Quantifying Vibrio cholerae Colonization and Diarrhea in the Adult Zebrafish Model
Summary
Zebrafish are a natural Vibrio cholerae host and can be used to recapitulate and study the entire infectious cycle from colonization to transmission. Here, we demonstrate how to assess V. cholerae colonization levels and quantify diarrhea in zebrafish.
Abstract
Vibrio cholerae is best known as the infectious agent that causes the human disease cholera. Outside the human host, V. cholerae primarily exists in the aquatic environment, where it interacts with a variety of higher aquatic species. Vertebrate fish are known to be an environmental host and are a potential V. cholerae reservoir in nature. Both V. cholerae and the teleost fish species Danio rerio, commonly known as zebrafish, originate from the Indian subcontinent, suggesting a long-standing interaction in aquatic environments. Zebrafish are an ideal model organism for studying many aspects of biology, including infectious diseases. Zebrafish can be easily and rapidly colonized by V. cholerae after exposure in water. Intestinal colonization by V. cholerae leads to the production of diarrhea and the excretion of replicated V. cholerae. These excreted bacteria can then go on to colonize new fish hosts. Here, we demonstrate how to assess V. cholerae-intestinal colonization in zebrafish and how to quantify V. cholerae-induced zebrafish diarrhea. The colonization model should be useful to researchers who are studying whether genes of interest may be important for host colonization and/or for environmental survival. The quantification of zebrafish diarrhea should be useful to researchers studying any intestinal pathogen who are interested in exploring zebrafish as a model system.
Introduction
Vibrio cholerae is an aquatic, Gram-negative bacterium that causes the human disease cholera as well as sporadic diarrhea1,2. V. cholerae is found in the environment in many areas of the globe, often associated with other aquatic organisms. These associating organisms include plankton, insect egg masses, shellfish, and vertebrate fish species3,4,5,6,7. Several studies have isolated V. cholerae from the intestinal tracts of fish in different geographical areas7,8,9,10. The presence of V. cholerae in fish indicates that fish may act as an environmental reservoir. Fish could also be implicated in transmitting the disease to humans and in the geographic spreading of V. cholerae strains6.
To better understand how V. cholerae interacts with fish, Danio rerio, better known as zebrafish, was developed as a model system for studying V. cholerae11. Zebrafish are native to southern Asia, including the Bay of Bengal region, which is thought to be the earliest reservoir of V. cholerae. Prior to the first cholera pandemic beginning in 1817, cholera had not been reported outside of what is now India and Bangladesh. Therefore, zebrafish and V. cholerae almost certainly associated with each other over evolutionary time scales, suggesting that zebrafish are a V. cholerae host in the natural environment12.
The zebrafish model for V. cholerae is simple to execute and can be used to study the entire pathogenic V. cholerae life cycle. Fish are exposed to V. cholerae by bathing in water that has been inoculated with a known number of V. cholerae. Within a few hours, intestinal colonization takes place, followed by the production of diarrhea. Diarrhea consists of mucin, proteins, excreted bacteria, and other intestinal contents. The degree of diarrhea can be quantified using a few simple measurements13. V. cholerae that has been excreted by infected fish can then go on to infect naïve fish, completing the infectious cycle. Therefore, the zebrafish model recapitulates the V. cholerae human disease process12,14.
The most frequently used V. cholerae animal models have historically been mice and rabbits14,15,16,17,18. These models have been instrumental in adding to our knowledge of V. cholerae pathogenesis. However, because mice and rabbits are not natural V. cholerae hosts, there are limitations to what aspects of the V. cholerae life cycle can be studied. The V. cholerae colonization of mice and rabbits typically requires the absence of intestinal microbiota or a pretreatment with antibiotics to damage the intestinal microbiota. Both models require either gavage to introduce the bacteria to the digestive tract or surgical manipulation to directly inject the bacteria into the intestines. Zebrafish have an advantage in that adult fish with an intact intestinal microbiota are readily colonized and the infectious process happens naturally, without any manipulation required.
The present work demonstrates the utilization of zebrafish as a model in V. cholerae infection. The infection, dissection, enumeration of colonizing V. cholerae, and the quantification of diarrhea caused by V. cholerae will be described12,13. This model is likely to be useful to scientists interested both in the V. cholerae disease process and in the V. cholerae environmental lifestyle.
Protocol
All methods described here have been approved by the Institutional Animal Care and Use Committee (IACUC) of Wayne State University. This method was first described in Runft et al.12
1. Determination of Intestinal Colonization Levels
NOTE: Intestinal colonization is the most useful metric in the zebrafish model as it can be used to compare the relative fitness of various V. cholerae strains or the effects of mutations or gene knockouts.
- Inoculation of zebrafish by immersion
NOTE: This means of exposure resembles the natural course of infection.- Preparation of V. cholerae
- Inoculate 5 mL of lysogeny broth (LB) with an isolated V. cholerae colony from an LB plate and incubate it at 37 °C with shaking (180 rpm) for 6–8 h (overnight culture).
- Inoculate 25 mL of fresh autoclaved LB medium in a 250 mL conical flask with 50 µL of the above overnight culture. Incubate it for 16–18 h at 37 °C with shaking (150 rpm).
- Read the optical density at 600 nm (OD600) of a 1/10 dilution of the overnight culture to estimate the number of bacteria per mL (the OD600 = 1 is ~109 CFU/mL.)
- Harvest the cells by centrifugation at 6,000 g for 10 min. Remove the media with a pipette or pour it off into a disinfectant solution. Resuspend the cells in sterile PBS to the desired concentration, typically between 1 x 107 to 1 x 1010 per ml of PBS.
NOTE: Here, we refer to autoclaved reverse-osmosis water containing 60 mg/L sea salts as sterile infection water.
- Inoculation and incubation
- Place four or five zebrafish into a 400 mL beaker containing 200 mL of sterile infection water.
- Add 1 mL of V. cholerae (from step 1.1.1.4.) to get the desired infection concentration (typically 5 x 104 to 5 x 107 CFU/mL) in 200 mL of infection water.
- Cover the beaker with a perforated lid to prevent the fish from jumping out; the top of a 200 µL tip box works well for this.
- Label each beaker and place them into a glass-front incubator set at 28 °C for the duration of the experiment.
- Procedure for transitory exposure
NOTE: A transitory exposure to V. cholerae (typically 6 h) followed by the removal of the inoculating bacteria is useful for studying the transmission and for the more accurate quantification of excreted bacteria.- After 6 h of exposure, pour out the beaker water through a fishnet to collect the fish. Discard the infected water into a container of bleach to kill the V. cholerae.
- Place the removed fish in a beaker of 200 mL of sterile infection water. Allow the fish to swim in this clean water for 5 min to remove the surface bacteria from the fish.
- Repeat the net procedure (step 1.2.2) and place the fish in a new beaker of 200 mL sterile infection water. Keep the fish in this beaker for the duration of the experiment.
- Preparation of V. cholerae
- Euthanasia
- When the experiment has reached its desired endpoint, take a sample of the infection water (15 mL is usually sufficient) to allow for excreted bacterial counts and the measurement of diarrhea (such as mucin assay, protein content, and/or OD), if desired (section 2).
- Pour off the remainder of the water through a fishnet (to collect the fish) into bleach to kill the V. cholerae in the water.
- Place the fish into a beaker containing infection water plus 336 µg/mL of Ethyl 3-aminobenzoate methanesulfonate (tricaine) and incubate the fish in this tricaine solution for 20 min at RT.
NOTE: The fishnets were sterilized with a 10% bleach solution.
- Dissection
- Preparation of fish
- Scoop a fish out of the tricaine solution with a disposable plastic spoon and place it on the dissecting surface.
- Position the fish with its ventral side facing upward and pin it through the lower jaw, with the blunt end of the pin angled away from the center of the body. Place another pin just posterior to the anus, also angled away from the body.
- Exposure of intestinal tract
- Swab the ventral surface of the fish with a lint-free wipe dipped in 70% ethanol.
- Sterilize a scalpel and Vannas scissors by dipping them in 70% ethanol and flaming them.
- Make a small incision lengthwise in the belly just under the skin by penetrating the scales and using the scalpel. Be careful not to cut too deeply, as the intestinal tract is located just under the skin.
- Using the scissors, extend the incision carefully along the length of the body, cutting no deeper than skin level and avoiding the anus.
- Make two lateral cuts with the scissors toward the head of the fish to allow an opening of the incision.
- Pin the skin on each side of the lateral incisions to the dissecting surface, angling the pins out, away from the body.
NOTE: The tips of the pins were previously sterilized by flaming them with alcohol.
- Removal of intestinal tract
NOTE: The intestinal tract should be visible as a pale, very thin tube on top of the other organs.- Flame-sterilize the forceps and then use them to remove the entire intestinal tract (typically 12–15 mm in length). Place the intestine into a homogenization tube containing glass beads (see steps 1.4.1–1.4.2) and 1 mL of 1x PBS or LB, on ice.
- Preparation of fish
- Homogenization
- Prepare the homogenization tubes in advance by pouring ~1.5 g of 1 mm glass beads into 2 mL screw cap tubes (fill them approximately half-way) and then sterilize them by autoclaving.
- Add 1 mL of sterile LB or 1x PBS.
- Once the zebrafish intestines have been added (step 1.3.3.1) to the tubes, screw the caps on very tightly, and then secure the tubes in the vortex homogenizer.
- Homogenize the samples for 1 min on the maximum setting, then cool them on ice for 1–2 min. Repeat this homogenization cycle once more for a complete homogenization.
Note: Several alternative methods for homogenization will also work.
- Quantifying intestinal colonization levels
- Prepare tubes (either small glass test tubes or 1.5 mL microcentrifuge tubes) for the serial dilution of the homogenate by adding 900 µL of LB or 1x PBS to each tube. For each fish, prepare 5–6 tubes and make 10-fold serial dilutions of the intestinal homogenate by adding 100 µL of homogenate to the first tube, vortexing it to mix, and then adding 100 µL from the first tube to the second tube.
- Repeat this procedure until all dilutions have been prepared.
- Plate 100–200 µL of each dilution on LB agar plates containing 100 µg/mL of streptomycin (if using Streptomycin resistant strains) and 40 µg/mL of X-gal (5-bromo-4-chloro-3-indolyl-β-D-galactopyranoside). Incubate the plates at 30 °C for 16–18 h.
- After the overnight incubation, count V. cholerae colonies on the plates, either using an automated colony counter or manually counting the colonies; it is helpful to mark the counted colonies with a marker tip.
- Determine the CFU per fish intestine by multiplying the plate count by the dilution factor of the suspension, taking into account the volume that was plated.
2. Measurement of Fish Diarrhea
NOTE: Four different metrics were used to assess fish diarrhea: the mucin levels in the water, the overall excreted protein levels in the water, the OD600 of the water, and the V. cholerae CFU in the water13. Diarrhea normally becomes visually evident approximately 6 h after the exposure of the zebrafish to V. cholerae as described above.
- Determine mucin levels
NOTE: Mucin secretion is induced during the V. cholerae colonization and is a good measure of excretion levels, especially early in infection13. The mucin assay is a variation on the microtiter periodic acid Schiff assay19.- Prepare the following materials: 50% (w/v) periodic acid stock solution and 0.1% periodic acid working solution (10 µL of the 50% periodic acid stock added to 5 mL of 7% acetic acid—use immediately after making), mucin standards, 96-well microtiter plates, Schiff’s reagent.
- Prepare mucin standards by suspending the following amounts of mucin (from a porcine stomach type III) in a sodium acetate buffer (100 mM sodium acetate, 5mM EDTA, pH to 5.5 with glacial acetic acid): 400 µg/mL, 300 µg/mL, 200 µg/mL, 150 µg/mL, 100 µg/mL, 75 µg/mL, 50 µg/mL, 25 µg/mL, and 10 µg/mL.
Prepare the plate by adding 100 µL of infection water to each well that will be used in the assay as follows: blank 1x PBS; mucin standards as described above, or a water sample from the fish infection. Do each sample in triplicate.
- Prepare mucin standards by suspending the following amounts of mucin (from a porcine stomach type III) in a sodium acetate buffer (100 mM sodium acetate, 5mM EDTA, pH to 5.5 with glacial acetic acid): 400 µg/mL, 300 µg/mL, 200 µg/mL, 150 µg/mL, 100 µg/mL, 75 µg/mL, 50 µg/mL, 25 µg/mL, and 10 µg/mL.
- Add 50 µL of fresh 0.1% periodic acid solution to each well with a multichannel pipette and mix it by pipetting up and down several times.
- Wrap the plate tightly in plastic wrap and incubate it at 37 °C for 1–1.5 h.
- Cool the plate down to room temperature for 5–10 min. Add 100 µL of Fuchsin solution (Schiff’s reagent) to each well, and pipet up and down to mix it.
- Wrap the plate in plastic wrap and incubate it at room temperature on a rocking platform until color has developed; the color is generally developed within 20 min. Read the absorbance at 560 nm using a plate reader.
- Plot a mucin standard curve (OD560 vs. µg/mL mucin standard). Determine the mucin levels of the unknowns by identifying where the OD560 of each unknown falls on the standard curve and reading the corresponding mucin concentration for that value.
- Prepare the following materials: 50% (w/v) periodic acid stock solution and 0.1% periodic acid working solution (10 µL of the 50% periodic acid stock added to 5 mL of 7% acetic acid—use immediately after making), mucin standards, 96-well microtiter plates, Schiff’s reagent.
- Determine protein levels
NOTE: Aside from mucin, overall protein levels in the water are another proxy for the levels of diarrhea (cloudy, liquid stool) produced by the fish. This procedure uses a standard Bradford assay to estimate protein levels. Protein levels are estimated based upon a bovine serum albumin (BSA) standard curve.- Mix 100 µL of one of the BSA standards (see note below) or 100 µL of infection water (water from the beaker containing the infected fish) with 900 µL of Bradford assay reagent (the Pierce protein assay reagent works well for this) in a disposable cuvette. Incubate all samples at room temperature for 2 min.
Note: The following BSA standards were used: 1,800 µg/mL, 1,000 µg/mL, 750 µg/mL, 500 µg/mL, 250 µg/mL, 125 µg/mL, 50 µg/mL, and 25 µg/mL. The BSA standards were diluted in autoclaved distilled water. - Read the OD660 of each standard or sample them using a spectrophotometer.
- Plot the BSA standard curve (OD660 vs. µg/mL). Determine the protein levels of the unknowns by identifying where the OD660 of each unknown falls on the BSA standard curve and read the corresponding mucin concentration for that value.
- Mix 100 µL of one of the BSA standards (see note below) or 100 µL of infection water (water from the beaker containing the infected fish) with 900 µL of Bradford assay reagent (the Pierce protein assay reagent works well for this) in a disposable cuvette. Incubate all samples at room temperature for 2 min.
- Measure OD600
NOTE: The presence of diarrhea (cloudy, liquid stool) is visibly evident after several h and a simple OD600 determination can be used to quantify this.- Invert the tube containing the infection water several times to evenly distribute the contents. Add 1 mL of the water sample to a disposable cuvette. Use 1 mL of sterile infection water as the negative control.
- Perform a “blank” measurement using the spectrophotometer at 600 nm using the negative control sample. Read each water sample at 600 nm and record the results.
- Determination of excreted V. cholerae CFU/mL after infection
NOTE: A major component of the diarrhea produced by the zebrafish is excreted V. cholerae. The determination of the CFU/mL can be used for purposes such as estimating the bacterial replication in the fish intestinal tract and as a measure of an infectious dose in transmission experiments. This measure is best done when a transitory infection has taken place. If a 24 h infection is used, then this assay will measure the combined inoculum plus the excreted V. cholerae.- Take a water sample at the desired time point and serially dilute it as described previously.
- Plate the serial dilutions on selective media (LB agar containing streptomycin or another appropriate antibiotic or DCLS) and incubate them at 30 °C for 24 h.
- Count the colonies. Calculate the CFU per mL of water as described in step 1.5.
Representative Results
V. cholerae colonization of zebrafish intestinal tracts
To provide an example of the typical colonization levels we observe, we inoculated 5 x 106 CFU of the pandemic EL Tor V. cholerae strain N16961 in 200 mL of water in a beaker containing several zebrafish. After 6 h of infection, the fish were washed in fresh water and transferred into a beaker of 200 mL of autoclaved infection water as described in the protocol. 18 h after the transfer (or 24 h after the primary infection), the fish were euthanized, and their intestines were taken to determine the colonization levels. Approximately 105 to 106 V. cholerae cells per fish intestine were typically observed in the intestinal tract 24 h post-infection (hpi) (Figure 1) with the 5 x 106 inoculum size.
Quantification of fish diarrhea
Diarrhea was quantified using the four simple assays described above. The first assay, the CFU of excreted V. cholerae, is shown in Figure 1. Serial dilutions of the water 24 hpi were plated to count the V. cholerae CFU. Relatively high levels of excreted V. cholerae are usually observed; in this example, approximately 105 V. cholerae per mL of water were detected. Uninfected fish do not produce detectible V. cholerae (data not shown).
The second assay used to quantify diarrhea is excreted mucin. Figure 2 shows the effects of three different V. cholerae infectious doses on the mucin levels in water 24 hpi. A higher infectious dose is correlated with a higher excretion of mucin into the water. As a control, four fish were placed in an identical beaker of water, but PBS was added instead of the V. cholerae suspension. The control fish excreted very little mucin.
The third diarrheal quantification assay is the OD600 of the water 24 hpi. As shown in Figure 3, the OD600 of this water was significantly higher in the beaker containing zebrafish infected with V. cholerae than in the beaker containing uninfected zebrafish.
Finally, the total protein levels were measured in the water. Water containing the infected fish contained total protein levels nearly twice that observed in the water containing the uninfected fish (Figure 4). Collectively, these four assays illustrate the effects V. cholerae infection has on zebrafish excretion.
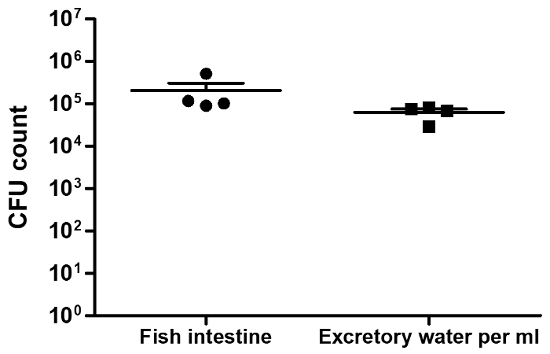
Figure 1: Intestinal colonization of zebrafish by V. cholerae. The fish were infected for 6 h, then washed and incubated for a total of 24 h. The left side of the figure illustrates the total CFU per intestine of four fish (black dots.) The right side of the figure indicates the V. cholerae CFU per mL measured in water 24 hpi (black squares.) The means of both data sets are shown with a large horizontal line and the standard deviation are indicated by error bars. Please click here to view a larger version of this figure.
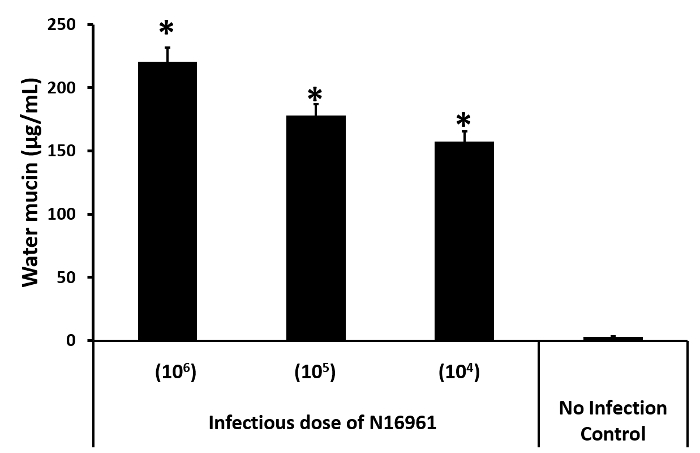
Figure 2: Mucin assay. Three different V. cholerae infectious doses were used, along with an uninfected control, as indicated under the x-axis. The mean values of the mucin detected in the water by the modified Periodic acid Schiff (PAS) assay are indicated by the black bars above each infectious dose. The error bars indicate the standard deviation. The asterisks indicate p <0.05 as determined by Student's unpaired t-test. Please click here to view a larger version of this figure.
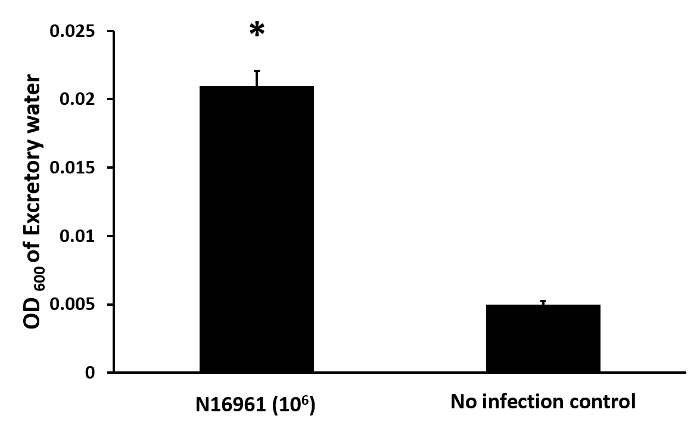
Figure 3: OD600 assay as a proxy for diarrhea. The OD600 of 1 mL of water from the beakers containing either V. cholerae infected or uninfected fish was measured. The black bars indicate the mean value and the error bars indicate the standard deviation. The asterisks indicate p <0.05 as determined by Student's unpaired t-test. Please click here to view a larger version of this figure.
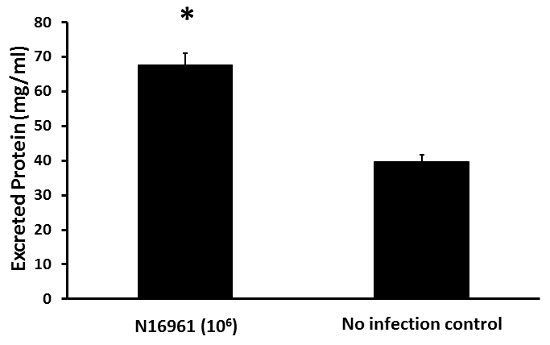
Figure 4: Total protein levels in water. The total protein was estimated by a Bradford assay as described. The black bars indicate the mean values and the error bars indicate the standard deviation. The asterisks indicate p <0.05 as determined by Student's unpaired t-test. Please click here to view a larger version of this figure.
Discussion
The zebrafish is a relatively new model for studying V. cholerae but holds much promise for the future discovery of previously unknown aspects of V. cholerae biology and pathogenesis11,12,13. The adult zebrafish model has the advantages of being both a natural V. cholerae host that contains intact, mature intestinal microbiota and an environmental model. Disadvantages of the model are that the two major human virulence factors, cholera toxin and toxin-coregulated pilus, are not required for zebrafish colonization or pathogenesis12. However, this could alternatively be viewed as another advantage that could enable the identification of novel V. cholerae colonization factors and facilitate the study of other V. cholerae toxins. This is especially relevant to the vast majority of non-O1/O139 "environmental" V. cholerae strains, most of which do not produce cholera toxin or toxin-coregulated pilus and yet can still cause disease20,21.
The problems most likely to arise using this model are related to the abundant intestinal microbiota. Plating on nonselective media will make this model essentially unusable as it will be impossible to identify the V. cholerae colonies. Even using selective or partially selective media such as LB plus streptomycin or DCLS, a background of colonies from intestinal microbiota will be present. It is essential to verify that the colonies that are being counted are bona fide V. cholerae by patching the colonies produced by the plating on less selective media onto a very selective medium such as TCBS. Alternatively, the insertion of a better selective marker into the genome of a strain of interest would greatly simplify the identification of V. cholerae from intestinal homogenates.
The advantage of the assays used to quantify zebrafish diarrhea is that they are simple and inexpensive to execute13. The disadvantage is that these assays are largely nonspecific, aside from counting excreted V. cholerae CFU directly, and it can become difficult to determine if the diarrhea is directly due to V. cholerae pathogenesis, or to some other stressor. The development of variations of these or other assays to improve their specificity will likely aid in future measurements of V. cholerae pathogenesis in zebrafish.
Declarações
The authors have nothing to disclose.
Acknowledgements
Thanks to Melody Neely, Jon Allen, Basel Abuaita, and Donna Runft for their efforts in helping to develop the zebrafish model. The research reported here was supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under award numbers R21AI095520 and R01AI127390 (to Jeffrey H. Withey). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Materials
| Instrument | |||
| Shaker incubator | New Brunswick Scientific, Edison, NJ | Excella E25 | |
| Incubator | NUAIRE, Plymouth, MN | Auto Flow | |
| Spectrophotometer | Thermo, Waltham, MA | Geaesys 6 | |
| Vortex homogenizer | Minibeadbeater24 | 112011 | |
| Weighing Machine | Ohaus, Columbia, MD | Adventurer Pro | |
| Heat Stirer | Corning, Corning, NY | PC-420D | |
| Burner | |||
| automated colony counter | REVSCI | 120417B | |
| Materials | |||
| 400 ml glass beakers | Pyrex | ||
| perforated lids | Microtip holder with holes from tip box | ||
| disposable plastic spoons | Office Depot, Boca Raton, FL | D15-25-7008 | |
| Fish Tank System | Aquaneering, San Diego, CA | ||
| RO Water Purifier | Aqua FX | TK001 | |
| Fish net | Marina | ||
| fish food | Tetra fin | ||
| Brine Shrimp | Red jungle brand | O.S.I. pro 80 | |
| Styrofoam board | |||
| Pins | |||
| Scalpels | Fine Scientific tools, Foster City, CA | 10000-10 | |
| Forceps | Fine Scientific tools, Foster City, CA | 11223-20 | |
| Vannas scissors | Fine Scientific tools, Foster City, CA | 15000-11 | |
| 2 ml screw cap tubes | Fisher Scientific, Hampton, NH | 02-681-375 | |
| 1 mm glass beads | Bio Spec | 11079110 | |
| Glass beads for spreading | Sigma, St. Louis, MO | 18406-500G | |
| Petri plate | Fisher Brand, Hampton, NH | FB0875713 | |
| 1.5 ml centrifuge tube | Midsci, Valley Park, MO | AVSS1700 | |
| 50 ml centrifuge tube | Corning Falcon, Corning, NY | 352098 | |
| Test tubes | Pyrex | 9820 | |
| Glass Pipette | Fisher Brand, Hampton, NH | 13675K | |
| Micro pipettes | Sartorius Biohit, Göttingen, Germany | m1000/m200/m20 | |
| Tips | Genesee Scientific, San Diego, CA | 24-150RS/24-412 | |
| Chemicals | |||
| Instant Ocean salts | |||
| phosphate buffered saline | VWR Life Science, Radnor, PA | K813-500ml | |
| Tricaine (ethyl 3-aminobenzoate methanesulfonate salt | Sigma, St. Louis, MO | A5040 | |
| 5-Bromo-4-chloro-3-indolyl-β-D-galactopyranoside | Sigma, St. Louis, MO | 10651745001 | |
| Schiff’s reagent | Sigma, St. Louis, MO | 84655-250 mL | |
| periodic acid | Fisher Scientific, Hampton, NH | 10450-60-9 | |
| Mucin from porcine stomach | Sigma, St. Louis, MO | M2378-100G | |
| Bovine serum albumin | Fisher Scientific, Hampton, NH | 9046-46-8 | |
| Pierce 660nm Protein Assay Reagent | Thermo, Waltham, MA | 22660 | |
| LB medium | |||
| Trypton | BD Biosciences, San Jose, CA | 211705 | |
| Teast Extract | BD Biosciences, San Jose, CA | 212750 | |
| NACL | Fisher Scientific, Hampton, NH | BP358-212 | |
| Agar | BD Biosciences, San Jose, CA | 214010 | |
| TCBS Agar | BD Biosciences, San Jose, CA | 265020 | |
| DCLS Agar | Sigma, St. Louis, MO | 70135-500gm | |
| Software | |||
| Microsoft office | |||
| Prism 5 |
Referências
- Harris, J. B., LaRocque, R. C., Qadri, F., Ryan, E. T., Calderwood, S. B. Cholera. The Lancet. 379 (9835), 2466-2476 (2012).
- Dutta, D., et al. Vibrio cholerae non-O1, non-O139 serogroups and cholera-like diarrhea, Kolkata, India. Emerging Infectious Diseases. 19 (3), 464-467 (2013).
- Huq, A., et al. Ecological relationships between Vibrio cholerae and planktonic crustacean copepods. Applied and Environmental Microbiology. 45 (1), 275-283 (1983).
- Halpern, M., Landsberg, O., Raats, D., Rosenberg, E. Culturable and VBNC Vibrio cholerae: interactions with chironomid egg masses and their bacterial population. Microbial Ecology. 53 (2), 285-293 (2007).
- Broza, M., Halpern, M. Pathogen reservoirs. Chironomid egg masses and Vibrio cholerae. Nature. 412 (6842), 40 (2001).
- Halpern, M., Izhaki, I. Fish as hosts of Vibrio cholerae. Frontiers in Microbiology. 8 (282), (2017).
- Senderovich, Y., Izhaki, I., Halpern, M. Fish as reservoirs and vectors of Vibrio cholerae. PLoS ONE. 5 (1), e8607 (2010).
- Traore, O., et al. Occurrence of Vibrio cholerae in fish and water from a reservoir and a neighboring channel in Ouagadougou, Burkina Faso. The Journal of Infection in Developing Countries. 8 (10), 1334-1338 (2014).
- Booth, L. V., Lang, D. A., Athersuch, R. Isolation of Vibrio cholerae non-01 from a Somerset farmworker and his tropical fish tank. Journal of Infection. 20 (1), 55-57 (1990).
- Torres-Vitela, M. A., et al. Incidence of Vibrio cholerae in fresh fish and ceviche in Guadalajara, Mexico. Journal of Food Protection. 60 (3), 237-241 (1997).
- Rowe, H. M., Withey, J. H., Neely, M. N. Zebrafish as a model for zoonotic aquatic pathogens. Developmental & Comparative Immunology. 46 (1), 96-107 (2014).
- Runft, D. L., et al. Zebrafish as a natural host model for Vibrio cholerae colonization and transmission. Applied and Environmental Microbiology. 80 (5), 1710-1717 (2014).
- Mitchell, K. C., Breen, P., Britton, S., Neely, M. N., Withey, J. H. Quantifying Vibrio cholerae enterotoxicity in a zebrafish infection model. Applied and Environmental Microbiology. , (2017).
- Klose, K. E. The suckling mouse model of cholera. Trends in Microbiology. 8 (4), 189-191 (2000).
- Formal, S. B., Kundel, D., Schneider, H., Kunevn, H., Sprinz, Studies with Vibrio cholerae in the ligated loop of the rabbit intestine. British Journal of Experimental Pathology. 42, 504-510 (1961).
- Williams, E. M., Dohadwalla, A. N., Dutta, N. K. Diarrhea and accumulation of intestinal fluid in infant rabbits infected with Vibrio cholerae in an isolated jejunal segment. The Journal of Infectious Diseases. 120 (6), 645-651 (1969).
- Spira, W. M., Sack, R. B., Froehlich, J. L. Simple adult rabbit model for Vibrio cholerae and enterotoxigenic Escherichia coli diarrhea. Infection and Immunity. 32 (2), 739-747 (1981).
- Ritchie, J. M., Rui, H., Bronson, R. T., Waldor, M. K. Back to the future: studying cholera pathogenesis using infant rabbits. mBio. 1 (1), (2010).
- Kilcoyne, M., Gerlach, J. Q., Farrell, M. P., Bhavanandan, V. P., Joshi, L. Periodic acid-Schiff’s reagent assay for carbohydrates in a microtiter plate format. Analytical Biochemistry. 416 (1), 18-26 (2011).
- Balaji, V., Sridharan, G., Jesudason, M. V. Cytotoxicity of non O1, non O139 Vibrios isolated from fresh water bodies in Vellore, south India. Indian Journal of Medical Research. 110, 155-159 (1999).
- Hasan, N. A., et al. Nontoxigenic Vibrio cholerae non-O1/O139 isolate from a case of human gastroenteritis in the U.S. Gulf Coast. Journal of Clinical Microbiology. 53 (1), 9-14 (2015).

