Utilization of Capsules for Negative Staining of Viral Samples within Biocontainment
Summary
This protocol provides instruction for negative staining virus samples which can easily be used in BSL-2, -3, or -4 laboratories. It includes the use of an innovative processing capsule, which protects the transmission electron microscopy grid and provides the user easier handling in the more turbulent environments within biocontainment.
Abstract
Transmission electron microscopy (TEM) is used to observe the ultrastructure of viruses and other microbial pathogens with nanometer resolution. Most biological materials do not contain dense elements capable of scattering electrons to create an image; therefore, a negative stain, which places dense heavy metal salts around the sample, is required. In order to visualize viruses in suspension under the TEM they must be applied to small grids coated with a transparent surface only nanometers thick. Due to their small size and fragility, these grids are difficult to handle and easily moved by air currents. The thin surface is easily damaged, leaving the sample difficult or impossible to image. Infectious viruses must be handled in a biosafety cabinet (BSC) and some require a biocontainment laboratory environment. Staining viruses in biosafety levels (BSL)-3 and -4 is especially challenging because these environments are more turbulent and technicians are required to wear personal protective equipment (PPE), which decreases dexterity.
In this study, we evaluated a new device to assist in negative staining viruses in biocontainment. The device is a capsule that works as a specialized pipette tip. Once grids are loaded into the capsule, the user simply aspirates reagents into the capsule to deliver the virus and stains to the encapsulated grid, thus eliminating user handling of grids. Although this technique was designed specifically for use in BSL-3 or -4 biocontainment, it can ease sample preparation in any lab environment by enabling easy negative staining of virus. This same method can also be applied to prepare negative stained TEM specimens of nanoparticles, macromolecules and similar specimens.
Introduction
Transmission electron microscopy (TEM) is an effective tool for viewing the morphology and ultrastructure of biological specimens that are too small to be seen with a traditional light microscope 1,2,3,4. TEMs shoot electrons through a very thin specimen producing a higher resolution image as electrons have a much shorter wavelength than light. Regions of the sample that bend or block electrons appear dark, while regions that are electron lucent appear white.
Lack of electron dense matter makes viruses difficult to view under a TEM because they cannot scatter electrons. Negative staining is the most common method used to create contrast and view viruses with a TEM. The first negative staining procedure was proposed by Brenner and Horne in 1959, based on an experiment where Hall (1955) and Huxley (1957) observed the appearance of biological structures in reverse contrast when immersed in an electron-dense substance 5. The process of negative staining has been virtually unchanged over the past half century. Negative staining involves briefly applying a heavy metal salt solution to a sample on a TEM grid in an attempt to surround the virus with dense material without infiltrating the virus 6. This creates a dark border and reveals the particle's shape 5. This study uses two reagents for negative staining, uranyl acetate (UA) and potassium phosphotungstic acid (PTA). Both of these stains are commonly used to negatively stain small biological samples, such as viruses, protein complexes, and nanoparticles 7,8,9.
The conventional negative staining technique is the manual droplet negative staining technique7. This method requires precise handling of small, fragile TEM grids with forceps to apply small amounts of virus sample, stain, and rinses. The typical preparation protocol involves applying a droplet of sample suspension onto the surface of a film-coated TEM grid (Figure 1A). After attachment of the sample to the film surface, the grid is rinsed to remove non-adherent viruses and stained with either UA or PTA for a few seconds to a minute, depending on the type of sample. Excess liquid is wicked away from the grid by touching a piece of filter paper to the edge of the grid.
The manual droplet method requires that each grid be individually made. If not handled carefully, coated TEM grids are easily punctured, bent, or contaminated. Processing multiple samples can lead to difficulties in tracking the grids and ensuring consistent staining for each sample. This manual staining procedure is much more difficult when conducted in biosafety level (BSL)-3 and -4 biocontainment laboratories, due to the required personal protective equipment (PPE) required for these environments. PPE is cumbersome and the biocontainment environment is much more turbulent compared to a regular laboratory. Personnel working in BSL-3 biocontainment laboratories are required to wear 2 pairs of gloves and work in a biosafety cabinet (BSC). This double layer of gloves reduces tactile sensitivity and restricts fine motor movement. The airflow of the BSC that protects the user and helps prevent sample contamination can cause the samples and stains to dry too quickly thus affecting the stain quality. The strong turbulent airflow in the BSC can also quickly blow away a grid that is not well secured. In BSL-4 biocontainment laboratories, there are additional safety requirements. Personnel are required to wear a positive pressure suit, which further restricts physical movement and the ability to clearly see and manipulate grids. The technician working in BSL-4 also wears at least 2 pairs of gloves, with the outer pair being a thick glove which greatly reduces dexterity and tactile sensation. Finally, the forceps used to handle TEM grids are sharp, thereby posing a risk to the technician due to their ability to puncture gloves. With capsules containing grids, forceps are not necessary, thus providing a safe, forceps-free alternative for manipulating grids in biocontainment. Finally, the capsules also provide an effective way to store grids during processing, osmium vapor decontamination, and during storage; thereby keeping the grids organized and safe from damage.
In this report, we introduce a new method for negative staining TEM grids in biocontainment laboratories that utilizes mPrep/g capsules, a capsule-based device for grid handling and staining 10,11,12. The capsule accommodates two TEM grids, minimizes direct handling, and reduces the potential for grid damage. The capsule attaches directly to a single or multichannel pipette in the same manner as a pipette tip, allowing the application of various liquids to grids contained within. This enables simultaneous preparation of multiple samples with duplicate grids (Figure 1B). To negative stain with capsules the virus sample is aspirated into the capsule and held for 10 min to let the viruses adsorb onto the grid surfaces. The grids with adsorbed virus are subsequently washed with deionized (dI) water and stained with either UA or PTA for a few seconds to 1 min. This process uses the same protocol steps and reagents as the manual droplet method; the difference being that all work occurs inside the capsule with no physical handling of the grids. (Figures 1C, 1D).
The purpose of this study was to evaluate capsules as a new method for negative staining of virus samples in biocontainment environments. This study also examined the quality of TEM images produced from two different virus inactivation procedures: 1) rapid inactivation, with 1% osmium tetroxide vapor, and 2) a 24 h inactivation with 2% glutaraldehyde. Both of these were conducted using the capsules. Finally, we evaluated two commonly used negative stains, UA and PTA, for use in the capsule. 13
Protocol
1. Experiment Preparation in a BSL-2 Environment Prior to Working with the Virus Samples
- Prepare or purchase Formvar and carbon coated TEM copper grids, usually 200-400 mesh.
- Insert the coated TEM grids into capsules.
- Use a magnified lens to make this process easy to perform. One or two grids may be inserted into each capsule. Pre-loaded capsules can be purchased to eliminate this step if desired.
- Transfer the capsules with inserted TEM coated grids, together with other supplies and reagents, to biocontainment where the viruses will be negatively stained.
2. The Capsule Method for Negative Staining in Biocontainment using Aqueous Glutaraldehyde and 1% Osmium Tetroxide Vapor Inactivation
- Inside the biocontainment BSC, aspirate 40 µL of virus suspension into the capsule attached to a pipette.
NOTE: The pipette remains attached to the capsule until the process is completed. Virus preparation is according to our previous publication14. - Place the pipette on its side for 10 min with grids oriented horizontally. This is to promote an even distribution of virus particles onto the coated grids.
- Inactivate virus within the capsule inside the biocontainment BSC.
- Pick up the pipette and depress the plunger to dispense the virus solution into a waste container.
- Aspirate 40 µL of 2% glutaraldehyde fixative into the capsules.
Caution: Glutaraldehyde is a hazardous chemical and requires appropriate protection. Glutaraldehyde can be used for brief periods in a normal BSC, but extended open reagent requires working in a ducted BSC or chemical fume hood. - Place the pipette on its side for 20 min. This is to ensure samples are well fixed.
- Expel the fixative and aspirate 40 µL of dI water into the capsules to wash away the fixative. Repeat this wash step for a total of 3 rinse cycles.
- Aspirate 40 µL of either 1% uranyl acetate (UA) or 1% potassium phosphotungstic acid (PTA) into the capsules and allow to sit for 30 s.
NOTE: Staining time may vary from 10 s to 1 min based on virus sample.
Caution: UA is an alpha emitter, and a cumulative toxin. Handle it with appropriate protection. - Remove the capsule from the pipette and blot dry the grids by touching a piece of filter paper to the edge of the grids while the grids remain within the capsule.
- Osmium Tetroxide Vapor inactivation procedure.
- Place the capsule, with the lid open, into a 50 mL centrifuge tube containing filter paper soaked in a 1% osmium tetroxide solution .
Caution: Osmium tetroxide is extremely toxic with low vapor pressure. It must be used in a ducted BSC or chemical fume hood. Handle it with appropriate protection. Post warning information in the working area. - Seal the 50-mL centrifuge tube for 1 h to allow complete permeation of the osmium tetroxide vapor. Then, subsequently decontaminate and transfer the tube out of the biocontainment to the BSL-2 EM facility.
- Place the capsule, with the lid open, into a 50 mL centrifuge tube containing filter paper soaked in a 1% osmium tetroxide solution .
- Remove EM grids from the capsule.
- In the BSL-2 EM facility, remove the capsule from the centrifuge tube and place it onto a pipette.
- Aspirate 40 µL of dI water into the capsule and dispense the water into a waste container three times.
- Remove the capsule from the pipette and blot dry the grids using filter paper to touch the edge of the grids.
- After air drying, store the grids for subsequent TEM imaging.
3. The Capsule Method for Inactivation in Biocontainment with 2% Glutaraldehyde, Followed by Negative Staining in a BSL-2 Laboratory
- Virus inactivation procedure.
- Inside the biocontainment BSC, mix the virus suspension well with the same volume of 4% glutaraldehyde to achieve a final concentration of 2% glutaraldehyde.
Caution: Glutaraldehyde is a hazardous chemical and requires appropriate protection. Glutaraldehyde can be used for brief periods in a normal BSC, but extended open reagent requires working in a ducted BSC or chemical fume hood. - Inactivate viruses with fixative for a minimum of 24 h before packaging, decontamination, and transfer it to the BSL-2 EM facility.
- Inside the biocontainment BSC, mix the virus suspension well with the same volume of 4% glutaraldehyde to achieve a final concentration of 2% glutaraldehyde.
- In the BSL-2 EM facility, aspirate the virus and fixative mixture into the capsule, containing two TEM grids, attached to a pipette.
- Place the pipette horizontally for 10 min with the grids oriented horizontally.
NOTE: This is to promote an even distribution of virus particles onto the TEM grids. - Pick up the pipette and depress the plunger to expel the virus to a waste container. Aspirate 40 µL of dI water into the capsules and expel it into the waste container for 3 rinse cycles.
- Aspirate 40 µL of either 1% UA or 1% PTA into the capsules for 30 s.
NOTE: Staining time varied from 10 s to 1 min based on virus sample.
Caution: UA is an alpha emitter, and a cumulative toxin. Handle it with appropriate protection. - Remove the capsule from the pipette and blot dry the grids by touching the edge of the grids to a piece of filter paper. Air dry the grids and store them for subsequent TEM imaging.
Representative Results
The capsule method produce good quality negative staining for TEM imaging:
First, we evaluated the quality of images generated by using both the manual droplet method and the capsule methods for negative staining Zaire ebolavirus. Ebolaviruses are members of the Filoviridae family, along with Marburg virus. Ebolavirus is typically 80 to 100 nm in diameter and can be over 1,000 nm in length. Ebolavirus must be handled in a BSL-4 biocontainment environment. Figure 2 shows images generated using the manual droplet method and the capsule method for negative staining. Figure 2A (manual droplet method) and Figure 2B (capsule method) show ebolavirus samples that have clearly defined details with nucleocapsid structures in the center of the virion, and visible ebolavirus glycoproteins on the surface. Thus, both the capsule and droplet methods have the ability to produce similar quality TEM images.
Compare and evaluate EM image quality after rapid inactivation with aqueous glutaraldehyde and 1% osmium tetroxide vapor versus 24 hour inactivation with 2% aqueous glutaraldehyde, using the capsule method:
We evaluated the quality of TEM images generated from two different methods of sample inactivation using Chikungunya virus. Chikungunya virus is a member of the Alphavirus genus in the family Togaviridae. It is spherical with a diameter of 60-70 nm. The virion contains an envelope rich in glycoproteins which form trimeric spikes on the viral surface. Chikungunya virus must be handled in BSL-3. Rapid inactivation is achieved using 2% glutaraldehyde for 20 min followed by a 1 h exposure to 1% osmium tetroxide vapor, with the entire negative staining process occurring in a capsule within a BSC in the biocontainment laboratory (Figure 1C). However, when using 2% glutaraldehyde for 24 h to inactivate the virus, the inactivation occurs inside a biocontainment environment, but the 1% UA negative stain procedure is carried out using the capsule method in a BSL-2 laboratory (Figure 1D). These inactivation procedures do not produce the same quality images (Figure 3). It is clear from Figure 3 that fixation in glutaraldehyde without the presence of osmium tetroxide (Figure 3A, 3B) shows more ultrastructural detail than samples prepared with glutaraldehyde and osmium tetroxide (Figure 3C, 3D).
The capsule method works well using Uranyl acetate (UA) and phosphotungstic acid (PTA) as negative stains for aldehyde fixed samples.
Examples of UA and PTA negative staining using the capsule method are shown in Figure 4 on aldehyde fixed virus-like-particles (VLPs). The VLPs are proteins assembled into virus like structures, but do not contain any viral genetic material. They are typically used in vaccine development and for basic viral research. Negative staining is a valuable tool to evaluate VLP assembly and morphology. We used both UA and PTA for negative staining of VLPs with the capsule method. Both stains display high quality results with glycoproteins visible and clearly defined borders of the Ebola nano-VLPs 15 (Figure 4A, 4B) and Murine Leukemia VLPs 16 (Figure 4C, 4D).
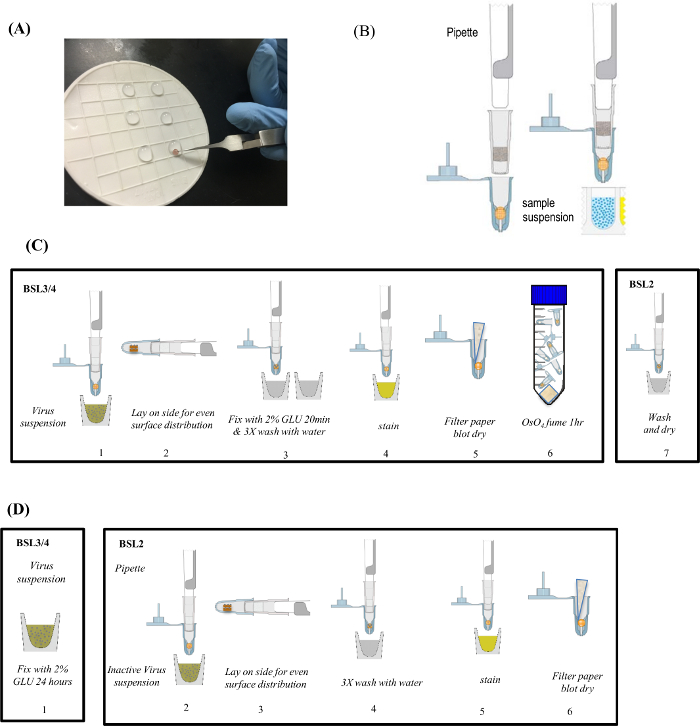
Figure 1: Negative Staining Methods Overview. (A) Manual droplet method is performed by sequentially moving TEM grids from droplet to droplet of specimen, rinses and stains. (B) Setting up the capsule method with grids and application of sample suspension. (C) Typical procedure using the capsule method in biocontainment with short-term inactivation with 1% osmium tetroxide vapor. (D) Recommended procedure using the capsule method in biocontainment with long-term inactivation with 2% glutaraldehyde. Figure was previously published and re-used with permissions from reference13. Please click here to view a larger version of this figure.
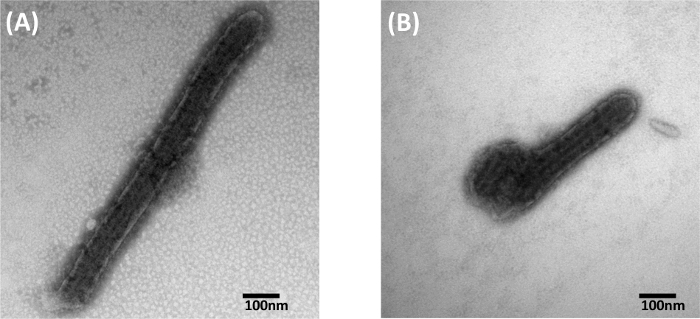
Figure 2: Comparison of 1% PTA Negative Stained Ebolavirus Particles. (A) Negative stained with the manual droplet method. (B) Negative stained using the capsule method. Figure was previously published and re-used with permissions from reference13. Please click here to view a larger version of this figure.
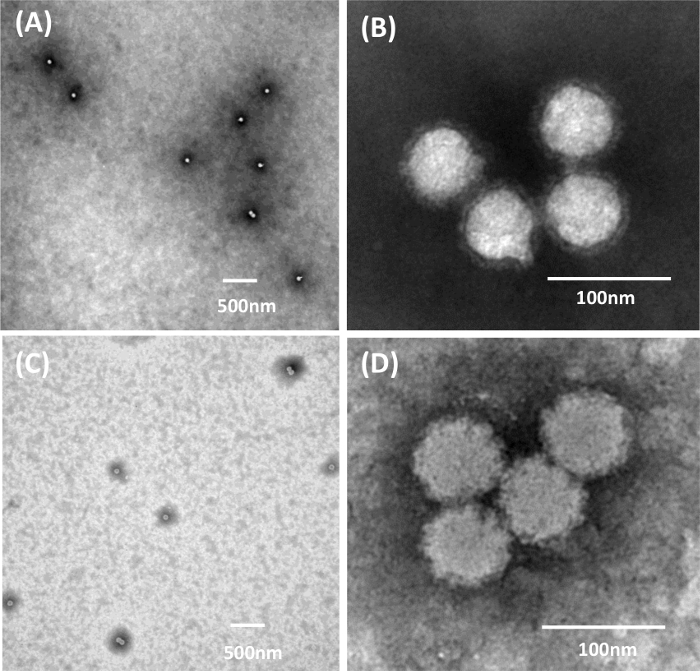
Figure 3: 1% UA Negative Staining with the Capsule Method of Chikungunya Virus using Different Inactivation Procedures. (A, B) inactivation for a minimum of 24 h with 2% glutaraldehyde only. (C, D) Rapid inactivation with 2% glutaraldehyde and 1% osmium tetroxide vapor. Figure was previously published and re-used with permissions from reference13. Please click here to view a larger version of this figure.
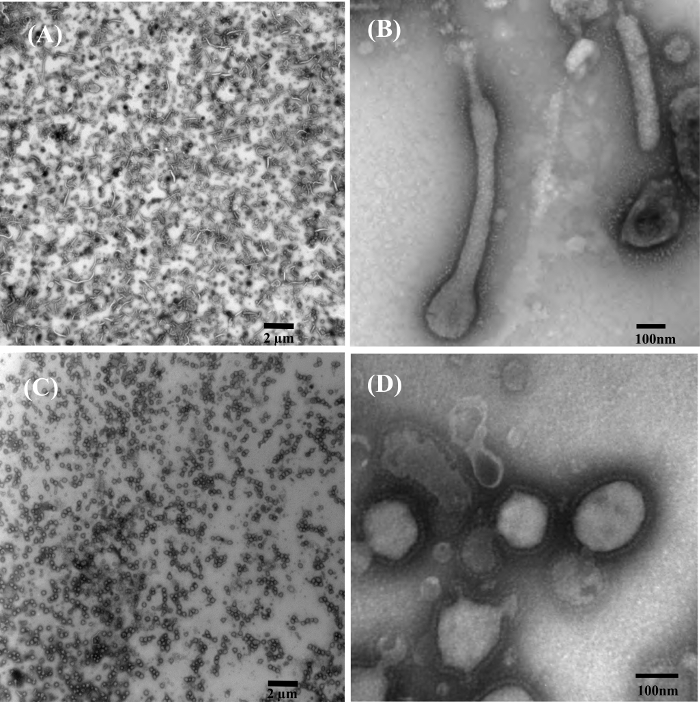
Figure 4: Examples of Phosphotungstic acid (PTA) and Uranyl Acetate (UA) Negatively Stained Virus-Like-Particles (VLPs) using the Capsule Method. (A) Low magnification overview of 1% PTA stained ebola nano-VLPs. (B) High magnification TEM image showing structural details of PTA stained ebola nano-VLPs. (C) Low magnification overview of 1% UA stained Murine Leukemia VLPs. (D) High magnification TEM image showing structural details of UA stained Murine Leukemia VLPs with ebolavirus glycoprotein on their surface. Figure was previously published and re-used with permissions from reference13. Please click here to view a larger version of this figure.
Discussion
Negative staining is a valuable TEM technique for evaluating and sizing viruses, protein complexes and nanoparticles. Droplet preparation of these specimens by manual moving of grids from reagent to negative stains has been the classic protocol for more than half a century. It is a simple process, but requires expertise gained through training for successful completion. Excellent negative staining is still considered a state-of-the-art skill set and highly desired in many TEM labs. The capsule method has several distinct advantages over the manual droplet method, especially in biocontainment laboratories. First, the manual droplet method requires substantial training and experience before it can be performed successfully, while the easy to use the capsule method can be accomplished by entry level technicians by simple pipetting. The biggest challenge with the manual droplet method is the successful handling the TEM grids with forceps in BSL-3 and BSL-4 PPE due to the greatly reduced tactile sensation and dexterity. Fragile coated TEM grids are easily damaged or punctured by forceps. Damage to the coated TEM grid is often not revealed until it is viewed with a TEM. A second advantage of capsules is that TEM grids are only directly handled when they are inserted into capsules and when they are removed for insertion into the electron microscope. Whether working in the biocontainment laboratory or in the BSL-2 EM facility, there is no direct grid handling. A third advantage of the capsule method is that both sides of the grid are covered with viruses and stains. This can be useful when the sample concentration is low; however this could cause too much sample if the concentration is high, or result in dilution of the sample prior to use.
Negative staining multiple grids using the manual droplet technique is time consuming because each sample needs to be individually prepared and stained. This leads to difficulties obtaining consistent, reproducible staining. Furthermore, keeping track of many individual grids is always a challenge. Each capsule holds two grids and multiple capsules can be attached to a multichannel pipette, streamlining the process. Therefore, capsules ensure that grids are stained using a very similar process, under consistent sample conditions, and at the same time. The capsules can be labeled to aid in organization, thus reducing potential for mixing up or misidentification of grids. Another common problem faced when using the manual droplet method is grid contamination. This can occur when the grids are left in the open air for too long or are dropped. The capsule protects the TEM grids from the open air and holds them securely so they are never dropped. Capsules provide greater experimental control and repeatability when preparing and negative staining because the capsule is a more controlled environment.
The capsules can be purchased pre-loaded if desired. Manually loading grids into the capsules takes some practice to ensure grids are not damaged. The capsule method also requires slightly more sample and stain than the manual droplet method. The capsule method requires at least 40 µL of sample, compared to the manual droplet method, which can be done with as little as 8 µL.
Virus inactivation is an important part of negative staining in BSL-3 and BSL-4, as it allows the inactivated virus to be taken out of the biocontainment laboratories for imaging in the EM facility. Inactivation using fixatives has the added benefit of fixing the virus, preventing degradation. There are two different ways to inactivate the virus sample, both of which were examined in this study. The first adheres the virus to coated TEM grids, fixes the viruses for 20 min in a 2% glutaraldehyde solution, followed by 1 h exposure of the grid to osmium tetroxide vapor (Figure 1C). This inactivation method is advantageous because it saves time by enabling the sample to be taken out of biocontainment laboratory after 1 h and 20 min. It also prevents dilution of virus before application to the coated TEM grid, so it may be necessary for samples suspected to be near the TEM detection limit. However, 1% osmium tetroxide vapor reduces the quality of TEM images as the osmium can further stain the sample (Figure 3). Previous reports show a 5 min exposure to osmium tetroxide vapor provides great results when negative staining; however, longer exposure required for absolute viral deactivation generated a mixture of positive and negative staining17. The second inactivation method involves a minimum of 24 h in a 2% glutaraldehyde solution, and does not require osmium tetroxide vapor treatment (Figure 1D). This second method takes longer to complete as the sample is not removed from the biocontainment laboratory for 24 h, but can be advantageous because it allows the negative staining to be done outside of biocontainment in a BSL-2 environment, and eliminates the need for hazardous osmium tetroxide. Our results demonstrate that 24 h of glutaraldehyde inactivation produces higher quality TEM images than when samples are treated with osmium tetroxide vapor (Figure 3).
Two negative stains were used in this experiment: UA and PTA. Both stains are used as a 1% solution. UA, the acetate salt of uranium, works well as a negative stain because dense uranium atoms scatter electrons5. PTA, a heteropoly acid, similarly works well as a negative stain due to dense tungsten atoms5. PTA is sometimes preferred over UA because it is much less toxic, and only a mild irritant if inhaled or contacted. When negative staining unfixed samples, the lower pH of UA must be considered as it can cause damage to the sample. The viruses used in this experiment require fixation for inactivation, so both UA and PTA achieved similar results and no distinct advantage could be detected for either stain. Both stains are easy to work with, and they both produce similar quality TEM images (Figure 4).
Overall, the capsule method is easier to use in a biocontainment environment and can be used as an alternative to the manual droplet method. Using capsules eases sample manipulation defects and yields good quality TEM images. The images produced using the capsule method are comparable to images produced using the manual droplet method. Short virus inactivation with osmium tetroxide improves speed, but should only be used if reduced image quality is acceptable, or it is suspected diluting the virus will put it below the TEM detection limit for the procedure. Both UA and PTA provide similar results with the fixed virus samples used in this study; however, results may be different when staining unfixed viruses.
Declarações
The authors have nothing to disclose.
Acknowledgements
We would like to acknowledge and thank Dr. John Carra and Rowena Schokman for providing purified Ebola nano-VLPs, Dr. Rajini Mudhasani for providing Chikungunya virus, and Dr. Charles (Jason) Shoemaker for providing Murine Leukemia VLPs expressing Ebolavirus glycoproteins. We would also like to thank MAJ Carl Soffler for facilitating the Summer Internship Program (SIP) and the Science and Engineering Apprenticeship Program (SEAP) and Dr. Catherine Wilhelmsen for the lab safety training.
Materials
| Formvar/carbon coated TEM grids | SPI | 3420C-MB | 200 mesh Cu Pk/100 |
| mPrep/g capsules | EMS | 85010-01 | box |
| mPrep/g couplers | EMS | 85010-11 | standard 16/Pk |
| glutaraldehdyde | EMS | 16320 | 50% solution, EM grade |
| Osmium Tetroxide | EMS | 19190 | 4% aqueous solution |
| Uranyl Acetate | EMS | 22400 | powder |
| Potassium phosphotungstic acid | EMS | 19500 | powder |
| filter paper | Whatman | 1450-090 | size 50 |
| Tranmission Electron Microscope | JEOL | JEM-1011 | TEM |
Referências
- Gentile, M., Gelderblom, H. R. Electron microscopy in rapid viral diagnosis: an update. New Microbiol. 37 (4), 403-422 (2014).
- Kruger, D. H., Schneck, P., Gelderblom, H. R. Helmut Ruska and the visualisation of viruses. Lancet. 355 (9216), 1713-1717 (2000).
- Curry, A., Appleton, H., Dowsett, B. Application of transmission electron microscopy to the clinical study of viral and bacterial infections: present and future. Micron. 37 (2), 91-106 (2006).
- Goldsmith, C. S., Miller, S. E. Modern uses of electron microscopy for detection of viruses. Clin Microbiol Rev. 22 (4), 552-563 (2009).
- Kiselev, N. A., Sherman, M. B., Tsuprun, V. L. Negative staining of proteins. Electron Microsc Rev. 3 (1), 43-72 (1990).
- Brenner, S., Horne, R. W. A negative staining method for high resolution electron microscopy of viruses. Biochim Biophys Acta. 34, 103-110 (1959).
- Harris, J. R. Negative staining of thinly spread biological samples. Methods Mol Biol. 369, 107-142 (2007).
- Bradley, D. E. Ultrastructure of bacteriophage and bacteriocins. Bacteriol Rev. 31 (4), 230-314 (1967).
- Suzuki, H., et al. Effects of two negative staining methods on the Chinese atypical rotavirus. Arch Virol. 94 (3-4), 305-308 (1987).
- Benmeradi, N., Payre, B., Goodman, S. L. Easier and Safter Biological Staining: High Contrast Uranyless Staining of TEM Grids using mPrep/g Capsules. Microsc Microanal. 21, 721 (2015).
- Goodman, S. L., Wendt, K. D., Kostrna, M. S., Radi, C. Capsule-Based Processing and Handling of Electron Microscopy Specimens and Grids. Microscopy Today. 23 (5), 30-37 (2015).
- Goodman, S. L., Kostrna, M. S. Reducing Reagent Consumption and Improving Efficiency of Specimen Fixation and Embedding, Grid Staining and Archiving using mPrep Capsule Processing. Microsc Microanal. 17, 174-175 (2011).
- Monninger, M. K., et al. Preparation of viral samples within biocontainment for ultrastructural analysis: Utilization of an innovative processing capsule for negative staining. J Virol Methods. 238, 70-76 (2016).
- Rossi, C. A., et al. Evaluation of ViroCyt(R) Virus Counter for rapid filovirus quantitation. Viruses. 7 (3), 857-872 (2015).
- Carra, J. H., et al. A thermostable, chromatographically purified Ebola nano-VLP vaccine. J Transl Med. 13, 228 (2015).
- Rein, A. Murine leukemia viruses: objects and organisms. Adv Virol. , 403419 (2011).
- Barland, M., Rojkind, Negative staining with osmium tetroxide vapour. Nature. 212 (5057), 84-85 (1966).

