Generation and On-Demand Initiation of Acute Ictal Activity in Rodent and Human Tissue
Summary
Acute seizure models are important for studying the mechanisms underlying epileptiform events. Furthermore, the ability to generate epileptiform events on-demand provides a highly efficient method to study the exact sequence of events underlying their initiation. Here, we describe the acute 4-aminopyridine cortical seizure models established in mouse and human tissue.
Abstract
Controlling seizures remains a challenging issue for the medical community. To make progress, researchers need a way to extensively study seizure dynamics and investigate its underlying mechanisms. Acute seizure models are convenient, offer the ability to perform electrophysiological recordings, and can generate a large volume of electrographic seizure-like (ictal) events. The promising findings from acute seizure models can then be advanced to chronic epilepsy models and clinical trials. Thus, studying seizures in acute models that faithfully replicate the electrographic and dynamical signatures of a clinical seizure will be essential for making clinically relevant findings. Studying ictal events in acute seizure models prepared from human tissue is also important for making findings that are clinically relevant. The key focus in this paper is on the cortical 4-AP model due to its versatility in generating ictal events in both in vivo and in vitro studies, as well as in both mouse and human tissue. The methods in this paper will also describe an alternative method of seizure induction using the Zero-Mg2+ model and provide a detailed overview of the advantages and limitations of the epileptiform-like activity generated in the different acute seizure models. Moreover, by taking advantage of commercially available optogenetic mouse strains, a brief (30 ms) light pulse can be used to trigger an ictal event identical to those occurring spontaneously. Similarly, 30 – 100 ms puffs of neurotransmitters (Gamma-Amino Butyric Acid or glutamate) can be applied to the human tissue to trigger ictal events that are identical to those occurring spontaneously. The ability to trigger ictal events on-demand in acute seizure models offers the newfound ability to observe the exact sequence of events that underlie seizure initiation dynamics and efficiently evaluate potential anti-seizure therapies.
Introduction
Acute seizure models can successfully reproduce electrographic signatures reminiscent of ictal events observed in the electroencephalogram (EEG) of individuals experiencing a seizure. Researchers use these ictal-like events (herein referred to as 'ictal events') as surrogates for the seizure event1. Clinically, ictal events serve as a reliable proxy for seizure events since seizures are a neurological disorder which originates from the brain. In the epilepsy monitoring unit, neurologists rely upon the detection of ictal events to confirm the brain's epileptogenic region and isolate it for resection2. In the intensive care unit, physicians monitor ictal activity to assess if any seizure activity persists in sedated patients3. Controlling seizures remains to be a challenging issue for the medical community, as 30% of epilepsy patients are drug resistant to the available medication4,5, and 10% of medical cases involving drug-induced seizures are unresponsive to the standard treatment3. This presents a serious concern for the society, as 10% of the American population is prospected to experience one seizure event in their lifetime and 3% are expected to develop epilepsy6.
Studying seizures in chronic epilepsy models is expensive, laborious, and often take months to prepare7. It is also difficult to perform electrophysiological recordings in freely moving animals. Human clinical trials face similar issues, as well as additional complexities related to patient consent, variability in participants' backgrounds, and the moral and ethical considerations involved8. Acute seizure models, on the other hand, are favorable because they are relatively convenient to prepare, cost-efficient, and capable of generating large volumes of ictal events for study9. Additionally, the tissue is fixed in a stable position, so the conditions are ideal for performing the electrophysiological recordings necessary to study seizure dynamics and the related underlying pathophysiology. Acute seizure models remain favorable over in silico (computer) models because they are based on biological material comprised of the brain's constituent neuronal network with all its inherent factors and synaptic connectivity, that may not be captured by even the most detailed computer models10. These features make acute seizure models poised to be efficient at screening for potential anti-seizure therapies and making preliminary findings before advancing them for further investigation in chronic epilepsy models and clinical trials.
Typically, acute seizure models are derived from the normal brain tissue that has been subjected to hyper-excitable conditions. To induce clinically relevant ictal events in healthy brain tissue, it is important to understand that the brain functions optimally in a critical state11 where excitation (E) and inhibition (I) are balanced12. A disruption of the E-I balance can lead to the hyper-excitable seizure state in which ictal events precipitate. Accordingly, within this conceptual framework, there are two major strategies to generate ictal events in brain slices (in vitro) or in whole-brain (in vivo) preparations: either decreased inhibition ("disinhibition") or increased excitation ("non-disinhibition"). However, ictal events are highly ordered and synchronized events that require the influence of GABAergic interneurons to orchestrate the neural network activity13,14. For this reason, non-disinhibition models are the most effective for generating ictal events in isolated neural networks, such as in an in vitro brain slice15, whereas in vitro disinhibition models commonly lead to spiking activity reminiscent of interictal-like spiking. Furthermore, within this conceptual framework, a momentary synchronizing event can also reliably trigger an ictal event16. In fact, an ictal event can be triggered by any minor perturbation applied to the neural system17 when it is at a critical state transition ("bifurcation") point18. Traditionally, these perturbations were induced by electrical stimulation. The recent development of optogenetics in neuroscience, however, now offers a more elegant strategy to induce critical state transitions16.
The methods described in this paper demonstrate how to generate ictal events on-demand in acute seizure models for both in vitro (step 1 of the Protocol) and in vivo studies (step 2 of the Protocol). They involve the choice of brain region, seizure induction method, study type, and species; however, the focus will be on the recommended choice of an acute 4-AP cortical seizure model because of its versatility in a wide variety of study types. The acute in vitro 4-AP seizure model is based on the standard protocol to prepare high-quality brain slices for electrophysiological recordings and imaging studies19. These protocols have already been used to make in vitro coronal brain slices from the somatosensory-motor cortex of mice16,20 and humans21. Modifications to generate ictal events in these types of brain slices have been previously demonstrated16 and the full details are described in the Protocol below. The acute in vivo 4-AP cortical seizure model is based on the standard protocol to prepare a craniotomy for imaging studies22. The modification is that no (glass slide) window is installed following the craniotomy. Instead, proconvulsant agents (4-AP) are topically applied to the exposed cortex to induce ictal events while the animal is under general anesthesia. To our knowledge, our group was the first to develop this acute in vivo cortical seizure model in mice16,23. The acute in vivo 4-AP cortical seizure model prepared from adult mice was developed to complement the in vitro slice model from juvenile tissue. The replication of findings in the adult in vivo seizure model helps to generalize the findings from slice models by addressing the inherent concerns regarding the non-physiological conditions of a 2D brain slice (versus a 3-D whole-brain structure) and the physiological differences between juvenile and adult tissue.
The method of on-demand ictal event initiation is demonstrated using either puffs of neurotransmitters with a picospritzer or optogenetic strategies. To the best of our knowledge, our group is the first to initiate ictal events in human tissue using neurotransmitters via a picospritzer16. For optogenetic strategies, the C57BL/6 mice strain is the conventional strain used for expressing transgenes. The expression of channelrhodopsin-2 (ChR2) in either GABAergic interneurons or glutamatergic pyramidal cells will provide the optional ability to generate ictal events on-demand with brief light pulses. Suitable optogenetic mice strains include the commercially available C57BL/6 variant that expresses ChR2 in either interneurons, using the mouse vesicular GABA transporter promotor (VGAT)24, or pyramidal cells, using the mouse thymus cell antigen 1 promotor (Thy1)25. These commercially available VGAT-ChR2 and Thy1-ChR2 mice offer the opportunity to activate GABAergic neurons or glutamatergic neurons, respectively, in the neocortex with blue (470 nm) light. The ability to generate ictal events on demand in acute seizure models can offer novel opportunities to study seizure initiation dynamics and efficiently evaluate potential anti-seizure therapies.
Protocol
All research involving patients was performed under a protocol approved by the University Health Network Research Ethics Board in accordance with the Declaration of Helsinki. Procedures involving animals were in accordance with guidelines by the Canadian Council on Animal Care and approved by the Krembil Research Institute Animal Care Committee.
1. Protocol I: Acute In vitro Seizure Model
- Preparation of dissection solutions and artificial cerebrospinal fluid
- Carbogenate ultrapure water (which is water filtered with a resistivity of 18.2 MΩ·cm) with carbogen (95% O2/5% CO2) for 5 min using air stone bubble diffusers (aerators for aquariums).
Note: Air stones can be connected to the carbogen tank’s regulator via standard silicone tubing. If air stones are not available, seal shut the end of the silicone tubing and poke small holes in the seal to allow the carbogen gas to bubble out and carbogenate the solution. - Prepare a dissection solution by dissolving the solutes from Table 1 in ultrapure water. Add CaCl2 to a solution that has been carbogenated for at least 5 min to help it dissolve.
Note: Alternatively, add CaCl2 first, so that it can dissolve easily in an unsaturated solution. The solution, in liquid form, should be 300 – 320 mOsm/L and have a pH of 7.4 after being saturated with carbogen.- Chill the dissection solution to 0 – 4 °C. Leave the solution in the fridge overnight or place the solution in the freezer for 1 h and continually monitor the temperature.
Note: The dissection solution can be stored for up to 3 nights; discard afterward. - Carbogenate the dissection solution for 5 – 10 min before use.
Note: Ideally, the solution should either appear as slush or be transitioning to slush prior to use.
- Chill the dissection solution to 0 – 4 °C. Leave the solution in the fridge overnight or place the solution in the freezer for 1 h and continually monitor the temperature.
- Prepare rodent artificial cerebrospinal fluid (ACSF) by dissolving the solutes from Table 2 (or Table 3 for human ACSF) in ultrapure water. Carbogenate the ACSF solution while it is in a water bath heated to 35 °C until use. Add CaCl2 to a solution that has been carbogenated for at least 5 min to help it dissolve.
Note: Alternatively, add CaCl2 first so that it can dissolve easier in an unsaturated solution. The solution, in liquid form, should be 290 – 320 mOsm/L and have a pH of 7.4 after being saturated with carbogen. Solutions should be made in 1, 2, or 4 L volumes depending on the duration of the experiments. Make 4 L for a full day (8 h) of experiments. The ACSF should be made fresh each day; do not store it for longer than 1 d.
- Carbogenate ultrapure water (which is water filtered with a resistivity of 18.2 MΩ·cm) with carbogen (95% O2/5% CO2) for 5 min using air stone bubble diffusers (aerators for aquariums).
- Mouse dissection to collect brain tissue
Note: For human tissue, proceed to the next step (step 1.3) on making brain slices with the vibratome. The cube-shaped blocks (1 cm3) of brain tissue should be obtained from the neurosurgeon using procedures previously described26,27.- Collect all the tools and materials necessary for animal dissections. Set up the workspace to prepare for animal dissections.
- Check the carbogen tank (95%O2/5%CO2) to ensure it is not empty. Replace the gas cylinder prior to experiments if there is less than 500 psi of pressure remaining.
- Calibrate the vibratome (vibrating blade tissue slicer) according to the manufacturer’s instruction manual. Adjust the vibratome setting to have a cutting amplitude of 1 mm and a cutting speed of 0.12 mm/s.
Note: The optimal settings may differ for each individual vibrating tissue blade cutter; however, in general, the amplitude should be high and the cutting speed should be low. - Fill the brain slice incubation chamber (i.e., the brain slice keeper) with ACSF and bubble it with carbogen. Then place the incubation chamber in a water bath set at 35 °C.
Note: Use rodent ACSF for rodent brain slices and human ACSF for human brain slices.
- Obtain a juvenile mouse of either sex, between the age of 13 d (p13) to 21 d (p21, where p0 is the date of birth).
- Anesthetize the mouse with an intraperitoneal injection of sodium pentobarbital (55 mg/kg of body weight). Once the mouse is deeply anesthetized, as indicated by the absence of the toe pinch reflex, promptly use a vet-approved guillotine instrument to decapitate the mouse in one swift movement.
Note: Prepare the sodium pentobarbital injection according to the procedures recommended by institutional guidelines. - Hold the mouse’s nose to stabilize the decapitated head and gently make a midline incision starting between the mouse’s eyes and along the entire length of the scalp to expose the skull. Ensure the skull is not compressed by the pressure applied by the razor. Use the corner of the razor’s edge to increase cut precision.
- Spread apart the flaps of the mouse’s scalp using fingers to expose the skull. Then, use the fresh corner of a razor’s edge to make an incision along the midline of the skull; pay careful attention to avoid any contact with the cerebral cortex.
- Insert splinter forceps into the mouse’s eye sockets to stabilize the decapitated head and place it in a Petri dish filled with cold (0 – 4 °C) dissection solution. Ensure that the head is fully submerged in the dissection solution.
- Use a second set of splinter forceps to gently peel away the mouse’s scalp, remove the nose bone by peeling it, and remove the back (caudal side) of the skull.
Note: The nose bone of juvenile mice is very soft and easily broken. - Use a micro spatula to cut the optic nerves, trigeminal nerves, and spinal cord. Then, use the micro spatula to gently separate the brain from the cranium. Leave the brain fully submerged in the Petri dish filled with dissection solution.
- Collect all the tools and materials necessary for animal dissections. Set up the workspace to prepare for animal dissections.
- Preparation of cortical slices from the somatosensory-motor cortex
- Fill the vibratome’s buffer tray with ~150 mL of cold (0 – 4°C) dissection solution.
Note: Optionally, keep the buffer tray in the freezer until needed to maintain a low temperature during the slicing procedure. - Glue the brain tissue from mouse or human onto the vibratome stage (specimen holder/tray) using instant adhesive glue. For mice, cut off a small caudal portion of the brain (i.e., the cerebellum) so that it can be easily glued flat onto the specimen holder (allowing the rostral side of the brain to face the ceiling).
Note: Alternatively, horizontal mouse brain slices can be prepared by gluing the dorsal side of the brain onto the specimen holder (the ventral side of the brain should face the ceiling)28. - Gently place the specimen holder (with brain tissue) into the buffer tray. Ensure that the dorsal portion of the brain is facing the vibratome’s blade.
Note: It is critical to minimize the amount of time the brain is exposed to air.
- Fill the vibratome’s buffer tray with ~150 mL of cold (0 – 4°C) dissection solution.
- Brain tissue sectioning and collection
- Slice the brain into 450 μm thick slices using the vibratome in the dorsal to ventral direction. Ensure that the brain remains completely anchored to the specimen tray while slicing it.
- Make the first cut in the mouse brain to remove the olfactory bulb. Then, make subsequent cuts until the somatosensory-motor area is observed (located approximately halfway between the olfactory bulb and bregma).
Note: Optionally, slice the brain in thinner (200 μm) slices until the corpus callosum appears in the coronal view of the brain, which indicates that the somatosensory-motor area is close.
- Make the first cut in the mouse brain to remove the olfactory bulb. Then, make subsequent cuts until the somatosensory-motor area is observed (located approximately halfway between the olfactory bulb and bregma).
- Use a wide-bore transfer pipette to collect coronal slices (450 μm) that contain the somatosensory-motor area and submerge them in a Petri dish containing cold (0 – 4 °C) dissection solution.
- Use a new razor blade and cut off any excess tissue from the slices. For coronal slices from mice, perform a transverse cut just below the neocortical commissure (i.e., corpus callosum). Do not cut in a sawing motion; simply apply pressure on the blade into the tissue and use a detailing brush to gently separate the tissue. Make sure to minimize the movement of the coronal slice.
- Use a wide-bore transfer pipette to transfer the dorsal portion of the coronal slices that contain the neocortex (layer 1 – 6) to a second Petri dish filled with warm (35 °C) ACSF for a moment (~1 s). Then, promptly transfer the slices to an incubation chamber containing warm (35 °C) carbogenated ACSF.
Note: The purpose of the transfer into the Petri dish with ACSF is to minimize the transfer of dissection solution to the incubation chamber while transferring brain slices with the wide-bore pipette. Utilize the incubation chamber’s multiple wells to keep different brain slices organized. - Discard the rest of the brain and animal carcass according to the institutional guidelines.
- Slice the brain into 450 μm thick slices using the vibratome in the dorsal to ventral direction. Ensure that the brain remains completely anchored to the specimen tray while slicing it.
- Incubation and maintenance
- Leave the brain slices slightly submerged in the incubation chamber at 35 °C for 30 min. Then, remove the incubation chamber from the water bath and allow it to return to room temperature (20 – 25 °C). Wait 1 h for the brain slices to recover before performing electrophysiological recordings.
Note: Ensure there are no air bubbles that collect underneath the brain slices in the incubation chamber. Air bubbles are air interfaces that cause tissue damage. The mouse brain slices can be maintained for 6 – 8 h, while human brain tissue can be stored for up to 24 h in a well-carbogenated incubation chamber with the appropriate ACSF.
- Leave the brain slices slightly submerged in the incubation chamber at 35 °C for 30 min. Then, remove the incubation chamber from the water bath and allow it to return to room temperature (20 – 25 °C). Wait 1 h for the brain slices to recover before performing electrophysiological recordings.
- Electrophysiological recordings of the superficial cortical layer
Note: Ictal events are observed in the extracellular local field potential (LFP) recordings from brain slices. The LFP of the brain slice can be observed and recorded with either an interface type chamber29 or a submerged multi-electrode array (MEA) system16. The procedure has been previously described16 and additional details are described below.- Use a wide-bore transfer pipette or a detailing brush to move a brain slice onto slightly larger pre-cut lens paper that is held in place using a dental tweezer. Transfer the lens paper (that the brain slice is resting upon) to the recording chamber and secure it in position with a harp screen.
- Run warm (35 °C), carbogenated ACSF perfusate through the recording chamber over the brain slice at a rate of 3 mL/min (~1 drip/s). Use a digital thermometer to ensure the recording chamber is 33 – 36°C.
- Pull glass electrodes with an impedance of 1 – 3 MΩ from borosilicate glass tubing (with an outer diameter of 1.5 mm) using a puller. Backfill the glass electrodes with ACSF (~10 µL) using a Hamilton syringe. Immediately discard the electrode if the tip is damaged.
Note: Bleach the silver wire (5 min) and only allow a minimal portion (i.e., the tip) of the silver wire to be submerged in the ACSF back-filled glass electrodes to minimize noise and drift during the recordings. Remove any excess ACSF from the glass electrode with a Hamilton syringe if necessary. - Use a 20X stereo microscope to accurately guide the recording glass electrode into the superficial cortical layer (2/3) using manual manipulators. Record/view the electrical activity of the brain slice on a computer with standard software.
Note: Viable (high-quality) brain slices will exhibit a robust evoked potential in response to the applied electrical stimuli (100 µs, 30 – 300 μA) or light pulses (30 ms, 10 mW/mm2) for optogenetic tissue.
- Induction of seizure-like activities
- Perfuse ACSF containing 100 µM 4-aminopyrimidine (4-AP) over the brain slice. Dissolve 80 mg of 4-AP in 8.5 mL of water to make a stock solution of 100 mM 4-AP. Add 100 µL of 100 mM 4-AP stock solution to 100 mL of ACSF to achieve an ACSF perfusate with 100 µM 4-AP.
Note: Alternatively, use Zero-Mg2+ ACSF (Table 4), a modified ACSF solution containing no added Mg2+, to perfuse the brain slice. For human tissue, use a combination of 100 µM 4-AP in Zero-Mg2+ Human ACSF (Table 5) to achieve optimal results. The average time for ictal events to appear is 15 min; however, it may take up to 40 min for some brain slices.
- Perfuse ACSF containing 100 µM 4-aminopyrimidine (4-AP) over the brain slice. Dissolve 80 mg of 4-AP in 8.5 mL of water to make a stock solution of 100 mM 4-AP. Add 100 µL of 100 mM 4-AP stock solution to 100 mL of ACSF to achieve an ACSF perfusate with 100 µM 4-AP.
- On-demand seizure generation: an optogenetic strategy for optogenetic mice
- Apply a brief (30 ms) pulse of blue (470 nm) light (with a minimum 1 mW/mm2 output intensity) to initiate an ictal event. Use a manual manipulator to position a 1,000 µm core diameter optical fiber (0.39 NA) directly above the recording region.
Note: Set the rate of the photo-stimulation to match the desired rate of the ictal event occurrence (i.e., 1 pulse every 50 s). However, the rate cannot be overly exaggerated from the intrinsic rate at which ictal events occur.
- Apply a brief (30 ms) pulse of blue (470 nm) light (with a minimum 1 mW/mm2 output intensity) to initiate an ictal event. Use a manual manipulator to position a 1,000 µm core diameter optical fiber (0.39 NA) directly above the recording region.
- On-demand seizure generation: a non-optogenetic strategy for human tissue
- Position the brain slice so that the superficial layers are upstream, and the deep layers are downstream to the flow of the perfusate through the recording chamber.
- Pull a glass electrode (the same type as used for the LFP recordings) and gently touch its tip with a delicate task wiper to create a small opening. Backfill the electrode with ~25 µL of 100 mM GABA (dissolved in water) and attach it to the picospritzer. Alternatively, backfill the electrode with 200 µM glutamate (dissolved in water).
Note: To estimate the volume being puffed from the picospritzer (~50 µL), apply a test puff onto a plastic plate (preferably gridded). Then, apply a droplet of known volumes (i.e., 10 µL, 20 µL, 50 µL, or 100 µL) with a pipette for a comparison with the test puff. - Apply puffs of the neurotransmitter (GABA or glutamate) to the human tissue with a picospritzer (10 – 20 psi for 30 – 100 ms) to initiate an ictal event. Apply a single puff of 100 mM GABA onto the deep layer (5) of the brain tissue to generate ictal events in the superficial layer (2/3). Alternatively, apply a single puff of 200 µM glutamate directly onto the superficial layer to generate ictal events in the superficial layer.
Note: Set the rate of the picospritzer puff to match the desired rate of the ictal event occurrence (i.e., 1 pulse every 50 s). However, the rate cannot be overly exaggerated from the intrinsic rate at which ictal events occur.
2. Protocol II: Acute In vivo Seizure Model
- Surgical preparation and surgery
- Obtain an adult mouse (p35 – p60) of either sex.
- Deeply anesthetize mouse with Ketamine (95 mg/kg) and Xylazine (5 mg/kg). Perform the toe-pinch reflex test to ensure the mouse is sedated. Alternatively, use isoflurane (4% for induction, 1.5 – 2% for surgery) to anesthetize the mouse.
Note: The choice of anesthetics can impact the seizure activity30,31. - Mount the mouse into a stereotaxic frame by securing its head with ear bars. Place a heated pad underneath the mouse for the duration of the surgery.
- Shave the top of the mouse’s head using a rodent trimmer to expose the scalp. Inject 0.3 mL of lidocaine with a 25 G 5/8 in needle into the periosteum to avoid excessive bleeding or pain. Apply eye ointment to the mouse’s eyes to prevent them from drying out during the experiment.
- Pinch and lift the center of the mouse’s scalp to assess if its reflexes are gone. Subsequently, perform a horizontal cut to expose the bregma to lambda region of the skull. Gently scrape the entire exposed area of the skull with a cotton swab to create a dry surface.
- Perform a craniotomy of 4 mm in diameter at coordinates 2.0 mm lateromedial and -2.0 mm rostrocaudal to expose the somatosensory cortex. Mark the location with a circle (4 mm in diameter) using a permanent marker. Drill around the circle with a pneumatic dental drill until the remaining layer of the craniotomy bone easily gives way when pushed down. Apply saline solution onto the layer of bone before removing the craniotomy with splinter forceps. Subsequently, apply warm saline solution to the exposed region.
- Recording methods and chemical induction of seizures
- Pull glass electrodes with an impedance of 1 – 3 MΩ from borosilicate glass tubing (with an outer diameter of 1.5 mm) using a puller. Backfill the glass electrodes with ~10 µL of ACSF or saline using a Hamilton syringe. Immediately discard the electrode if the tip is damaged.
Note: Bleach the silver wire (5 min) and only allow a minimal portion (i.e., the tip) of the silver wire to be submerged in the ACSF back-filled glass electrodes to minimize noise and drift during the recordings. Remove any excess ACSF from the glass electrode with a Hamilton syringe if necessary. - Use a manual manipulator to guide the glass electrode into the superficial cortical layer (2/3) in the somatosensory-motor area. Record/view the electrical activity of the brain slice on a computer with standard software.
Note: Layer 2/3 is approximately 0.3 mm deep from the surface32. - Topically apply ~0.5 mL 1.5 mM 4-AP saline onto the exposed cortex of the mouse with a syringe until it completely covers the craniotomy. Dissolve 14 mg of 4-AP in 100 mL of isotonic saline to make a stock solution of 1.5 mM 4-AP saline.
Note: Prepare the 4-AP saline before the experiment begins. On average, ictal events appear 15 – 30 min after topical application.
- Pull glass electrodes with an impedance of 1 – 3 MΩ from borosilicate glass tubing (with an outer diameter of 1.5 mm) using a puller. Backfill the glass electrodes with ~10 µL of ACSF or saline using a Hamilton syringe. Immediately discard the electrode if the tip is damaged.
- On-demand generation of seizures in optogenetic mice
- Apply a brief (30 ms) pulse of blue (470 nm) light (with a minimum 10 mW/mm2 output intensity) to initiate an ictal event. Use a manual manipulator to position a 1,000 µm core diameter optical fiber (0.39 NA) directly above the recording region.
Note: Set the rate of the photo-stimulation to match the desired rate of the ictal event occurrence (i.e., 1 pulse every 300 s). However, the rate cannot be overly exaggerated from the intrinsic rate at which ictal events occur.
- Apply a brief (30 ms) pulse of blue (470 nm) light (with a minimum 10 mW/mm2 output intensity) to initiate an ictal event. Use a manual manipulator to position a 1,000 µm core diameter optical fiber (0.39 NA) directly above the recording region.
Representative Results
The application of 100 µM 4-AP to good-quality (undamaged) 450 µm-sized cortical brain slices from a juvenile VGAT-ChR2 mouse reliably induced recurrent ictal events (> 5 s) within 15 min (Figure 1Ai). The application of 100 µM 4-AP to slices of poor-quality resulted in bursting events or spiking activity (Figure 1Aii). On average, 40% of the slices from each dissected mouse brain successfully generated ictal events. Moreover, 83% (25/30) of the dissected mice resulted in at least one brain slice that successfully generated ictal events. In brain slices with spontaneously occurring ictal events, the application of a brief 30 ms light pulse on the brain slice reliably triggered an ictal event that was identical in morphology (Figure 1Aiii and 1Aiv). The same findings were made in brain slices from Thy1-ChR2 mice (Figure 1B). Thus, regardless of which neuronal-subpopulation was activated, any brief synchronizing event in the isolated cortical neural network led to the onset of an ictal event. These ictal events were comprised of a sentinel (preictal) spike (Figure 2Aii and 2Bii), tonic-like firing (Figure 2Aiii and 2Biii), clonic-like firing (Figure 2Aiv and 2Biv), and bursting activity toward the end (Figure 2Av and 2Bv); they were similar in nature to the electrographic signatures associated with clinical seizures33. Moreover, these juvenile mice were physiologically adult-like as the addition of 10 µM bumetanide (BUM), an NKCC1 blocker, had no effect on the resulting ictal events (Figure 2).
The in vitro 4-AP cortical slice model reliably generated consistent ictal events for ~1 h (Figure 1Ai), whereas the Zero-Mg2+ model typically generated ictal events for ~10 min before rapidly transforming into burst-like activity (Figure 3A). However, if a non-4-AP method of seizure induction is required, the Zero-Mg2+ model can be modified with the addition of 5 – 10 µM baclofen (a GABAB receptors agonist) to transform the bursting activity back into ictal events (Figure 3B). In general, methods of non-disinhibition to increase excitability (i.e., 4-AP or Zero-Mg2+ ACSF) reliably reproduced ictal events in cortical brain slices. In contrast, methods of disinhibition [i.e., bicuculline (BMI), a GABAA receptor antagonist] resulted in spiking activity reminiscent of interictal activity or bursting activity, rather than ictal events (Figure 4A). Similarly, acute seizure models prepared from 100 µM 4-AP-treated hippocampal slices generated interictal-like spiking activity or status epilepticus-like conditions in the CA3 (Figure 4B). The in vivo 4-AP cortical model correspondingly generated recurrent ictal events (> 5 s). Ictal events were observed in the superficial layer (2/3) within ~30 min of topically applying 1.5 mM 4-AP onto the exposed cortex of adult VGAT-ChR2 mice. The application of a brief 30 ms light pulse onto the exposed cortex reliably triggered ictal events that were morphologically similar to those spontaneously occurring (Figure 5).
The application of Zero-Mg2+ human ACSF with 100 µM 4-AP to 'non-epileptic' cortical brain slices (450 µm) from temporal lobe epilepsy patients reliably generated recurrent ictal events (> 5 s) within ~30 min (Figure 6Ai and 6Bi). Slices of poor quality generated either spiking activity or no activity (Figure 1Aii). The viability of brain slices was deemed 'good quality' when a brief electrical stimulus (100 µs, 30 – 300 µA) induced a robust, evoked response in the LFP at the beginning of the experiment. Once ictal events began to precipitate, the application of a brief puff (75 ms at 20 psi) of 100 mM GABA onto the brain slice reliably triggered ictal events that were identical in morphology to those occurring spontaneously (Figure 6Aii and 6Aiii). A lower concentration of GABA, 100 – 200 µM, will likely be effective as well for in vitro experiments34; however, a higher concentration of GABA, 100 mM, is recommended for in vivo experiments35. The same observations were reproduced when a brief puff of 200 µM glutamate was applied to human brain slices (Figure 6Bii and 6Biii). Thus, regardless of which post-synaptic receptors were activated, a brief synchronizing event in the isolated human cortical neural network reliably triggered an ictal event.
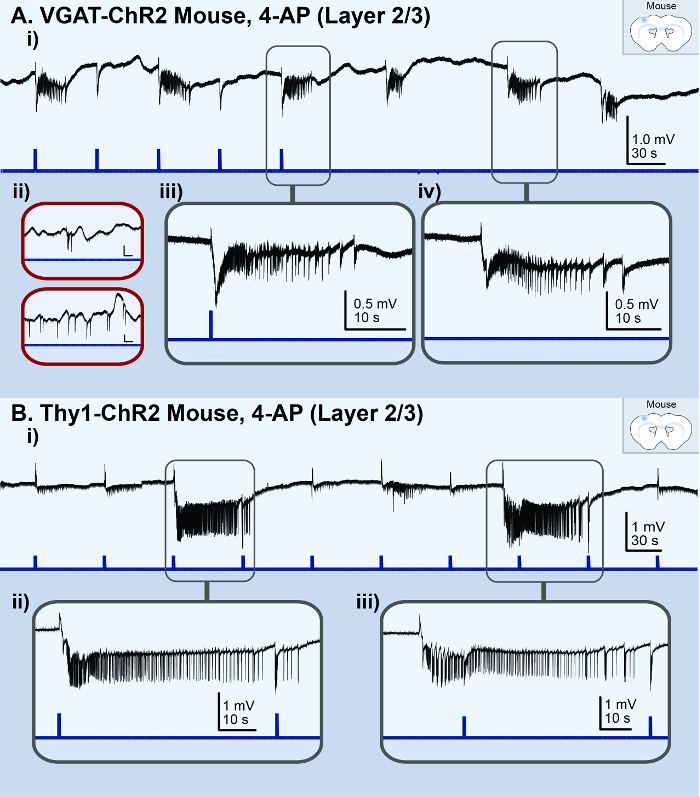
Figure 1: Acute in vitro 4-AP cortical seizure model. The black lines represent the local field potential (LFP) recording; the blue lines represent the light stimulus. (A) These panels are based on the results from a VGAT-ChR2 mouse model. They illustrate ictal events observed in the LFP recording from the superficial layer (2/3) of a high-quality cortical brain slice treated with 100 µM 4-AP. i) This panel shows an overview of the LFP recording. ii) These are examples of the LFP recording from poor-quality brain slices. The vertical scale bar is 0.4 mV, the horizontal scale bar is 20 s. iii) This is a zoomed-in view of a light-triggered ictal event. iv) This is a zoomed-in view of a spontaneous ictal event. (B) These panel are based on the results from a Thy1-ChR2 mouse model. They illustrate ictal events observed in the LFP recording from the superficial layer (2/3) of a cortical brain slice treated with 100 µM 4-AP. i) This panel shows an overview of the LFP recording. ii) This is a zoomed-in view of a light-triggered ictal event. iii) This is a zoomed-in view of a spontaneous ictal event. Please click here to view a larger version of this figure.
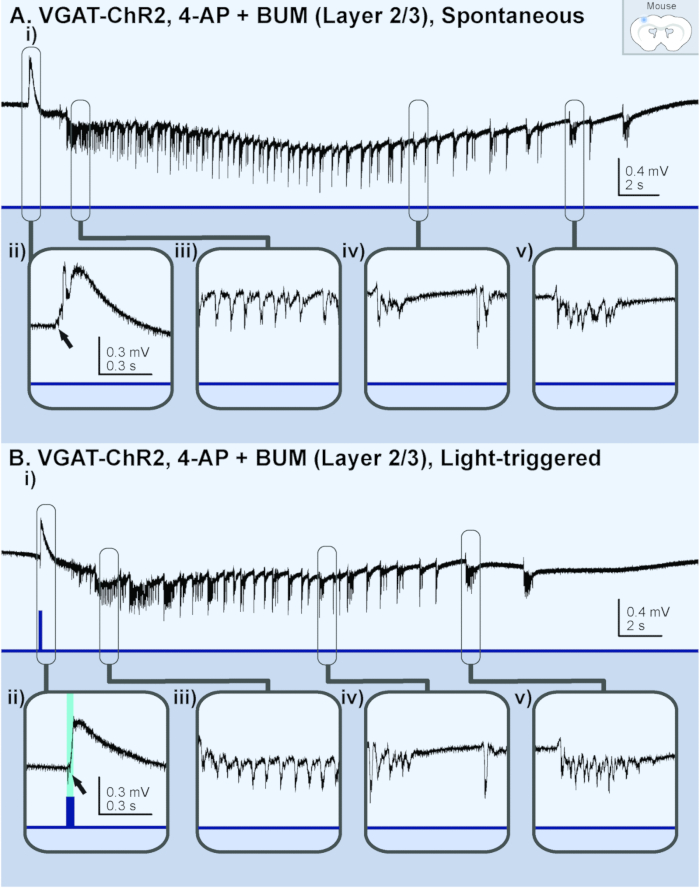
Figure 2: Ictal events generated in a brain slice (layer 2/3) from a juvenile (p13) VGAT-ChR2 mouse perfused with 4-AP and bumetanide (BUM). The black lines represent the local field potential (LFP) recording; the blue lines represent the light stimulus. (A) These panels show a spontaneous ictal event. i) This panel shows an overview of the entire ictal event. The following panels show ii) a sentinel spike, iii)tonic-like firing, iv) clonic-like firing, and v) bursting activity. (B) These panels show a light-triggered ictal event. i) This panel shows an overview of the entire ictal event. The following panels show ii) a light-triggered sentinel spike from the same slice recording, iii) tonic-like firing, iv) clonic-like firing, and v) bursting activity. Please click here to view a larger version of this figure.
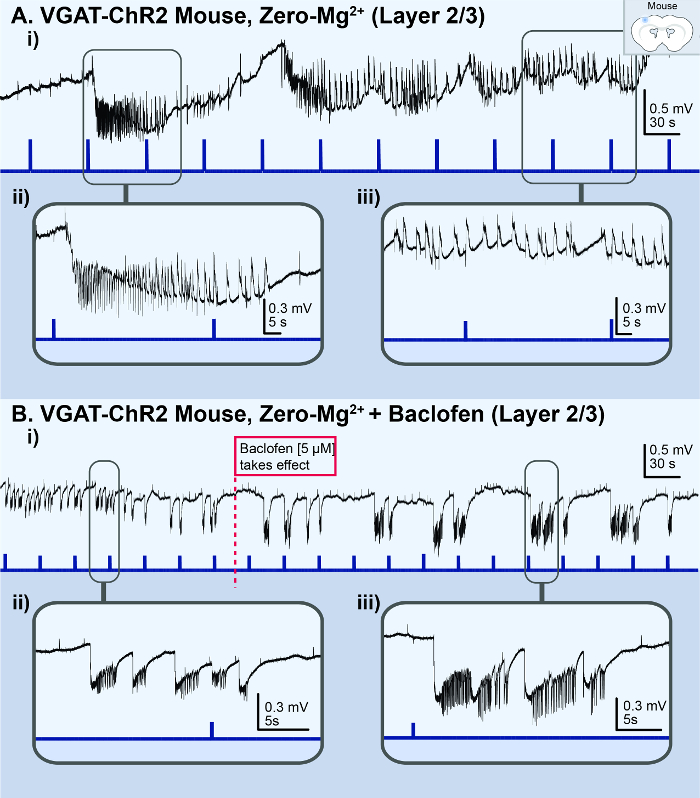
Figure 3: Acute in vitro Zero-Mg2+ cortical seizure model. The black lines represent the local field potential (LFP) recording; the blue lines represent the light stimulus. (A) These panels are based on the results from a VGAT-ChR2 mouse model. i) This panel illustrates the status epilepticus-like conditions observed in the LFP recording from the superficial layer (2/3) of a cortical brain slice treated with Zero-Mg2+ ACSF. ii) This is a zoomed-in view of a light-triggered ictal event. iii) This is a zoomed-in view of the status epilepticus-like bursting activity. (B) These panels show the same slice recording with the addition of baclofen. i) The application of 5 µM baclofen to the Zero-Mg2+ ACSF transforms the bursting activity back into distinct, recurrent ictal events. ii) This is a zoomed-in view of the bursting activity. iii) This is a zoomed-in view of the distinct ictal event. Please click here to view a larger version of this figure.
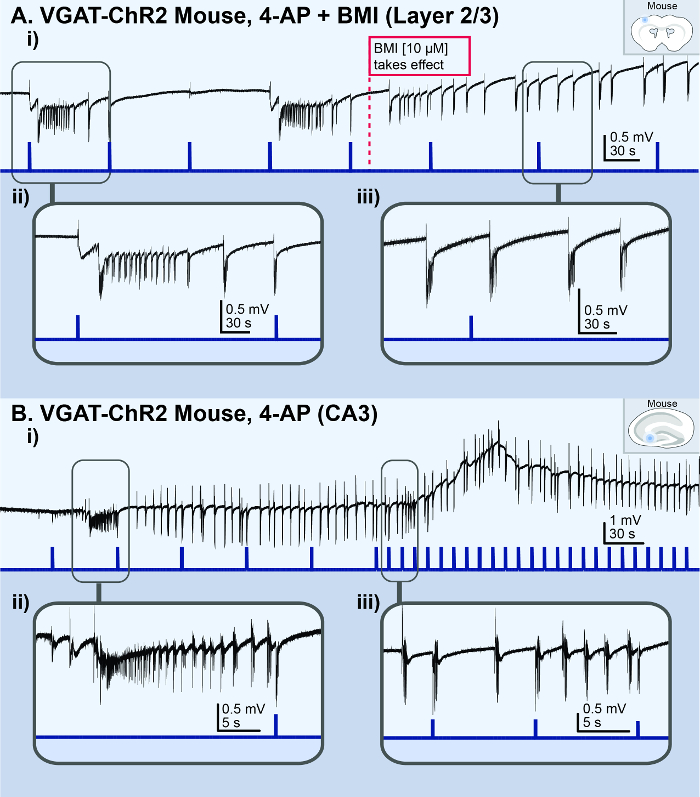
Figure 4: Acute in vitro bursting/spiking models. The black lines represent the local field potential (LFP) recording; the blue lines represent the light stimulus. (A) These panels show the results from a cortical slice from a VGAT-ChR2mouse, illustrating the bursting activity observed in the superficial layer (2/3) following the addition of 10 µM BMI to 100 µM 4AP. i) This is an overview of the LFP recording. The dotted red line indicates when the BMI took effect. ii) This is a zoomed-in view of a light-triggered ictal event. iii) This is a zoomed-in view of the spontaneous bursting activity. (B) These panels show the results from a hippocampal slice from a VGAT-ChR2 mouse, illustrating a status epilepticus-like event observed in the CA3 area following the application of 100 µM 4AP. i) This is an overview of the LFP recording. ii) This is a zoomed-in view of an ictal event. iii) This is a zoomed-in view of light-triggered and spontaneous bursting events. Please click here to view a larger version of this figure.
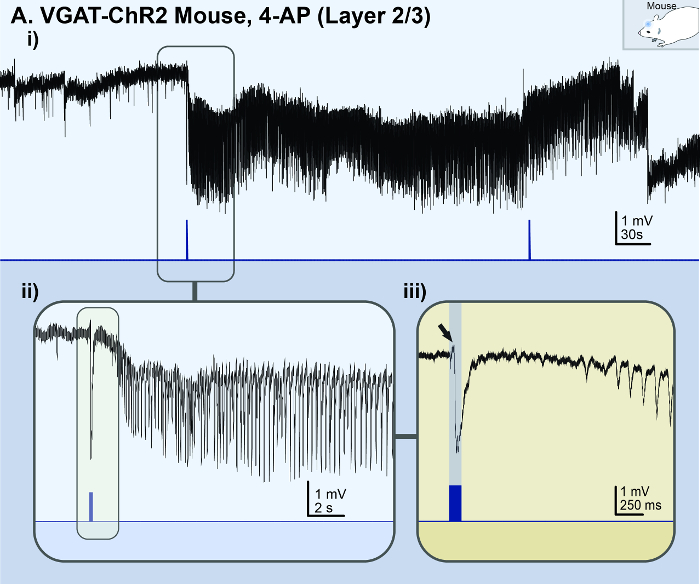
Figure 5: Acute in vivo 4-AP cortical seizure model. The black lines represent the local field potential (LFP) recording; the blue lines represent the light stimulus. (A) These panels show the results of an adult (p56) VGAT-ChR2 mouse model with 1.5 mM 4-AP topically applied to the exposed cortex. i) This panel illustrates a light-triggered ictal event observed in the superficial layer (2/3) of the somatosensory-motor area. ii) This is a zoomed-in view of the light-triggered ictal event from panel Ai. iii) This is a super zoomed-in view of the onset of the light-triggered ictal event (indicated by the black arrow). This figure is the unfiltered version of a figure from Chang et al.16. Please click here to view a larger version of this figure.
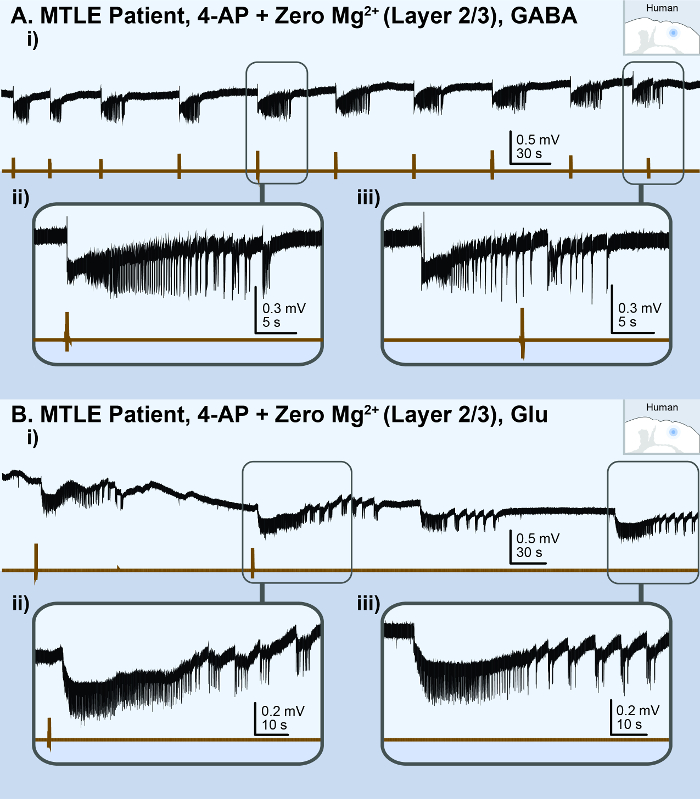
Figure 6: Acute in vitro human cortical seizure model. The black lines represent the local field potential (LFP) recording; the brown lines represent the picospritzer puff. (A) These panels show the results of a cortical brain slice from a medial temporal lobe epilepsy (MTLE) patient, illustrating the ictal events observed in the superficial layer (2/3) following a perfusion with 100 µM 4-AP and Zero-Mg2+ human ACSF. i) This is an overview of the LFP recording. The following panels show ii) a zoomed-in view of a 100 mM GABA puff-triggered ictal event and iii) a zoomed-in view of a spontaneous ictal event. (B) These panels show the results of a cortical brain slice from another MTLE patient, illustrating the ictal events observed in the superficial layer (2/3) following a perfusion with 100 µM 4-AP and Zero-Mg2+ human ACSF. i) This is an overview of the LFP recording. The following panels show ii) a zoomed-in view of a 200 µM glutamate puff-triggered ictal event and iii) a zoomed-in view of a spontaneous ictal event. Please click here to view a larger version of this figure.
| # | Reagent | Conc. [mM] | MW (g/mol) | 1L (g) | 2L (g) |
| 1 | Sucrose | 248 | 342.3 | 84.89 | 169.78 |
| 2 | Sodium Bicarbonate (NaHCO2) | 26 | 84.01 | 2.18 | 4.37 |
| 3 | Dextrose (D-glucose) | 10 | 180.16 | 1.8 | 3.6 |
| 4 | Potassium Chloride (KCl) | 2 | 74.55 | 0.15 | 0.3 |
| 5 | Magnesium Sulfate (MgSO4·7H2O) | 3 | 246.47 | 0.74 | 1.48 |
| 6 | Sodium phosphate monobasic monohydrate (H2NaPO4·H2O) | 1.25 | 137.99 | 0.17 | 0.34 |
| 7 | Calcium Chloride (CaCl2·2H2O) | 1 | 147.01 | 0.15 | 0.29 |
Table 1: Recipe for dissection solution. These are instructions to make 1 L or 2 L volumes. MW = the molecular weight of the solute.
| # | Reagent | Conc. [mM] | MW (g/mol) | 2L (g) | 4L (g) |
| 1 | Sodium Chloride (NaCl) | 123 | 58.4 | 14.37 | 28.73 |
| 2 | Sodium Bicarbonate (NaHCO2) | 26 | 84.01 | 4.37 | 8.74 |
| 3 | Dextrose (D-glucose) | 10 | 180.16 | 3.6 | 7.21 |
| 4 | Potassium Chloride (KCl) | 4 | 74.55 | 0.6 | 1.19 |
| 5 | Magnesium Sulfate (MgSO4·H2O) | 1.3 | 246.47 | 0.64 | 1.28 |
| 6 | Sodium phosphate monobasic monohydrate (HNaPO4·H2O) | 1.2 | 137.99 | 0.33 | 0.66 |
| 7 | Calcium Chloride (CaCl2·2H2O) | 1.5 | 147.01 | 0.44 | 0.88 |
Table 2: Recipe for rodent artificial cerebral spinal fluid (ACSF). These are instructions to make 2 L or 4 L volumes. MW = the molecular weight of the solute.
| # | Reagent | Conc. [mM] | MW (g/mol) | 2L (g) | 4L (g) |
| 1 | Sodium Chloride (NaCl) | 123 | 58.4 | 14.38 | 28.75 |
| 2 | Sodium Bicarbonate (NaHCO2) | 25.2 | 84.01 | 4.23 | 8.46 |
| 3 | Dextrose (D-glucose) | 10 | 180.16 | 3.6 | 7.21 |
| 4 | Potassium Chloride (KCl) | 4 | 74.55 | 0.6 | 1.19 |
| 5 | Magnesium Sulfate (MgSO4·H2O) | 1 | 246.47 | 0.49 | 0.99 |
| 6 | Sodium phosphate monobasic monohydrate (HNaPO4·H2O) | 1.2 | 137.99 | 0.33 | 0.66 |
| 7 | Calcium Chloride (CaCl2·2H2O) | 1 | 147.01 | 0.29 | 0.59 |
Table 3: Recipe for human artificial cerebral spinal fluid (human ACSF). These are instructions to make 2 L or 4 L volumes. MW = the molecular weight of the solute.
| # | Reagent | Conc. [mM] | MW (g/mol) | 2L (g) | 4L (g) |
| 1 | Sodium Chloride (NaCl) | 123 | 58.4 | 14.37 | 28.73 |
| 2 | Sodium Bicarbonate (NaHCO2) | 26 | 84.01 | 4.37 | 8.74 |
| 3 | Dextrose (D-glucose) | 10 | 180.16 | 3.6 | 7.21 |
| 4 | Potassium Chloride (KCl) | 4 | 74.55 | 0.6 | 1.19 |
| 5 | Magnesium Sulfate (MgSO4·H2O) | Nominally Free | 246.47 | 0 | 0 |
| 6 | Sodium phosphate monobasic monohydrate (HNaPO4·H2O) | 1.2 | 137.99 | 0.33 | 0.66 |
| 7 | Calcium Chloride (CaCl2·2H2O) | 1.5 | 147.01 | 0.29 | 0.59 |
Table 4: Recipe for Zero-Mg2+ rodent artificial cerebral spinal fluid (Zero-Mg2+ ACSF). These are instructions to make 2 L or 4 L volumes. MW = the molecular weight of the solute.
| # | Reagent | Conc. [mM] | MW (g/mol) | 2L (g) | 4L (g) |
| 1 | Sodium Chloride (NaCl) | 123 | 58.4 | 14.38 | 28.75 |
| 2 | Sodium Bicarbonate (NaHCO2) | 25.2 | 84.01 | 4.23 | 8.46 |
| 3 | Dextrose (D-glucose) | 10 | 180.16 | 3.6 | 7.21 |
| 4 | Potassium Chloride (KCl) | 4 | 74.55 | 0.6 | 1.19 |
| 5 | Magnesium Sulfate (MgSO4·H2O) | Nominally Free | 246.47 | 0 | 0 |
| 6 | Sodium phosphate monobasic monohydrate (HNaPO4·H2O) | 1.2 | 137.99 | 0.33 | 0.66 |
| 7 | Calcium Chloride (CaCl2·2H2O) | 1 | 147.01 | 0.29 | 0.59 |
Table 5: Recipe for Zero-Mg2+ human artificial cerebral spinal fluid (Zero-Mg2+ human ACSF). These are instructions to make 2 L or 4 L volumes; MW = the molecular weight of the solute.
Discussion
The brain slices are treated with a proconvulsant drug or an altered ACSF perfusate to increase the neural network's excitability and promote a precipitation of ictal events (electrographic seizure-like events). For mice, the preferred coronal slices of the somatosensory-motor area should contain the cingulate cortex, area 2 (CG), but not the retrosplenial area (RS); these anatomical markers help identify the range of coronal slices that are best for inducing ictal events. An optional modification for mice tissue is to cut the two hemispheres of the brain slice in half for matched-pair experimental designs, as the two hemispheres are virtually identical (similar experimental units). When preparing brain slices to generate ictal events, it is imperative to maintain the integrity of the neural network and its synaptic connections, because ictal events are a neural network phenomenon. Three points in step 1 of the Protocol that are critical for slice quality are 1) the slicing procedure, 2) incubation, and 3) oxygenation. Firstly, the slicing procedure requires a balance between speed and technique. It is crucial to minimize the time between the decapitation (or surgical resection) and incubation, while also being careful with every contact and movement of the brain slice to avoid damage. Secondly, the quality of the tissue is very sensitive to the incubation temperature and duration. It is important to use a timer and thermometer to ensure the incubation is at 35 °C for 30 min. Thirdly, the viability of the brain tissue is sensitive to exposure to anything other than oxygenated (artificial) cerebral spinal fluid. The brain slice will expire if it is not perfused with carbogenated ACSF for an extended period (~1 min).
Brain slices from mice aged p13 – p16 offer the highest probability of successfully generating ictal events. The reason is that mice ≤ p16 do not require a transcardial perfusion prior to dissections. This effectively reduces the chance for errors and speeds up the dissection process, which is a huge benefit, because the amount of time between the decapitation and incubation is inversely correlated with the brain slice's viability. Meanwhile, mice > p13 have reduced amounts of NKCC1 that are comparable to an adult36. In general, juvenile (< p21) tissue is more viable than adult tissue due to an exceptional ability to recover from the damaging slicing procedure. This week-long window between p13 and p21 offers the opportunity to exploit the mice's adult-like physiology and juvenile-like ability to easily generate ictal events37,38. However, if experiments require studying ictal events in brain slices from adult mice, an NMDG-based ACSF with HEPES, thiourea, and ascorbate will help promote the viability of adult tissue24,39,40,41. For human tissue, the addition of antioxidants, such as α-tocopherol, to the dissection solution can benefit tissue viability, especially during long-distance transportation (> 30 min) between the operating room and the laboratory for slicing8,26. For all brain slices, the favorable conditions to generate ictal events are to record from 450 µm thick slices at 36 °C. Brain slices need to be at least 350 µm thick to contain enough neurons in the neural network to generate the structured ictal event. However, slices cannot be thicker than 500 µm, as that will make it difficult for oxygen to diffuse into the center of the tissue. Slices that are 450 µm represent an optimal thickness, where an ample amount of neuronal network connectivity is maintained without impeding the perfusion of oxygen throughout the tissue. Lastly, the precipitation of ictal events is optimal at 33 – 36 °C; if the recording chamber is not at least 33 °C, it will be difficult for ictal events to occur.
A limitation of the acute seizure model is that they do not generate seizures. They only generate ictal events, which are the electrographic signature of a seizure. Ictal events have no associated behavioral components, such as the loss of consciousness or motor convulsions that define a seizure. Consequently, acute seizure models cannot be used to confirm the effectiveness of potential anti-seizure drug candidates or gain insights into epileptogenesis; such research questions should be addressed by chronic epilepsy models and clinical trials. Acute seizure models should be used only for their intended purpose of performing fundamental preliminary studies on seizure mechanisms. Only the most promising findings from acute seizure models should be advanced on to higher models that are more expensive, laborious to prepare, and require much more complex ethical considerations.
To make progress in epilepsy and seizure research, it is imperative to have a reliable acute seizure model that can accurately replicate the electrographic seizure activity observed clinically in the EEG of seizure patients. To reliably reproduce ictal events in brain slices, non-disinhibition methods of increasing excitability are required, whereas methods of disinhibition (i.e., the GABAA receptor antagonist BMI) typically result in spiking activity reminiscent of the interictal activity, rather than ictal events (Figure 3A). The preferred method of non-disinhibition is to apply the proconvulsant agent 4-AP because it can reliably generate consistent ictal events for 1 h. In contrast, the Zero-Mg2+ cortical model generates ictal events for only ~10 min before rapidly transforming into burst-like activity (Figure 2A). If using the Zero-Mg2+ model, the addition of 5 – 10 µM baclofen, a GABAB receptor agonist, will help to transform the bursting activity back into ictal events (Figure 2B), as previously shown in hippocampal slices42. Moreover, the in vitro 4-AP seizure model is preferred, because findings from that model can be replicated in its in vivo counterpart, the in vivo 4-AP cortical seizure model (Figure 4). It is not feasible to modify the entire cerebral spinal fluid of a live adult mouse to recreate the acute in vivo Zero-Mg2+ environment.
The application of 4-AP in cortical brain slices can accurately reproduce the seizure activity observed clinically15,33. In contrast, 4-AP-treated hippocampal slices are predisposed to generating interictal-like spiking activity43 and status epilepticus-like conditions (Figure 3B). Thus, for the purposes of studying seizures, the acute in vitro 4-AP cortical model is preferred over the in vitro 4-AP hippocampal model. Furthermore, there are virtually no opportunities to record from viable human hippocampal slices, as most hippocampal resections have the CA1 and CA3 damaged21. In contrast, non-pathological human cortical tissue is more readily accessible as that is the secondary outcome of subcortical neurosurgical procedures such as temporal lobe epilepsy surgery. Non-epileptic 'control' neocortical tissue can also be acquired from tumor resection surgeries. For these reasons, the cortex is the preferred site for modeling seizure activity because of its transportability between mouse and human tissue to confirm clinical relevance. Finally, the C57BL/6 strain of mice is preferred because they readily express transgenes, and optogenetic variants are commercially available. Optogenetic mice models allow for the on-demand initiation of ictal events via a minimally invasive, brief light stimulation. This makes the study of seizures incredibly efficient by eliminating wait times and allowing for the targeted activation of neuronal subpopulations. Furthermore, the ability to trigger seizures on-demand allows for new ways to definitively demarcate the exact point of seizure initiation and potentially study the effectiveness of anti-seizure drug candidates. A user-friendly MATLAB-based program was specifically developed to detect and classify the various types of epileptiform events that occur in the in vitro and in vivo 4-AP seizure models. This detection program is available for download from the Valiante Lab’s GitHub repository (https://github.com/Valiantelab/ChangValiante2018).
Declarações
The authors have nothing to disclose.
Acknowledgements
This work was supported by the Canadian Institutes of Health Research (MOP 119603 to Peter L. Carlen and Taufik A. Valiante), the Ontario Brain Institute (to Taufik A. Valiante), and the Mightex Student Research Grant (to Michael Chang). We would like to thank Liam Long for his assistance in filming the video manuscript. We would like to acknowledge Paria Baharikhoob, Abeeshan Selvabaskaran, and Shadini Dematagoda for their assistance in compiling the figures and tables in this manuscript. Figures 1A, 3A, 4A, and 6A are all original figures made from data published in Chang et al.16.
Materials
| Sodium pentobarbital | N/A | N/A | Purchased through the Toronto Western Hospital's Suppliers |
| 1 mm syringe | N/A | N/A | Purchased through UT Med Store |
| 25G 5/8” sterile needle | N/A | N/A | Purchased through UT Med Store |
| Single edge razor blade (2x) | N/A | N/A | Purchased through UT Med Store |
| Instant adhesive glue | N/A | N/A | Purchased through UT Med Store |
| Lens paper | N/A | N/A | Purchased through UT Med Store |
| Glass petri dish (2x) | N/A | N/A | Purchased through UT Med Store |
| Splinter forceps (2x) | N/A | N/A | Purchased through UT Med Store |
| PVC handle micro spatula | N/A | N/A | Purchased through UT Med Store |
| Micro spoon with flat end | N/A | N/A | Purchased through UT Med Store |
| Detailing brush 5/0 | N/A | N/A | Purcahsed from a boutique art store |
| Wide bore transfer pipette | N/A | N/A | Purchased through UT Med Store |
| Dental Tweezer | N/A | N/A | Purchased through UT Med Store |
| Thermometer (digital) | N/A | N/A | Purchased on Amazon.ca |
| Check carbogen tank (95%O2/5%CO2) | N/A | N/A | Purchased through the Toronto Western Hospital's Suppliers |
| Vibratome | Leica | N/A | Purchased through the Toronto Western Hospital's Suppliers |
| brain slice incubation chamber (a.k.a. brain slice keeper) | Scientific Systems Design Inc | N/A | |
| Sodium Chloride (NaCl) | N/A | N/A | Purchased through UT Med Store |
| Sodium Bicarbonate | N/A | N/A | Purchased through UT Med Store |
| Dextrose | N/A | N/A | Purchased through UT Med Store |
| Potassium Chloride (KCl) | N/A | N/A | Purchased through UT Med Store |
| Magnesium Sulfate (MgSO4 H2O) | N/A | N/A | Purchased through UT Med Store |
| Sodium phosphate monobasic monohydrate (HNaPO4·H2O) | N/A | N/A | Purchased through UT Med Store |
| Calcium Chloride (CaCl2·2H2O) | N/A | N/A | Purchased through UT Med Store |
| Sucrose | N/A | N/A | Purchased through UT Med Store |
Referências
- Jefferys, J. G. R. Advances in understanding basic mechanisms of epilepsy and seizures. Seizure. 19 (10), 638-646 (2010).
- Fujiwara, H., et al. Resection of ictal high-frequency oscillations leads to favorable surgical outcome in pediatric epilepsy. Epilepsia. 53 (9), 1607-1617 (2012).
- Chen, H. Y., Albertson, T. E., Olson, K. R. Treatment of drug-induced seizures. British Journal of Clinical Pharmacology. 81 (3), 412-419 (2015).
- Kwan, P., Brodie, M. J. Early Identification of Refractory Epilepsy. New England Journal of Medicine. 342 (5), 314-319 (2000).
- Giussani, G., et al. A population-based study of active and drug-resistant epilepsies in Northern Italy. Epilepsy & Behavior. 55, 30-37 (2016).
- Pellock, J. M. Overview: definitions and classifications of seizure emergencies. Journal of Child Neurology. 22 (5_suppl), 9S-13S (2007).
- Löscher, W. Critical review of current animal models of seizures and epilepsy used in the discovery and development of new antiepileptic drugs. Seizure. 20 (5), 359-368 (2011).
- Jones, R. S., da Silva, A. B., Whittaker, R. G., Woodhall, G. L., Cunningham, M. O. Human brain slices for epilepsy research: Pitfalls, solutions and future challenges. Journal of Neuroscience Methods. 260, 221-232 (2016).
- Castel-Branco, M., Alves, G., Figueiredo, I., Falcão, A., Caramona, M. The maximal electroshock seizure (MES) model in the preclinical assessment of potential new antiepileptic drugs. Methods and Findings in Experimental and Clinical Pharmacology. 31 (2), 101-106 (2009).
- Wendling, F., Bartolomei, F., Modolo, J., Pitkänen, A., Buckmaster, P., Galanopoulou, A. S., Moshé, S. Neocortical/Thalamic In Silico Models of Seizures and Epilepsy. Models of Seizures and Epilepsy. , 233-246 (2017).
- Cocchi, L., Gollo, L. L., Zalesky, A., Breakspear, M. Criticality in the brain: A synthesis of neurobiology, models and cognition. Progress in Neurobiology. 158, 132-152 (2017).
- Xue, M., Atallah, B. V., Scanziani, M. Equalizing excitation-inhibition ratios across visual cortical neurons. Nature. 511 (7511), 596-600 (2014).
- Engel, J. . Seizures and epilepsy. , (2013).
- Panuccio, G., Curia, G., Colosimo, A., Cruccu, G., Avoli, M. Epileptiform synchronization in the cingulate cortex. Epilepsia. 50 (3), 521-536 (2009).
- Avoli, M., de Curtis, M. GABAergic synchronization in the limbic system and its role in the generation of epileptiform activity. Progress in Neurobiology. 95 (2), 104-132 (2011).
- Chang, M., et al. Brief activation of GABAergic interneurons initiates the transition to ictal events through post-inhibitory rebound excitation. Neurobiology of Disease. 109, 102-116 (2018).
- Jiruska, P., et al. High-frequency network activity, global increase in neuronal activity, and synchrony expansion precede epileptic seizures in vitro. The Journal of Neuroscience. 30 (16), 5690-5701 (2010).
- Jirsa, V. K., Stacey, W. C., Quilichini, P. P., Ivanov, A. I., Bernard, C. On the nature of seizure dynamics. Brain. 137 (Pt 8), 2210-2230 (2014).
- Colbert, C. M. Preparation of cortical brain slices for electrophysiological recording. Ion Channels: Methods and Protocols. 337, 117-125 (2006).
- Li, H., Prince, D. A. Synaptic activity in chronically injured, epileptogenic sensory-motor neocortex. Journal of Neurophysiology. 88 (1), 2-12 (2002).
- Köhling, R., Avoli, M. Methodological approaches to exploring epileptic disorders in the human brain in vitro. Journal of Neuroscience Methods. 155 (1), 1-19 (2006).
- Mostany, R., Portera-Cailliau, C. A Craniotomy Surgery Procedure for Chronic Brain Imaging. Journal of Visualized Experiments. (12), e680 (2008).
- Ritter, L. M., et al. WONOEP appraisal: optogenetic tools to suppress seizures and explore the mechanisms of epileptogenesis. Epilepsia. 55 (11), 1693-1702 (2014).
- Zhao, S., et al. Cell type-specific channelrhodopsin-2 transgenic mice for optogenetic dissection of neural circuitry function. Nature Methods. 8 (9), 745-752 (2011).
- Arenkiel, B. R., et al. In vivo light-induced activation of neural circuitry in transgenic mice expressing channelrhodopsin-2. Neuron. 54 (2), 205-218 (2007).
- Heinemann, U., Pitkänen, A., Buckmaster, P., Galanopoulou, A. S., Moshé, S., et al. Brain slices from human resected tissue. Models of Seizures and Epilepsy. , 285-299 (2017).
- Florez, C., et al. In vitro recordings of human neocortical oscillations. Cerebral Cortex. 25 (3), 578-597 (2015).
- Lein, P. J., Barnhart, C. D., Pessah, I. N. Acute hippocampal slice preparation and hippocampal slice cultures. Methods in Molecular Biology. , 115-134 (2011).
- Haas, H. L., Schaerer, B., Vosmansky, M. A simple perfusion chamber for the study of nervous tissue slices in vitro. Journal of Neuroscience Methods. 1 (4), 323-325 (1979).
- Poulton, T. J., Ellingson, R. J. Seizure associated with induction of anesthesia with isoflurane. Anesthesiology: The Journal of the American Society of Anesthesiologists. 61 (4), 471-476 (1984).
- Borris, D. J., Bertram, E. H., Kapur, J. Ketamine controls prolonged status epilepticus. Epilepsy Research. 42 (2-3), 117-122 (2000).
- DeFelipe, J., Alonso-Nanclares, L., Arellano, J. I. Microstructure of the neocortex: comparative aspects. Journal of Neurocytology. 31 (3-5), 299-316 (2002).
- Velasco, A. L., Wilson, C. L., Babb, T. L., Engel, J. Functional and anatomic correlates of two frequently observed temporal lobe seizure-onset patterns. Neural Plasticity. 7 (1-2), 49-63 (2000).
- Vlachos, A., Reddy-Alla, S., Papadopoulos, T., Deller, T., Betz, H. Homeostatic regulation of gephyrin scaffolds and synaptic strength at mature hippocampal GABAergic postsynapses. Cerebral Cortex. 23 (11), 2700-2711 (2012).
- Kirmse, K., et al. GABA depolarizes immature neurons and inhibits network activity in the neonatal neocortex in vivo. Nature Communications. 6, 7750 (2015).
- Stein, V., Hermans-Borgmeyer, I., Jentsch, T. J., Hübner, C. A. Expression of the KCl cotransporter KCC2 parallels neuronal maturation and the emergence of low intracellular chloride. Journal of Comparative Neurology. 468 (1), 57-64 (2004).
- Wong, B. Y., Prince, D. A. The lateral spread of ictal discharges in neocortical brain slices. Epilepsy Research. 7 (1), 29-39 (1990).
- Trevelyan, A. J., Sussillo, D., Watson, B. O., Yuste, R. Modular propagation of epileptiform activity: evidence for an inhibitory veto in neocortex. Journal of Neuroscience. 26 (48), 12447-12455 (2006).
- Brahma, B., Forman, R., Stewart, E., Nicholson, C., Rice, M. Ascorbate inhibits edema in brain slices. Journal of Neurochemistry. 74 (3), 1263-1270 (2000).
- MacGregor, D. G., Chesler, M., Rice, M. E. HEPES prevents edema in rat brain slices. Neuroscience Letters. 303 (3), 141-144 (2001).
- Ting, J. T., Daigle, T. L., Chen, Q., Feng, G., Martina, M., Taverna, S. Acute brain slice methods for adult and aging animals: Application of targeted patch clamp analysis and optogenetics. Patch-clamp Methods and Protocols. , 221-242 (2014).
- Swartzwelder, H. S., Lewis, D., Anderson, W., Wilson, W. Seizure-like events in brain slices: suppression by interictal activity. Brain Research. 410 (2), 362-366 (1987).
- Lees, G., Stöhr, T., Errington, A. C. Stereoselective effects of the novel anticonvulsant lacosamide against 4-AP induced epileptiform activity in rat visual cortex in vitro. Neuropharmacology. 50 (1), 98-110 (2006).

