI and DNA content gates are optional. They were included in this strategy to select live cells and reduce cross-contamination, respectively. As summarized in Table 1, quantification of cells in the DNA content gate reflects the relative proportion of cells in different stages of spermatogenesis. As expected, 1C cells are the most abundant and represent spermatid populations, followed by 2C cells (SPC II) and 4C cells (SPC I). Importantly, this pattern is preserved across species and small differences may reflect interspecific variability in germ cells and the cycles of the seminiferous epithelium21.
After Ho-FACS, cell integrity can be assessed using Trypan blue staining. The proportion of live cells after 1 h of FACS ranged from 27% (SCP I) to 67% (SPD) for the mouse, indicating that duration of sorting and exposure to Hoechst increases cell death. For users looking to culture the sorted cells, Hoechst concentration and duration of Ho-FACS should be adjusted to improve cell survival. Nonetheless, RNA sequencing data has been generated for single cells isolated using this method (Jung et al., unpublished), indicating that these cells are suitable for molecular studies of germ cell biology. This dataset was used to detect potential contamination of germ cell populations with somatic cells (Supplementary Figure 6), which was estimated to be less than 5%.
Morphologic evaluation of sorted germ cell populations
As germ cells undergo drastic morphological changes throughout spermatogenesis, it is possible to estimate the purity of isolated populations by microscopy. As a reference, Figure 4 illustrates the general morphology of different male germ cells types from 4 mammalian species (from Lima, et al. 5). Chromatin distribution and condensation, as well as cell size and shape, show unique and well characterized patterns in different cell types. SPG have distinct pericentric heterochromatin, which stains brightly for Hoechst, and are small and round in shape (Spg in Figure 4B). Spermatocytes are larger granulated cells. Nuclei of SPC I are easily identifiable, with chromatin variations characteristic of meiotic cells (Spc I in Figure 4B), whereas SPC II are binucleated or in diakinesis (Spc II in Figure 4B). Finally, SPD are small haploid cells with round or elongated shape (Spd in Figure 4B). Notably, rSPD are similar to SPG in size and shape, but clearly distinguished by the presence of localized chromocenters. Based on these characteristics, sorted cell populations were evaluated for the enrichment of specific cell types (Table 1). SPD and SPC II populations were highly enriched for the cell type of interest, whereas SPC I and SPG showed some degree of contamination with other cell types, especially for the Guinea Pig and Mini Pig samples. Interestingly, for the dog sample the gating strategy was sufficient to discriminate round from elongating spermatids (Figure 3C; Supplementary Figure 3; Table 1). However, this was not the case for most species, where spermatid subpopulations appear to overlap.
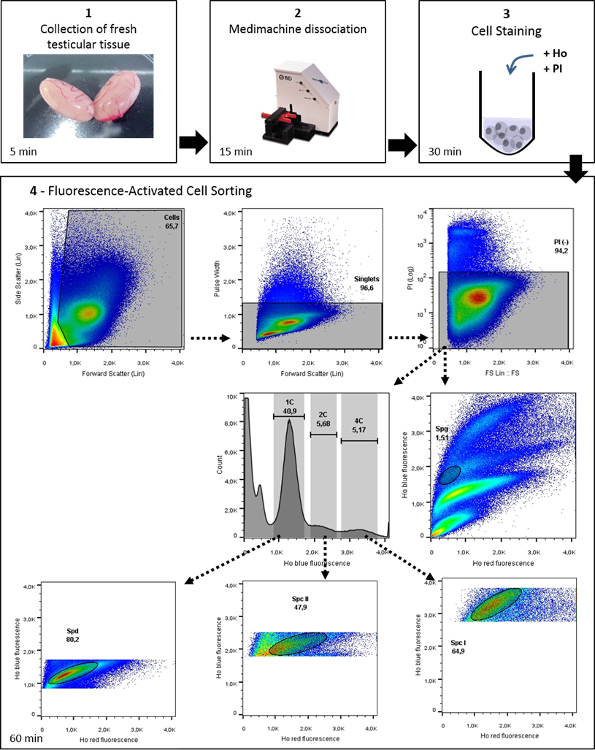
Figure 1: Workflow of Ho-FACS isolation of mammalian male germ cells. This image illustrates the general protocol for germ cell isolation of mammalian germ cells, with representative data from the application of this protocol to rat testis. Ho: Hoechst. From Lima, et al. 5 with permission. Please click here to view a larger version of this figure.
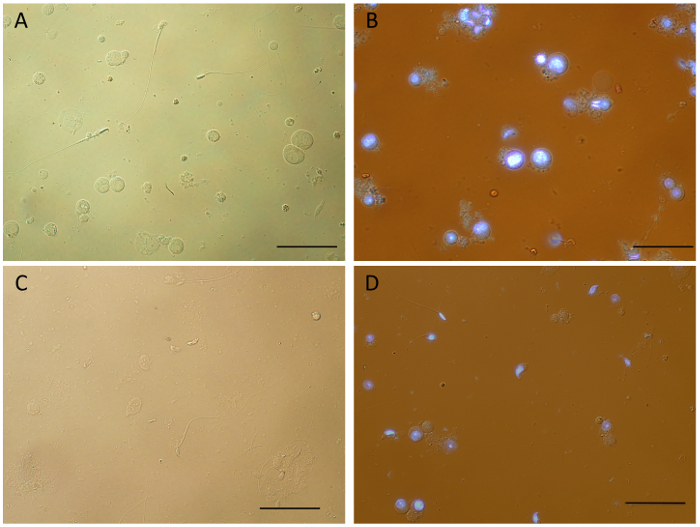
Figure 2: Single cell suspensions generated by mechanical dissociation of mouse testicular tissue. Samples obtained from fresh tissue (A-B) show a variety of intact germ cell types and very little debris. When compared to unstained cells (A), some cell clumping was observed for Hoechst stained cell suspensions (B), which can be reduced by adding DNase (final concentration of 10 µg/mL) to the sample immediately after dissociation (D). Frozen testicular tissue can also be used to obtain single cell suspensions by mechanical dissociation (C). However, it appears that the process of flash freezing and thawing prior to tissue dissociation results in more debris, cell death and overall less cellular yield. After dissociation (See Protocol), samples were spun for 10 min at 4 °C and re-suspended in 1x DMEM before mounting the slides. Black bars = 50 µm. Images obtained using an upright white light microscope equipped with a UV lamp. Please click here to view a larger version of this figure.
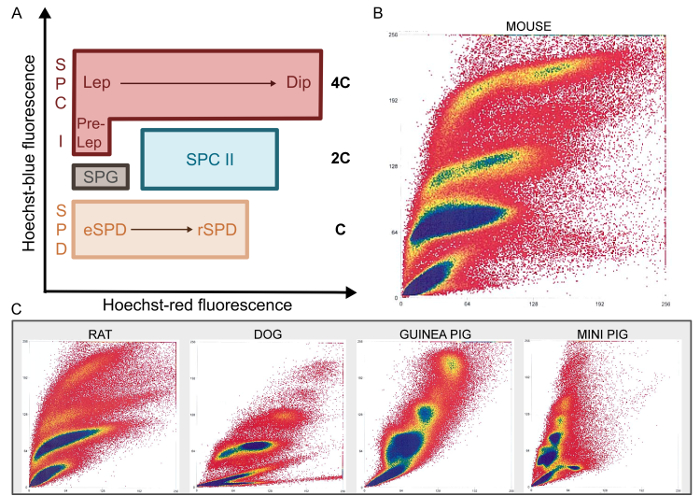
Figure 3: Ho-FACS of mammalian germ cells. Specific populations present unique patterns of Hoechst fluorescence during FACS (A). As Hoechst blue fluorescence reflects variations in chromatin content clusters of haploid (C); diploid (2C) and tetraploid (4C) cells are located in areas of the FACS plot with increasing Hoechst blue fluorescence. Plots generated as a function of Hoechst blue/red fluorescence show clusters of distinct cell populations for the different mammalian species tested (B-C). SPG: Spermatogonia; SPC I: Primary spermatocytes; Pre-Lep: Pre-Leptotene spermatocytes; Lep: Leptotene spermatocytes; Dip: Diplotene spermatocytes; SPC II: Secondary spermatocytes; SPD: Spermatids; eSPD: elongating spermatids; rSPD: round spermatids. Adapted from Lima, et al. 5 with permission. Please click here to view a larger version of this figure.
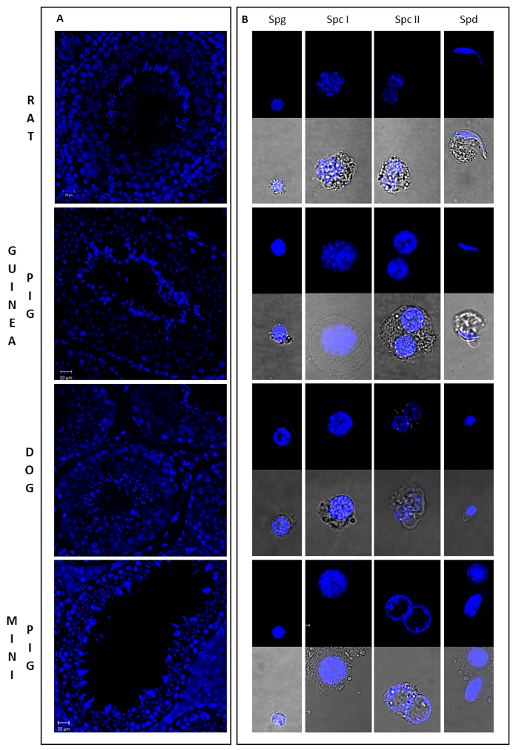
Figure 4: Morphology of germ cells in different developmental stages from four mammalian species. This figure shows sections of testicular tissue stained with Hoechst (A) and the general aspect of different male germ cell types sorted by Ho-FACS (B) from rat, guinea pig, dog and mini pig. As cell size, shape and chromatin conformation varies dramatically throughout spermatogenesis, these can be used to assign cells to different stages of differentiation. Spermatogonia (Spg) are small and round cells with distinct pericentric heterochromatin and exhibit a high intensity of Hoechst fluorescence. Spermatocytes are the largest germ cells and show variable chromatin conformations. Nuclei of primary spermatocytes (Spc I) exhibit chromatin variations characteristic of meiotic cells, whereas secondary spermatocytes (Spc II) are binucleated or in diakinesis. Spermatids (Spd) have round or elongated shape, depending on the stage of spermiogenesis and are small haploid cells. Although round spermatids and spermatogonia are similar in size and shape, the former can be distinguished by the presence of localized chromocenters. Slides were prepared for each sorted cell type after Ho-FACS and were visualized in a confocal microscope: 63X magnification lens, with (lower panel) or without (upper panel) white light transmission.From Lima, et al. 5 with permission. Please click here to view a larger version of this figure.
| % cells in DNA content gate | Purity of sorted germ cell populations (%) | |||||||||||
| Species | % Singlets | % Live cells | 1C | 2C | 4C | Spg | Spc I | Spc II | Spd | rSpd | eSpd | |
| Mouse | 98.4 | 92.5 | 34.7 | 3.9 | 3.72 | 74 | 82 | 87.5 | 95.2 | 95* | 92* | |
| Rat | 95.7 | 93.8 | 37.7 | 5.3 | 5.4 | 83 | 81 | 82 | 87 | . | . | |
| Guinea Pig | 96.2 | 92.1 | 39.3 | 7.6 | 5.8 | 48 | 68.7 | 85 | 87 | . | . | |
| Dog | 97.9 | 86.5 | 16.4 | 3 | 0.5 | 78 | . | 87 | . | 91 | 81 | |
| Mini Pig | 95.9 | 93.2 | 26.9 | 6.4 | 3.5 | 49 | 52 | 82 | 92 | . | . | |
| * Obtained by enzymatic dissociation and gated based on FSC&SSC parameters (from Lima et al. 2016, with permission) | ||||||||||||
Table 1: Statistics of Ho-FACS of male germ cell suspensions obtained by mechanical dissociation.
Supplementary Figure 1: Single cell suspensions obtained by mechanical dissociation of frozen testicular tissue. Mechanical dissociation allows the generation of single cell suspensions from testicular tissue of different mammalian species without the need of species-specific protocols. Different panels show the cell suspensions obtained for 4 mammalian species. Samples were obtained by mechanical dissociation of frozen testicular tissue and stained with Hoechst. SPG: Spermatogonia; SPC I: Primary Spermatocyte; SPC II: Secondary Spermatocyte; SPD: Spermatid; SPZ: Spermatozoa. Slides were visualized in a confocal microscope with (lower panel) or without (upper panel) white light transmission. Scale bars =20 µm. Please click here to download this file.
Supplementary Figure 2: Gating strategy for separation of live male germ cells by Ho-FACS of mouse testicular single cell suspensions. Live cells (PI negative) are sorted according to Hoechst fluorescence. Spermatogonia (SPG) are identified by plotting directly Hoechst-blue and red fluorescence intensities. A DNA content gate is used to sort cells based on Hoechst blue fluorescence: haploid (C); diploid (2C) and tetraploid (4C). Cells in each peak of the histogram are then sorted by plotting the function of Hoechst blue and red fluorescence. SPG: Spermatogonia; SPD: Spermatids; SPC II: Secondary spermatocytes; SPC I: Primary spermatocytes. Please click here to download this file.
Supplementary Figure 3: Gating strategy for separation of live male germ cells by Ho-FACS of dog testicular single cell suspensions. Live cells (PI negative) are sorted according to Hoechst fluorescence. Spermatogonia (SPG) are identified by plotting directly Hoechst-blue and red fluorescence intensities. A DNA content gate is used to sort cells based on Hoechst blue fluorescence: haploid (C); diploid (2C) and tetraploid (4C). Cells in each peak of the histogram are then sorted by plotting the function of Hoechst blue and red fluorescence. The peak containing haploid cells can be subdivided according to the range of Hoechst blue intensity and represent two subpopulations of spermatids: elongating spermatids (gate C') and round spermatids (gate C''). SPG: Spermatogonia; eSPD: elongating spermatids; rSPD: round spermatids; SPC II: Secondary spermatocytes; SPC I: Primary spermatocytes. Please click here to download this file.
Supplementary Figure 4: Gating strategy for separation of live male germ cells by Ho-FACS of guinea pig testicular single cell suspensions. Live cells (PI negative) are sorted according to Hoechst fluorescence. Spermatogonia (SPG) are identified by plotting directly Hoechst-blue and red fluorescence intensities. A DNA content gate is used to sort cells based on Hoechst blue fluorescence: haploid (C); diploid (2C) and tetraploid (4C). Cells in each peak of the histogram are then sorted by plotting the function of Hoechst blue and red fluorescence. SPG: Spermatogonia; SPD: Spermatids; SPC II: Secondary spermatocytes; SPC I: Primary spermatocytes. Please click here to download this file.
Supplementary Figure 5: Gating strategy for separation of live male germ cells by Ho-FACS of mini pig testicular single cell suspensions. Live cells (PI negative) are sorted according to Hoechst fluorescence. Spermatogonia (SPG) are identified by plotting directly Hoechst-blue and red fluorescence intensities. A DNA content gate is used to sort cells based on Hoechst blue fluorescence: haploid (C); diploid (2C) and tetraploid (4C). Cells in each peak of the histogram are then sorted by plotting the function of Hoechst blue and red fluorescence. SPG: Spermatogonia; SPD: Spermatids; SPC II: Secondary spermatocytes; SPC I: Primary spermatocytes. Please click here to download this file.
Supplementary Figure 6: Examination of somatic cell contamination in Ho-FACS sorted populations. t-distributed stochastic neighbor embedding (t-SNE) plot of approximately 600 single-cell transcriptome from three FACS subpopulation gates (A-B). On the t-SNE plot, each point represents the unique position of a single cell in a transcriptome space and each cell is labeled with its FACS subpopulation gate origin (A). Visual quantification of cell-type specific markers on the FACS single-cell t-SNE plot (B). Contamination from somatic cells is estimated to be less than 5% and is not specific to one Ho-FACS population gate. Please click here to download this file.
Discussion
Considering the highly-conserved chromatin dynamics during spermatogenesis in mammals, the goal of this work was to develop a protocol to isolate male germ cells in distinct stages of differentiation from different mammalian testicular tissues (Figure 1). One of the major obstacles in the application of a single workflow to different animal models is the need of species-specific adjustments, especially in regard to tissue dissociation protocols. Current methods mostly rely on enzymatic tissue digestion and protocols often vary even within species 12. To overcome this issue, the protocol described here shows the application of mechanical tissue dissociation to obtain single cell suspensions from testicular samples of different mammalian species. The results indicate that mechanical dissociation of fresh testicular tissue with this system performs well (Figure 2A-2B), similar to a previous application in rodents 20. Importantly, Hoechst staining does not seem to significantly affect the quality of single cell suspensions. Although more cell clumping is visible in stained samples (Figure 2B), treatment with DNase (Figure 2D) or NDA 20 can help prevent this issue. Previous reports suggest that mechanical dissociation of testicular tissue is better than or as efficient as enzymatic tissue digestion 5,20. In accordance, the data presented here indicates that mechanical dissociation is a reliable method to obtain single cell suspensions from testicular tissue for Ho-FACS processing.
Incubation with Hoechst is a crucial step as it influences the signal detected during flow cytometry. Long periods of staining provide better discrimination of germ cell types but can be toxic, resulting in cell death and changes in intracellular programming 22. Users intending to establish germ cell cultures with this method should evaluate the genotoxic effect of long-time exposure to Hoechst3,22 and determine the adequate concentration and time of staining. Here, 30 min of staining was sufficient to provide reliable separation of germ cell populations (Figure 3B-3C). The duration of staining can potentially be reduced to 10 min, as shown by Rodriguez-Casuriaga, et al. 20, and should be further evaluated by users for the species of interest.
As hypothesized, four germ cell populations can be consistently detected – SPG, SPC I, SPC II and SPD- based on chromatin variations of germ cells from the different species tested (Figure 3B-3C). Due to the lack of commercial antibodies that can be used transversely across species, purity of cell populations was assessed by morphologic evaluation of chromatin structure and cell size and shape. Alternatively, RT-qPCR can be performed for stage-specific markers to quantify the levels of contamination with unwanted cell types, given that RNA is preserved during this procedure. The results above show an overall enrichment of the targeted cell types, with high purities obtained for SPC II and SPD populations (Table 1), and support the gating strategy adopted in this workflow (Figure 1) for different mammalian species (Supplementary Figures 2-5). It is nonetheless advisable that users optimize the gating according to the species and populations of interest in order to reduce the cross contamination detected for some of the populations (Table 1). This can be achieved by longer incubation periods during staining 18, reduced flow rate 2 and reduced gate sizes. SPG are more challenging to isolate since they are the rarest cell type in the testis and also due to dye efflux over time 1,15. Although it is possible to detect distinct SPG populations by Ho-FACS, as shown here, studies focusing solely on stem cell biology would benefit from a more stringent technique such as Magnetic-Activated Cell Sorting (MACS; 23). Notably, subpopulations of SPD (eSPD and rSPD) could clearly be distinguished for the Dog but not for other species (Figure 3C and Supplementary Figure 3). Potentially, these subpopulations can be resolved by adjusting the gating settings, as described by the authors for the mouse 5. Cytograms generated for cell size and granularity (FSC VS SSC), prior to plotting the function of Hoechst blue/red fluorescence, discriminates elongating (low FSC + SSC), from round (high FSC + SSC) spermatid subpopulations. Separation of round and elongating spermatid subpopulations would allow a more in depth investigation of spermiogenesis, bringing new insights into specific molecular events occurring during sperm differentiation. Also, as described for the mouse 1,2,12, it is possible to obtain further resolution of meiotic stages, by adjusting FACS gates. Such approach would largely benefit comparative studies of meiotic events. Although cell integrity is disturbed during Ho-FACS, single cell RNA sequencing data obtained for cells sorted with this method (Jung et al., unpublished; Supplementary Figure 6) indicates that this is a reliable approach to obtain cells for molecular studies of germ cell biology. Nonetheless, users intending to perform reproductive assays, such as round spermatid injection (ROSI), using cells isolated by Ho-FACS must assess viability of sorted cells and may want to explore alternative methods 24,25.
In summary, this work describes a standardized method to isolate testicular germ cells from different mammals, building and adding refinements on previously established techniques 2,12,20. Such standardization allows for the use of one set of reagents and a single workflow on different mammalian species, facilitating data generation for comparative studies of germ cell biology. Also, mechanical tissue dissociation overcomes the hurdle of species-specific adjustments that may introduce methodological variability which is almost impossible to account for. Additionally, the reduced execution time and manipulation restricts perturbations of ex vivo cellular physiology to a minimum. In this regard, Ho-FACS is significantly faster when compared to other methods of germ cell isolation, such as STA-PUT and elutriation 6,7. Moreover, simultaneous separation of 4 germ populations in the same experiment is only possible by FACS. Given the properties of the Hoechst dye, cell structure and RNA species are preserved, and therefore cells can be used for further downstream molecular studies. Importantly, since Ho-FACS relies on chromatin attributes to discriminate germ cell types, the successful performance of this workflow in 5 different species strongly supports its application to other mammals. The results presented here suggest that spermatogenesis might be a unique process of cell differentiation that could be tackled in many mammalian species using a single protocol. As such, in the "omics" and systems biology era, this technology provides the means for an evolutionary approach on questions of male germ cell biology, including studies of epigenetics, regulation, and protein diversity throughout spermatogenesis.
Declarações
The authors have nothing to disclose.
Acknowledgements
The authors thank the Hillside Animal Hospital (St. Louis, MO) for dog testes; Jason Arand and Dr. Ted Cicero's lab at Washington University in St. Louis (WashU) for providing rat testes and Brianne Tabers for assisting with the collection; Jared Hartsock and Dr. Salt's Lab at WashU for the guinea pig testes; and Dr. Michael Talcott at the Division of Comparative Medicine at WashU for the miniature pig testis. The authors also acknowledge the Alvin J. Siteman Cancer Center at Washington University School of Medicine and Barnes-Jewish Hospital in St. Louis, MO, for the use of the Siteman Flow Cytometry Core, which provided staff-operated cell sorting service. The Siteman Cancer Center is supported in part by NCI Cancer Center Support Grant #P30 CA91842.
This research was funded by an FCT doctoral fellowship [SFRH/BD/51695/2011 to ACL], grants from the United States National Institutes of Health [R01HD078641 and R01MH101810 to DFC] and an FCT research contract [IF/01262/2014 to AML].
Materials
| 4% paraformaldehyde | VWR | #15710 | 4% PFA, For fixing sorted cells |
| Medimachine | BD Biosciences | #340588; | System for testis dissociation |
| 1X Dulbecco modified Eagle medium | Life Technologies | #31053 | DMEM, for testis dissociation |
| Medicon | BD Biosciences | #340591 | Disaggregation cartridge for testis dissociation |
| Fetal bovine serum | Thermo Scientific | #10082139 | FBS, for FACS ceollction |
| Hoechst 33342 | Invitrogen | #H3570 | Hoecsht, For FACS staining |
| 40µm cell strainer | GREINER BIO-ONE | #89508-342 | For testis dissociation |
| 100x 15mm petri dish | Falcon | #351029 | For testis dissection |
| 5mL polypropylene round-bottom tubes | Falcon | #352063 | For FACS collection |
| 1.5mL Eppendorf tubes | VWR | #20170-650 | For FACS collection |
| 3mL needless syringe | BD Biosciences | #309657 | For testis dissociation |
| 50mL conical tube | MIDSCI | #C50R | For testis dissociation |
| Propidium Iodide | Invitrogen | #L7011 | PI, For FACS staining |
| MoFlo Legacy cell sorter | Beckman Coulter | #ML99030 | For FACS |
| Summit Cell Sorting software | Beckman Coulter | #ML99030 | For FACS |
| Phosphate buffered saline | Thermo Scientific | #AM9625 | PBS, For microscopy |
| Microscopic glass slide | Fisher Scientific | #12-544-4 | For microscopy |
| Glass coverslip | Fisher Scientific | #12-548-87 | For microscopy |
| Disposable transfer pipette (3mL) | Samco Scientific | #225 | For testis dissociation |
| DNase I | Roche | #10104159001 | For testis dissociation |
| Carbon steel surgical blade | Miltex | #4-111 | For testis dissection |
| Countess automated cell counter | Invitrogen | #C10227 | For automatic cell counting |
| Trypan blue solution (0.4%) | Sigma | #T8154 | For viability staining |
Referências
- Bastos, H., et al. Flow cytometric characterization of viable meiotic and postmeiotic cells by Hoechst 33342 in mouse spermatogenesis. Cytometry A. 65 (1), 40-49 (2005).
- Gaysinskaya, V., Bortvin, A. Flow cytometry of murine spermatocytes. Curr Protoc Cytom. 72, (2015).
- Geisinger, A., Rodriguez-Casuriaga, R. Flow cytometry for gene expression studies in Mammalian spermatogenesis. Cytogenet Genome Res. 128 (1-3), 46-56 (2010).
- Getun, I. V., Torres, B., Bois, P. R. Flow cytometry purification of mouse meiotic cells. J Vis Exp. (50), (2011).
- Lima, A. C., et al. Multispecies Purification of Testicular Germ Cells. Biol Reprod. , (2016).
- Bryant, J. M., Meyer-Ficca, M. L., Dang, V. M., Berger, S. L., Meyer, R. G. Separation of spermatogenic cell types using STA-PUT velocity sedimentation. J Vis Exp. (80), (2013).
- Chang, Y. F., Lee-Chang, J. S., Panneerdoss, S., MacLean, J. A., Rao, M. K. Isolation of Sertoli, Leydig, and spermatogenic cells from the mouse testis. Biotechniques. 51 (5), (2011).
- Margolin, G., Khil, P. P., Kim, J., Bellani, M. A., Camerini-Otero, R. D. Integrated transcriptome analysis of mouse spermatogenesis. BMC Genomics. 15, 39 (2014).
- Grogan, W. M., Farnham, W. F., Sabau, J. M. DNA analysis and sorting of viable mouse testis cells. J Histochem Cytochem. 29 (6), 738-746 (1981).
- Mays-Hoopes, L. L., Bolen, J., Riggs, A. D., Singer-Sam, J. Preparation of spermatogonia, spermatocytes, and round spermatids for analysis of gene expression using fluorescence-activated cell sorting. Biol Reprod. 53 (5), 1003-1011 (1995).
- Gaysinskaya, V., Soh, I. Y., van der Heijden, G. W., Bortvin, A. Optimized flow cytometry isolation of murine spermatocytes. Cytometry A. 85 (6), 556-565 (2014).
- Goodell, M. A., Brose, K., Paradis, G., Conner, A. S., Mulligan, R. C. Isolation and functional properties of murine hematopoietic stem cells that are replicating in vivo. J Exp Med. 183 (4), 1797-1806 (1996).
- Watson, J. V., Nakeff, A., Chambers, S. H., Smith, P. J. Flow cytometric fluorescence emission spectrum analysis of Hoechst-33342-stained DNA in chicken thymocytes. Cytometry. 6 (4), 310-315 (1985).
- Lassalle, B., et al. ‘Side Population’ cells in adult mouse testis express Bcrp1 gene and are enriched in spermatogonia and germinal stem cells. Development. 131 (2), 479-487 (2004).
- Omran, H. M., Bakhiet, M., Dashti, M. G. DNA integrity is a critical molecular indicator for the assessment of male infertility. Mol Med Rep. 7 (5), 1631-1635 (2013).
- Rodriguez-Casuriaga, R., Folle, G. A., Santinaque, F., Lopez-Carro, B., Geisinger, A. Simple and efficient technique for the preparation of testicular cell suspensions. J Vis Exp. (78), (2013).
- Rodriguez-Casuriaga, R., Geisinger, A., Santinaque, F. F., Lopez-Carro, B., Folle, G. A. High-purity flow sorting of early meiocytes based on DNA analysis of guinea pig spermatogenic cells. Cytometry A. 79 (8), 625-634 (2011).
- Rodriguez-Casuriaga, R., et al. Rapid preparation of rodent testicular cell suspensions and spermatogenic stages purification by flow cytometry using a novel blue-laser-excitable vital dye. MethodsX. 1, 239-243 (2014).
- Rodriguez-Casuriaga, R., et al. Ultra-fast and optimized method for the preparation of rodent testicular cells for flow cytometric analysis. Biol Proced Online. 11, 184-195 (2009).
- Hess, R. A., Renato de Franca, L. Spermatogenesis and cycle of the seminiferous epithelium. Adv Exp Med Biol. 636, 1-15 (2008).
- Zhao, H., Traganos, F., Dobrucki, J., Wlodkowic, D., Darzynkiewicz, Z. Induction of DNA damage response by the supravital probes of nucleic acids. Cytometry A. 75 (6), 510-519 (2009).
- Gassei, K., Ehmcke, J., Dhir, R., Schlatt, S. Magnetic activated cell sorting allows isolation of spermatogonia from adult primate testes and reveals distinct GFRa1-positive subpopulations in men. J Med Primatol. 39 (2), 83-91 (2010).
- Hayama, T., et al. Practical selection methods for rat and mouse round spermatids without DNA staining by flow cytometric cell sorting. Mol Reprod Dev. 83 (6), 488-496 (2016).
- Zhu, L., et al. FACS selection of valuable mutant mouse round spermatids and strain rescue via round spermatid injection. Zygote. 23 (3), 336-341 (2015).

